Fish Consumption and Risk of Stroke in Chinese Adults: A Prospective Cohort Study in Shanghai, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Assessment of Diet
2.3. Follow-Up and Ascertainment of Stroke
2.4. Assessment of Covariates
2.5. Statistical Analyses
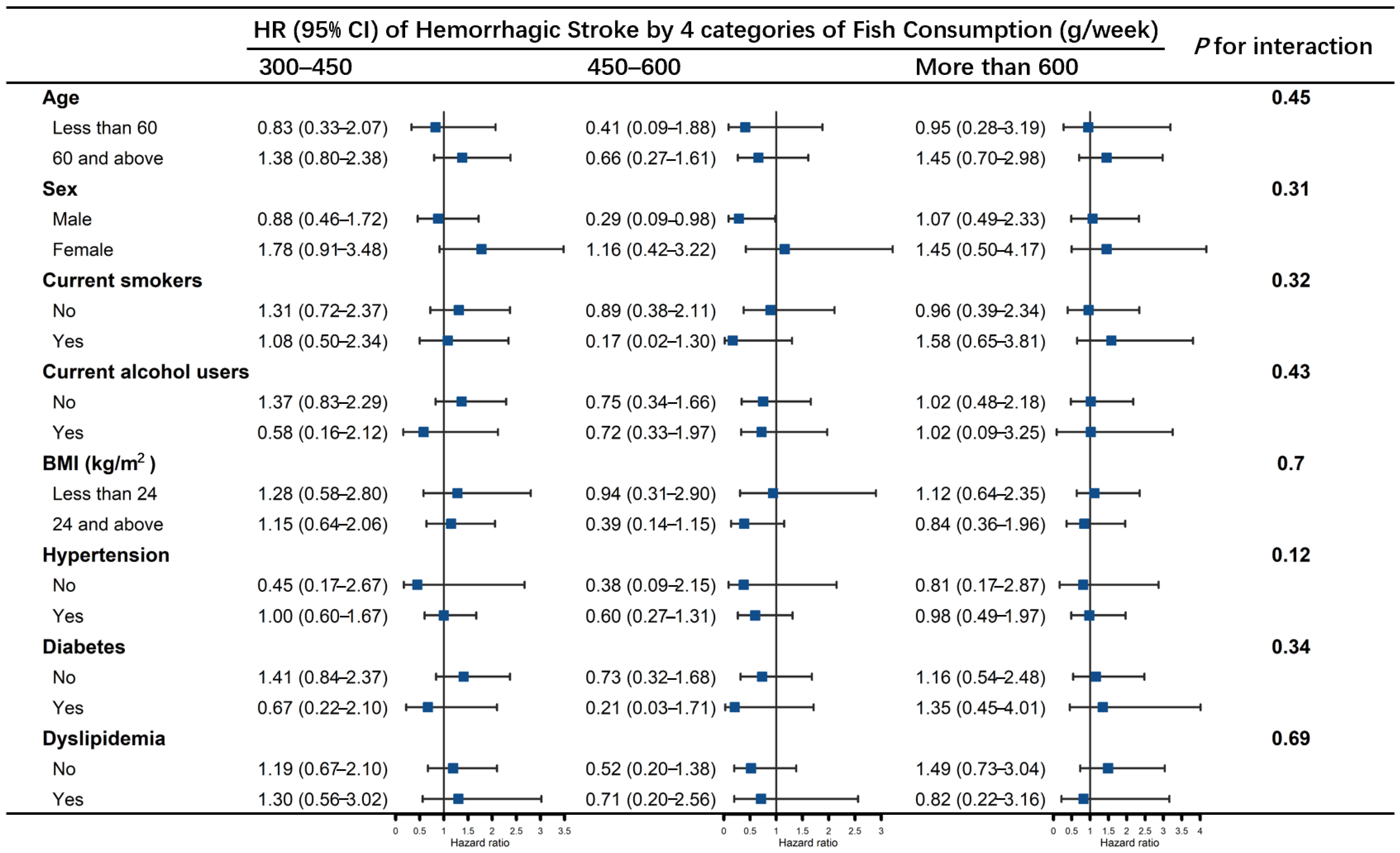
3. Results
3.1. Baseline Characteristics and the Incidence of Stroke
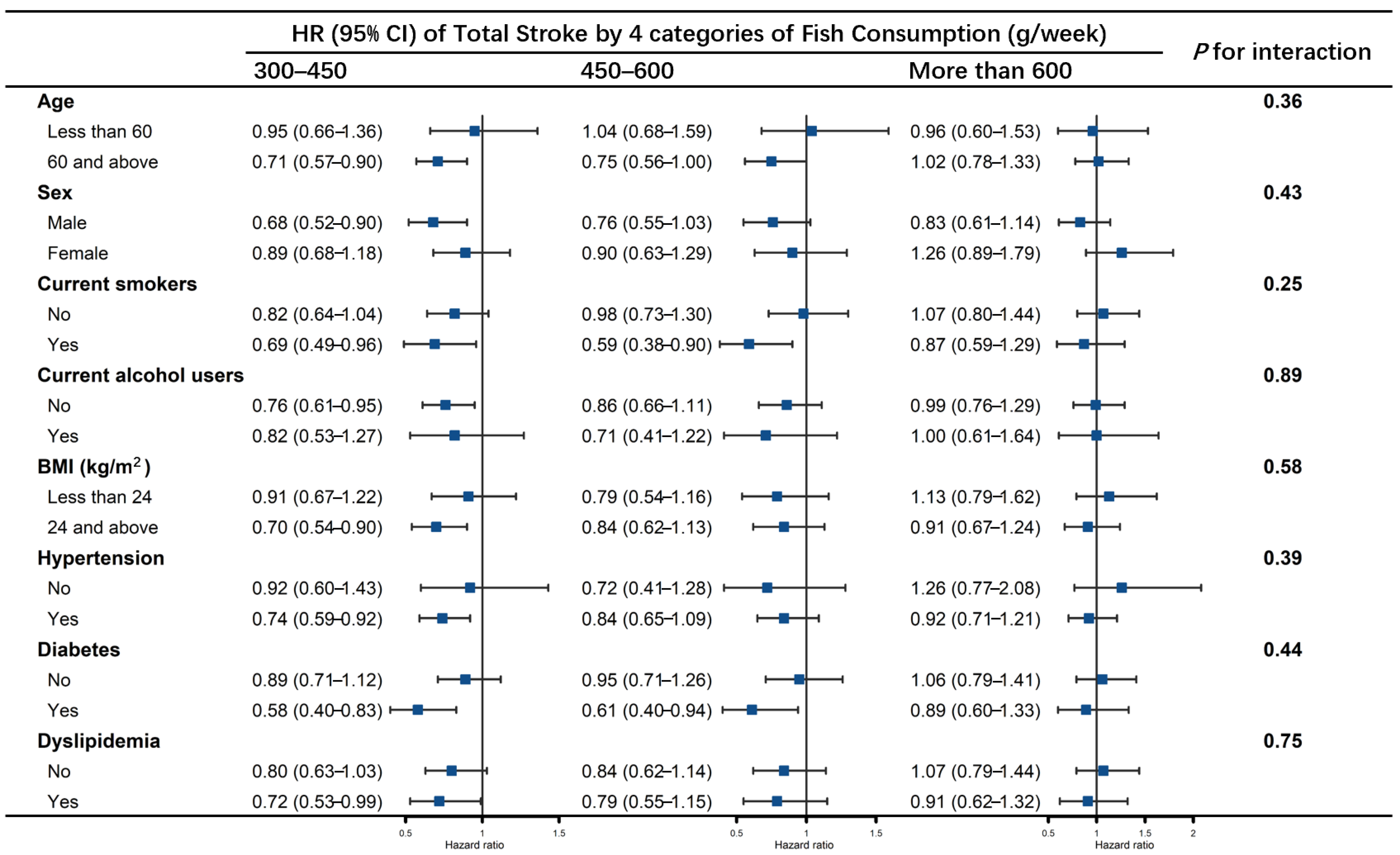
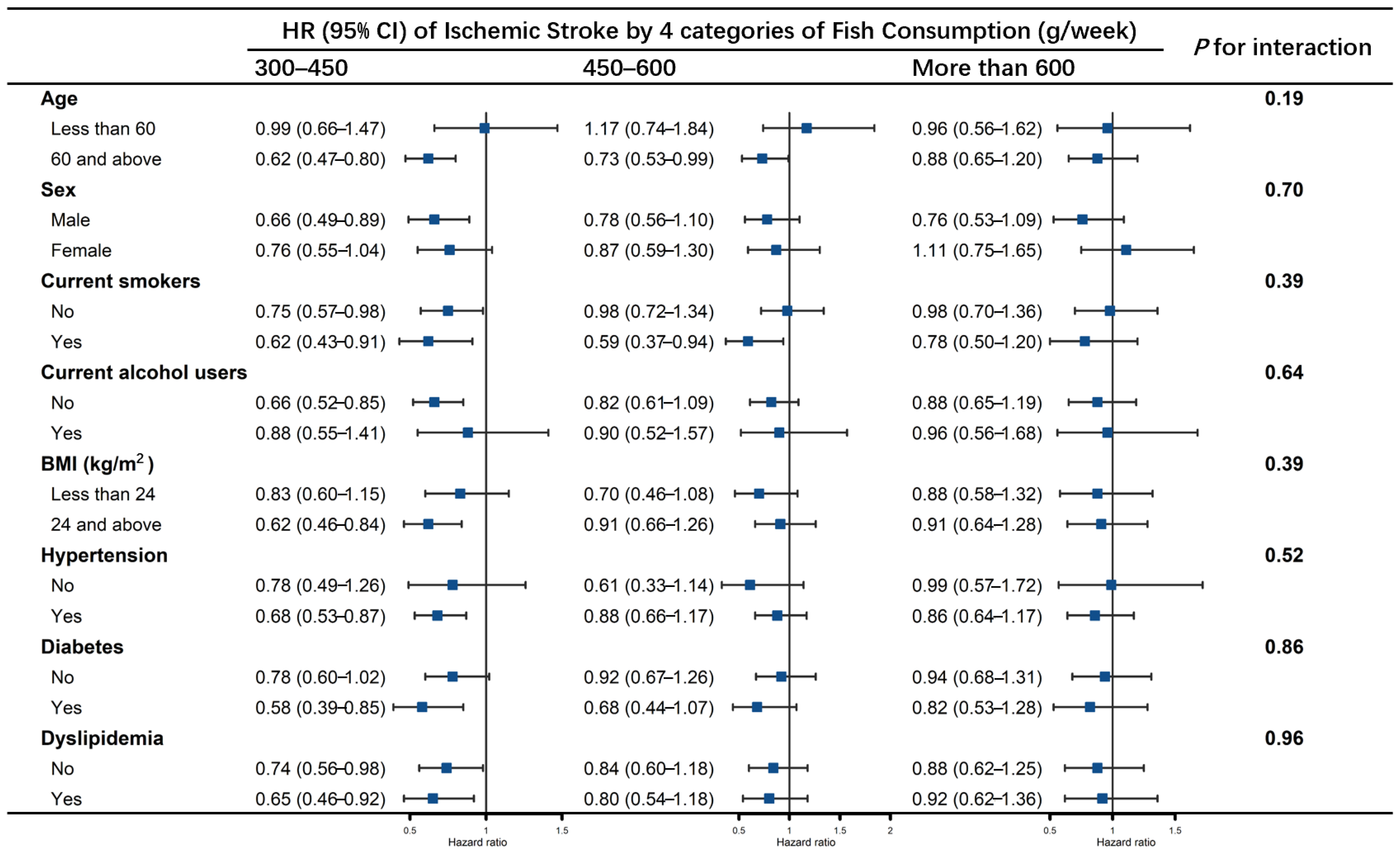
3.2. Association of Fish Consumption with Stroke
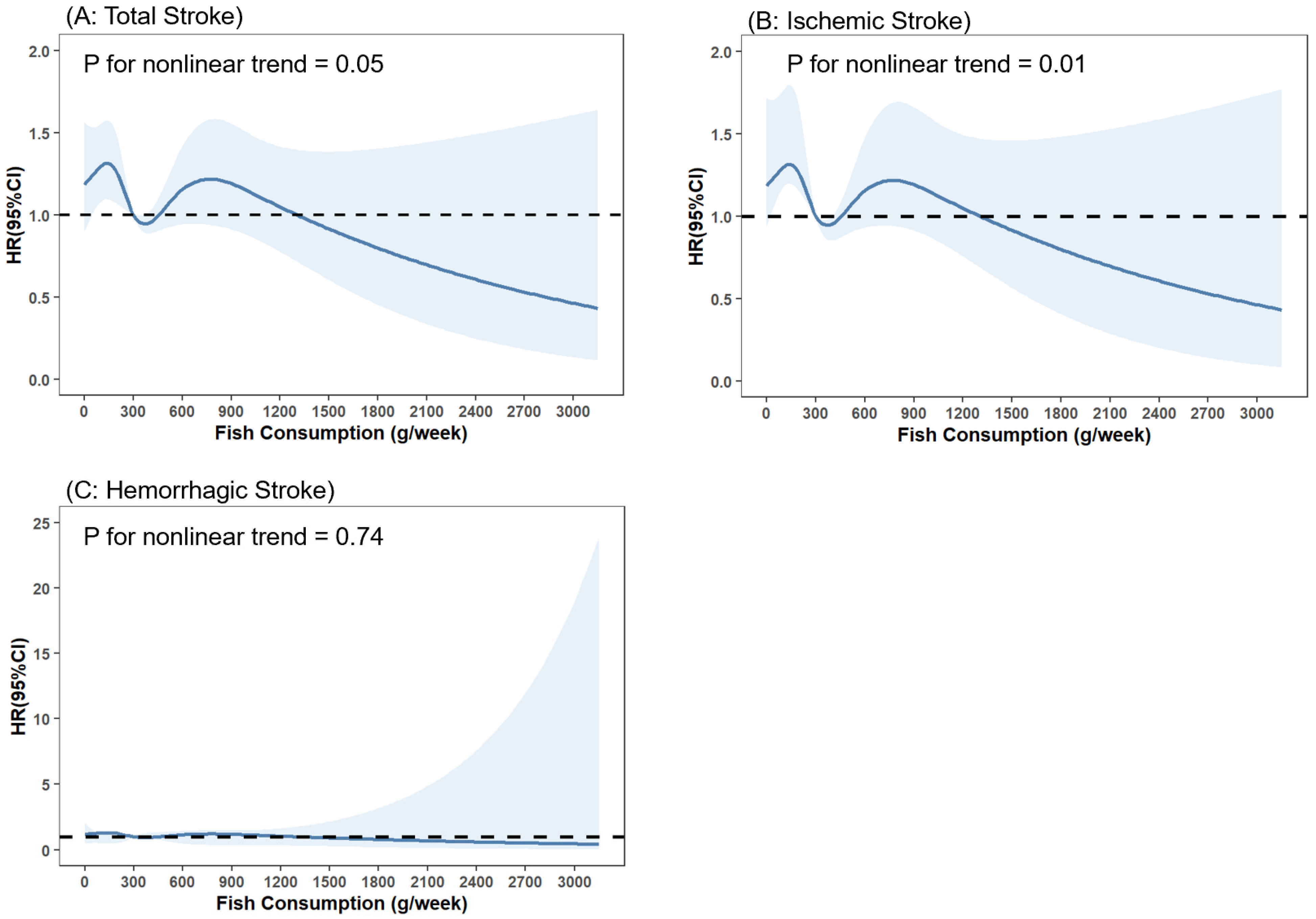
3.3. Dose–Response Analysis of Fish Consumption with Stroke
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PUFAs | polyunsaturated fatty acids |
| SRR | summary relative risk |
| SSACB | Shanghai Suburban Adult Cohort and Biobank study |
| CCDRR | Cardiovascular and Cerebrovascular Disease Registration and Reporting System |
| EMR | Electronic Medical Record System |
| CDSS | Cause-of-Death Surveillance System |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| FFQ | food frequency questionnaire |
| COPD | chronic obstructive pulmonary disease |
| FPG | fasting blood glucose |
| HbA1c | glycosylated hemoglobin, type A1c |
| eGFR | estimated glomerular filtration rate |
| HUA | hyperuricemia |
| HHcy | hyperhomocysteinemia |
| PA | physical activities |
| METs | metabolic equivalent tasks |
| IPAQ | International Physical Activity Questionnaire |
| PSQI | Pittsburgh Sleep Quality Index |
| EPIC-Spain | the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition |
| CDC | center for disease control and prevention |
| PUFAs | polyunsaturated fatty acids |
References
- Pan, X.F.; Marklund, M.; Wu, J.H. Fish consumption for cardiovascular health: Benefits from long-chain omega-3 fatty acids versus potential harms due to mercury. Heart 2019, 105, 1384–1385. [Google Scholar] [CrossRef]
