Antioxidant Efficacy of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Elderly Subjects: A Randomized, Double Blind, Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design Description
2.2. Eligibility Criteria for Participants
2.3. Settings and Locations
2.4. Intervention
2.5. Randomization and Masking
2.6. Primary and Secondary Objectives and Outcome Measures
2.6.1. Concentration of Glutathione in Erythrocytes
2.6.2. d-ROMs Hematic Concentration
2.6.3. Serum TNF-α Levels
2.6.4. Short Form Health Survey (SF-36) Questionnaire
2.6.5. Menopause Rating Scale (MRS)
2.6.6. Blood and Urine Analysis
- Complete blood cell count: White Blood Cells (WBC), Red Blood cells (RBC), Haemoglobin (Hb), Haematocrit (Hct), and Platelet count (PLT);
- Biochemistry test: Blood Urea Nitrogen (BUN, azotemia), Cholesterol, High-Density Lipoprotein Cholesterol (HDL-C), Low-Density Lipoprotein Cholesterol (LDL-C), Triglycerides, Albumin, Total bilirubin, Alkaline Phosphatase (ALP), Gamma-glutamyl Transferase (r-GT), Creatinine, High-sensitivity C-reactive Protein (hs-CRP);
- Urinalysis: Specific Gravity, pH, White Blood Cells (leukocytes, WBC), Occult blood (erythrocytes), Nitrite, Protein, Glucose, Ketone body, Urobilinogen, Bilirubin.
2.7. Statistical Analysis
3. Results