- He, K. Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease--eat fish or take fish oil supplement? Prog. Cardiovasc. Dis. 2009, 52, 95–114. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Hu, F.B.; Cho, E.; Rexrode, K.M.; Albert, C.M.; Manson, J.E. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 2003, 107, 1852–1857. [Google Scholar] [CrossRef]
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in Fish and Possible Associations with Cardiovascular Disease Risk Factors in Metabolic Syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef]
- Mohan, D.; Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.; Wei, L.; et al. Associations of Fish Consumption With Risk of Cardiovascular Disease and Mortality Among Individuals With or Without Vascular Disease From 58 Countries. JAMA Intern. Med. 2021, 181, 631–649. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Wu, J.H.; Mozaffarian, D. ω-3 fatty acids, atherosclerosis progression and cardiovascular outcomes in recent trials: New pieces in a complex puzzle. Heart 2014, 100, 530–533. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N. Fish consumption and the risk of stroke: A dose-response meta-analysis. Stroke 2011, 42, 3621–3623. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J. Am. Heart. Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef]
- Djoussé, L.; Akinkuolie, A.O.; Wu, J.H.; Ding, E.L.; Gaziano, J.M. Fish consumption, omega-3 fatty acids and risk of heart failure: A meta-analysis. Clin. Nutr. 2012, 31, 846–853. [Google Scholar] [CrossRef]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Fish consumption and risk of stroke in Swedish women. Am. J. Clin. Nutr. 2011, 93, 487–493. [Google Scholar] [CrossRef]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Jayedi, A.; Shab-Bidar, S. Fish Consumption and the Risk of Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Adv. Nutr. 2020, 11, 1123–1133. [Google Scholar] [CrossRef]
- Shao, M.Y.; Jiang, C.Q.; Zhang, W.S.; Zhu, F.; Jin, Y.L.; Woo, J.; Cheng, K.K.; Lam, T.H.; Xu, L. Association of fish consumption with risk of all-cause and cardiovascular disease mortality: An 11-year follow-up of the Guangzhou Biobank Cohort Study. Eur. J. Clin. Nutr. 2022, 76, 389–396. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, B.; Wang, R.; Zhu, M.; Shao, Y.; Wang, N.; Liu, X.; Zhang, T.; Jiang, F.; Wang, W.; et al. Cohort profile: Protocol and baseline survey for the Shanghai Suburban Adult Cohort and Biobank (SSACB) study. BMJ Open 2020, 10, e035430. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Chen, S.; Yang, X.; Liu, F.; Li, Y.; Li, J.; Cao, J.; Liu, X.; Chen, J.; et al. Association of Lipids With Ischemic and Hemorrhagic Stroke: A Prospective Cohort Study Among 267 500 Chinese. Stroke 2019, 50, 3376–3384. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Wang, N.; Zhu, M.; Liu, X.; Wang, R.; Jiang, F.; Chen, Y.; Zhao, Q.; Zhao, G. Central But Not General Obesity Is Positively Associated with the Risk of Hyperhomocysteinemia in Middle-Aged Women. Nutrients 2019, 11, 1614. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zhang, L.; Wang, X.; Hao, G.; Zhang, Z.; Shao, L.; Tian, Y.; Dong, Y.; Zheng, C.; et al. Status of Hypertension in China: Results From the China Hypertension Survey, 2012-2015. Circulation 2018, 137, 2344–2356. [Google Scholar] [CrossRef]
- Hou, X.; Lu, J.; Weng, J.; Ji, L.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; Zhu, D.; Ge, J.; et al. Impact of waist circumference and body mass index on risk of cardiometabolic disorder and cardiovascular disease in Chinese adults: A national diabetes and metabolic disorders survey. PLoS ONE 2013, 8, e57319. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef]
- Song, P.; Zha, M.; Yang, X.; Xu, Y.; Wang, H.; Fang, Z.; Yang, X.; Xia, W.; Zeng, C. Socioeconomic and geographic variations in the prevalence, awareness, treatment and control of dyslipidemia in middle-aged and older Chinese. Atherosclerosis 2019, 282, 57–66. [Google Scholar] [CrossRef]
- Borghi, C.; Domienik-Karłowicz, J.; Tykarski, A.; Widecka, K.; Filipiak, K.J.; Jaguszewski, M.J.; Narkiewicz, K.; Mancia, G. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk: 2021 update. Cardiol. J. 2021, 28, 72728. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Hengeveld, L.M.; Praagman, J.; Beulens, J.W.J.; Brouwer, I.A.; van der Schouw, Y.T.; Sluijs, I. Fish consumption and risk of stroke, coronary heart disease, and cardiovascular mortality in a Dutch population with low fish intake. Eur. J. Clin. Nutr. 2018, 72, 942–950. [Google Scholar] [CrossRef]
- Amiano, P.; Chamosa, S.; Etxezarreta, N.; Arriola, L.; Moreno-Iribas, C.; Huerta, J.M.; Egües, N.; Guevara, M.; Navarro, C.; Chirlaque, M.D.; et al. No association between fish consumption and risk of stroke in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain): A 13·8-year follow-up study. Public Health Nutr. 2016, 19, 674–681. [Google Scholar] [CrossRef]
- Nanri, A.; Mizoue, T.; Noda, M.; Takahashi, Y.; Matsushita, Y.; Poudel-Tandukar, K.; Kato, M.; Oba, S.; Inoue, M.; Tsugane, S. Fish intake and type 2 diabetes in Japanese men and women: The Japan Public Health Center-based Prospective Study. Am. J. Clin. Nutr. 2011, 94, 884–891. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Robinson, J.G.; Wallace, R.B.; Peterson, L.L.; Bao, W. Association of fried food consumption with all cause, cardiovascular, and cancer mortality: Prospective cohort study. BMJ 2019, 364, k5420. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Rajaram, S.; Haddad, E.H.; Mejia, A.; Sabaté, J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: A randomized controlled study. Am. J. Clin. Nutr. 2009, 89, 1657S–1663S. [Google Scholar] [CrossRef]
- Raatz, S.K.; Johnson, L.K.; Rosenberger, T.A.; Picklo, M.J. Twice weekly intake of farmed Atlantic salmon (Salmo salar) positively influences lipoprotein concentration and particle size in overweight men and women. Nutr. Res. 2016, 36, 899–906. [Google Scholar] [CrossRef]
- Theobald, H.E.; Chowienczyk, P.J.; Whittall, R.; Humphries, S.E.; Sanders, T.A. LDL cholesterol-raising effect of low-dose docosahexaenoic acid in middle-aged men and women. Am. J. Clin. Nutr. 2004, 79, 558–563. [Google Scholar] [CrossRef]
- Von Schacky, C. n-3 fatty acids and the prevention of coronary atherosclerosis. Am. J. Clin. Nutr. 2000, 71, 224S–227S. [Google Scholar] [CrossRef]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 12978. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids and cardiovascular disease: The epidemiological evidence. Env. Health Prev. Med. 2002, 6, 203–209. [Google Scholar] [CrossRef]
- Wennberg, M.; Bergdahl, I.A.; Stegmayr, B.; Hallmans, G.; Lundh, T.; Skerfving, S.; Strömberg, U.; Vessby, B.; Jansson, J.H. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br. J. Nutr. 2007, 98, 1038–1045. [Google Scholar] [CrossRef]
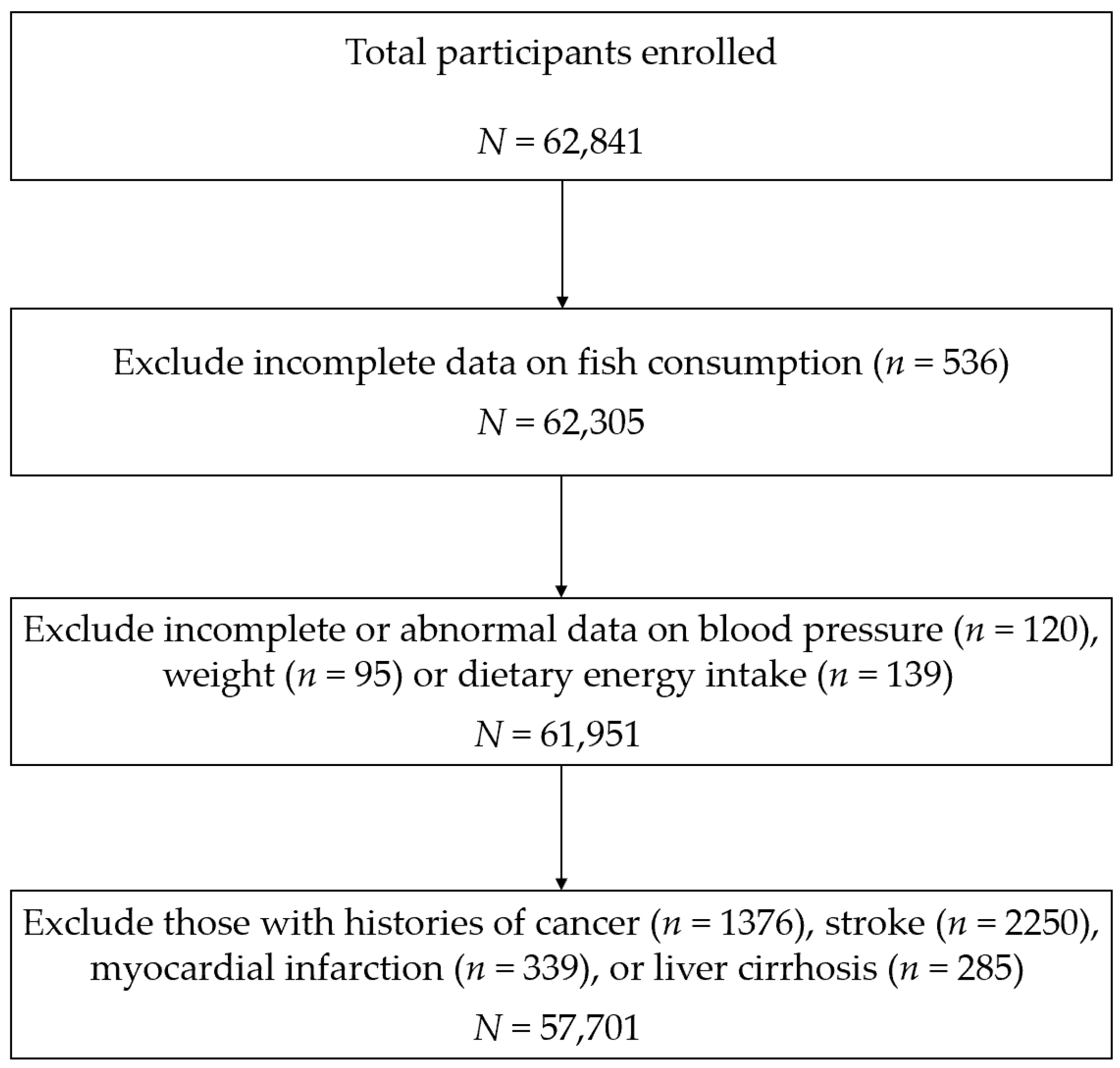
| Characteristics | Total (n = 57,701) | Fish Consumption (g/week) | p-Value | |||
|---|---|---|---|---|---|---|
| Less than 300 (n = 25,977) | 300–450 (n = 13,222) | 450–600 (n = 9223) | More than 600 (n = 9279) | |||
| Age (year) | 59 (51–65) | 60 (52–66) | 58 (50–64) | 57 (49–64) | 57 (49–64) | <0.001 |
| Men (%) | 22,914 (39.71) | 9780 (37.65) | 5093 (38.52) | 3895 (42.23) | 4146 (44.68) | <0.001 |
| Newly developed stroke (%) | ||||||
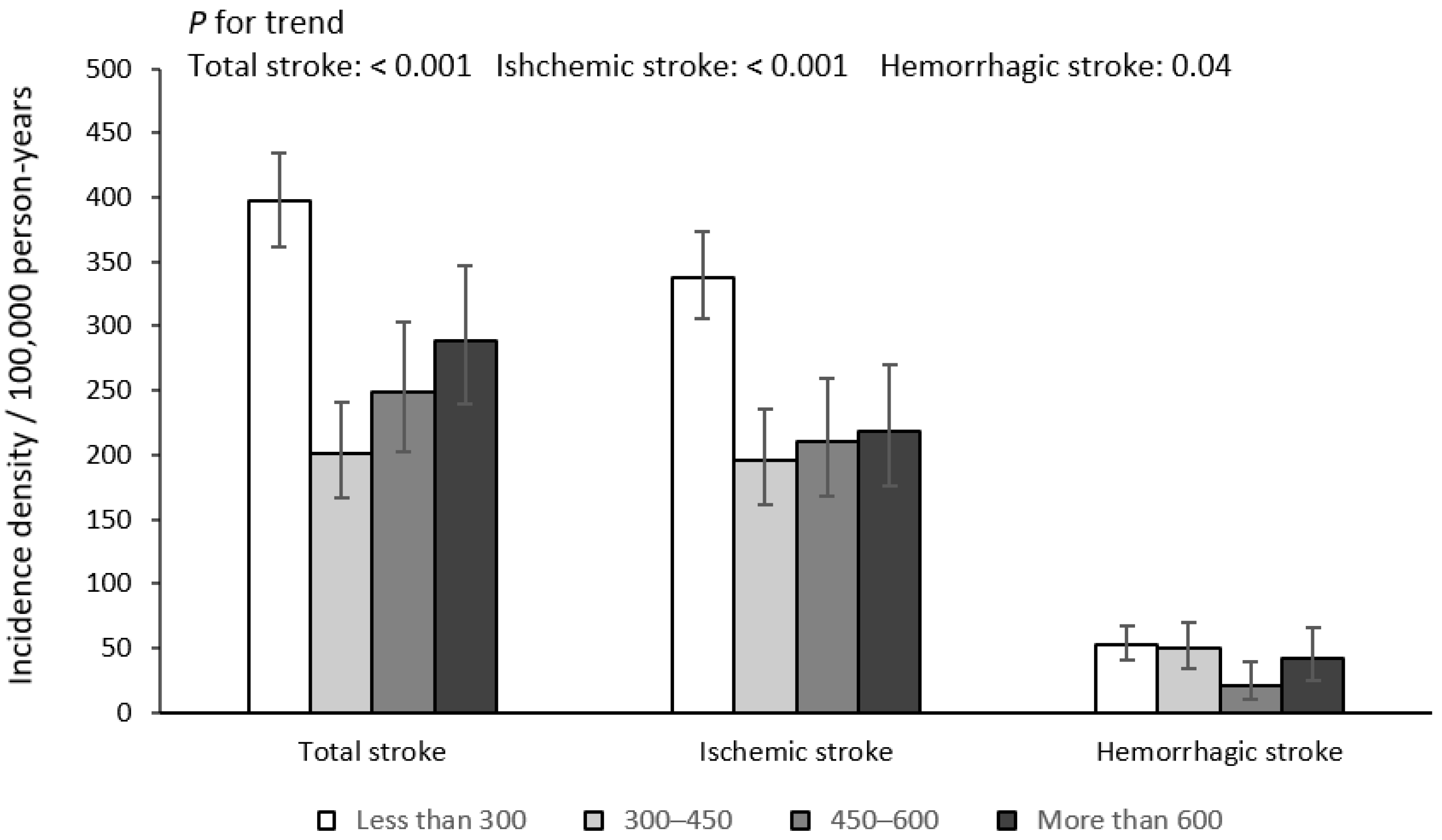
| Total stroke | 807 (1.40) | 457 (1.76) | 144 (1.09) | 95 (1.03) | 111 (1.20) | <0.001 |
| Ischemic stroke | 664 (1.15) | 389 (1.50) | 111 (0.84) | 80 (0.87) | 84 (0.91) | <0.001 |
| Hemorrhagic stroke | 113 (0.20) | 61 (0.23) | 28 (0.21) | 8 (0.09) | 16 (0.17) | 0.04 |
| Educational levels (%) | <0.001 | |||||
| Primary school or below | 20,712 (35.90) | 12,071 (46.47) | 4232 (32.01) | 2234 (24.22) | 2175 (23.44) | |
| Junior high school | 22,531 (39.05) | 8924 (34.35) | 5613 (42.45) | 4053 (43.94) | 3941 (42.47) | |
| Senior high school | 8927 (15.47) | 3038 (11.69) | 2126 (16.08) | 1821 (19.74) | 1942 (20.93) | |
| High school or above | 5531 (9.59) | 1944 (7.48) | 1251 (9.46) | 1115 (12.09) | 1221 (13.16) | |
| Marriage status (%) | <0.001 | |||||
| Married | 53,455 (92.64) | 23,715 (91.25) | 12,375 (93.59) | 8692 (94.24) | 8683 (93.58) | |
| Unmarried | 867 (1.50) | 451 (1.74) | 170 (1.29) | 110 (1.19) | 136 (1.47) | |
| Divorced and other | 3379 (5.86) | 1821 (7.01) | 677 (5.12) | 421 (4.56) | 460 (4.96) | |
| Retired (%) | 35,355 (61.27) | 16,715 (64.35) | 8006 (60.55) | 5306 (57.53) | 5328 (57.42) | <0.001 |
| Smoking status (%) | <0.001 | |||||
| Never | 44,642 (77.37) | 20,438 (78.68) | 10,331 (78.13) | 7021 (76.12) | 6852 (73.84) | |
| Former | 2110 (3.66) | 954 (3.67) | 442 (3.34) | 307 (3.33) | 407 (4.39) | |
| Current | 10,949 (18.98) | 4585 (17.65) | 2449 (18.52) | 1895 (20.55) | 2020 (21.77) | |
| Current alcohol users (%) | 7216 (12.51) | 3025 (11.64) | 1550 (11.72) | 1210 (13.12) | 1431 (15.42) | <0.001 |
| Current tea drinkers (%) | 16,724 (28.98) | 6559 (25.25) | 3844 (29.07) | 3044 (33.00) | 3277 (35.32) | <0.001 |
| PA level (%) | 0.25 | |||||
| Low | 19,233 (33.33) | 8776 (33.78) | 4180 (31.61) | 3133 (33.97) | 3144 (33.88) | |
| Moderate | 19,211 (33.29) | 8750 (33.68) | 4436 (33.55) | 3037 (32.93) | 2988 (32.20) | |
| High | 19,257 (33.37) | 8451 (32.53) | 4606 (34.84) | 3053 (33.10) | 3147 (33.92) | |
| PSQI score | 3 (2–5) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 3 (2–5) | <0.001 |
| BMI (kg/m2) | 23.94 (21.72–26.21) | 23.98 (21.76–26.29) | 23.92 (21.72–26.14) | 23.91 (21.67–26.11) | 23.86 (21.64–26.20) | <0.001 |
| History of chronic diseases (%) | ||||||
| Hypertension | 36,468 (63.20) | 16,764 (64.53) | 8343 (63.10) | 5667 (61.44) | 5694 (61.36) | <0.001 |
| CHD | 2454 (4.25) | 1187 (4.57) | 520 (3.93) | 389 (4.22) | 358 (3.86) | 0.003 |
| Diabetes | 8663 (15.01) | 4163 (16.03) | 1883 (14.24) | 1288 (13.97) | 1329 (14.32) | <0.001 |
| CKD | 8251 (14.30) | 3989 (15.36) | 1843 (13.94) | 1235 (13.39) | 1184 (12.76) | <0.001 |
| Dyslipidemia | 17,981 (31.16) | 7967 (30.67) | 4044 (30.59) | 2986 (32.38) | 2984 (32.16) | 0.001 |
| HUA | 9692 (16.80) | 4552 (17.52) | 2069 (15.65) | 1506 (16.33) | 1565 (16.87) | 0.03 |
| HHcy | 13,288 (23.03) | 6070 (23.37) | 2970 (22.46) | 2054 (22.27) | 2194 (23.64) | 0.70 |
| COPD | 295 (0.51) | 145 (0.56) | 59 (0.45) | 41 (0.44) | 50 (0.54) | 0.37 |
| Chronic bronchitis | 3797 (6.58) | 1905 (7.33) | 812 (6.14) | 544 (5.90) | 536 (5.78) | <0.001 |
| Asthma | 1203 (2.08) | 607 (2.34) | 271 (2.05) | 150 (1.63) | 175 (1.89) | <0.001 |
| Energy intake (kcal/day) | 1457.37 (1156.38–1831.30) | 1283.41 (1028.47–1609.73) | 1454.11 (1190.47–1769.58) | 1610.22 (1330.03–1956.11) | 1850.86 (1504.12–2332.94) | <0.001 |
| Fruit (g/day) | 80.00 (28.57–150.00) | 50.00 (25.71–100.00) | 100.00 (28.57–150.00) | 100.00 (42.86–150.00) | 100.00 (50.00–200.00) | <0.001 |
| Vegetables (g/day) | 242.86 (135.15–406.35) | 206.58 (104.11–334.29) | 242.86 (150.00–378.57) | 300.00 (200.00–428.57) | 350.00 (228.57–542.86) | <0.001 |
| Peanuts (g/week) | 23.03 (3.43–100.03) | 23.03 (1.75–93.03) | 39.97 (5.74–100.03) | 46.06 (9.24–140.00) | 46.06 (5.74–175.00) | <0.001 |
| Whole grains (g/day) | 6.58 (0.82–28.57) | 5.14 (0–14.29) | 6.58 (1.64–28.57) | 14.29 (2.47–28.57) | 14.29 (1.97–30.00) | <0.001 |
| Unprocessed meats (g/day) | 48.31 (28.58–85.15) | 35.27 (20.87–59.87) | 51.08 (35.15–79.65) | 67.57 (42.87–106.58) | 78.58 (45.01–128.99) | <0.001 |
| Processed meats (eat %) | 30,282 (52.48) | 11,827 (45.53) | 6409 (48.47) | 4772 (51.74) | 4411 (47.54) | <0.001 |
| Bean products (g/day) | 18.40 (6.58–41.72) | 14.29 (3.29–28.57) | 27.44 (14.29–41.72) | 28.57 (14.29–57.15) | 28.57 (14.29–71.43) | <0.001 |
| Salt (g/day) | 4.00 (2.67–6.25) | 4.17 (2.67–6.67) | 3.33 (2.67–5.56) | 3.33 (2.67–5.56) | 4.00 (2.67–6.40) | <0.001 |
| Oil (g/day) | 28.57 (20.00–41.67) | 33.33 (20.00–41.67) | 27.78 (20.00–41.67) | 27.78 (20.00–41.67) | 27.78 (18.52–41.67) | <0.001 |
| Fish Consumption (g/week) | p for Trend | ||||
|---|---|---|---|---|---|
| Less than 300 | 300–450 | 450–600 | More than 600 | ||
| Total stroke | |||||
| Model 1 | 1.00 | 0.74 (0.61–0.89) | 0.77 (0.62–0.96) | 0.88 (0.72–1.09) | 0.05 |
| Model 2 | 1.00 | 0.77 (0.64–0.94) | 0.82 (0.65–1.04) | 1.00 (0.80–1.27) | 0.55 |
| Model 3 | 1.00 | 0.78 (0.64–0.94) | 0.82 (0.65–1.04) | 1.00 (0.79–1.26) | 0.52 |
| Ischemic stroke | |||||
| Model 1 | 1.00 | 0.68 (0.55–0.84) | 0.78 (0.61–0.99) | 0.80 (0.66–1.02) | 0.01 |
| Model 2 | 1.00 | 0.70 (0.56–0.87) | 0.83 (0.64–1.07) | 0.91 (0.70–1.18) | 0.20 |
| Model 3 | 1.00 | 0.70 (0.57–0.88) | 0.82 (0.64–1.07) | 0.90 (0.69–1.17) | 0.18 |
| Hemorrhagic stroke | |||||
| Model 1 | 1.00 | 1.08 (0.69–1.69) | 0.49 (0.23–1.03) | 0.96 (0.55–1.68) | 0.47 |
| Model 2 | 1.00 | 1.22 (0.77–1.96) | 0.58 (0.27–1.25) | 1.24 (0.67–2.32) | 0.81 |
| Model 3 | 1.00 | 1.21 (0.76–1.94) | 0.58 (0.27–1.25) | 1.24 (0.66–2.30) | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Yi, K.; Wu, Y.; Su, X.; Xiang, Y.; Yu, Y.; Tang, M.; Tong, X.; Zaid, M.; Jiang, Y.; et al. Fish Consumption and Risk of Stroke in Chinese Adults: A Prospective Cohort Study in Shanghai, China. Nutrients 2022, 14, 4239. https://doi.org/10.3390/nu14204239
Cui S, Yi K, Wu Y, Su X, Xiang Y, Yu Y, Tang M, Tong X, Zaid M, Jiang Y, et al. Fish Consumption and Risk of Stroke in Chinese Adults: A Prospective Cohort Study in Shanghai, China. Nutrients. 2022; 14(20):4239. https://doi.org/10.3390/nu14204239
Chicago/Turabian StyleCui, Shuheng, Kangqi Yi, Yiling Wu, Xuyan Su, Yu Xiang, Yuting Yu, Minhua Tang, Xin Tong, Maryam Zaid, Yonggen Jiang, and et al. 2022. "Fish Consumption and Risk of Stroke in Chinese Adults: A Prospective Cohort Study in Shanghai, China" Nutrients 14, no. 20: 4239. https://doi.org/10.3390/nu14204239
APA StyleCui, S., Yi, K., Wu, Y., Su, X., Xiang, Y., Yu, Y., Tang, M., Tong, X., Zaid, M., Jiang, Y., Zhao, Q., & Zhao, G. (2022). Fish Consumption and Risk of Stroke in Chinese Adults: A Prospective Cohort Study in Shanghai, China. Nutrients, 14(20), 4239. https://doi.org/10.3390/nu14204239






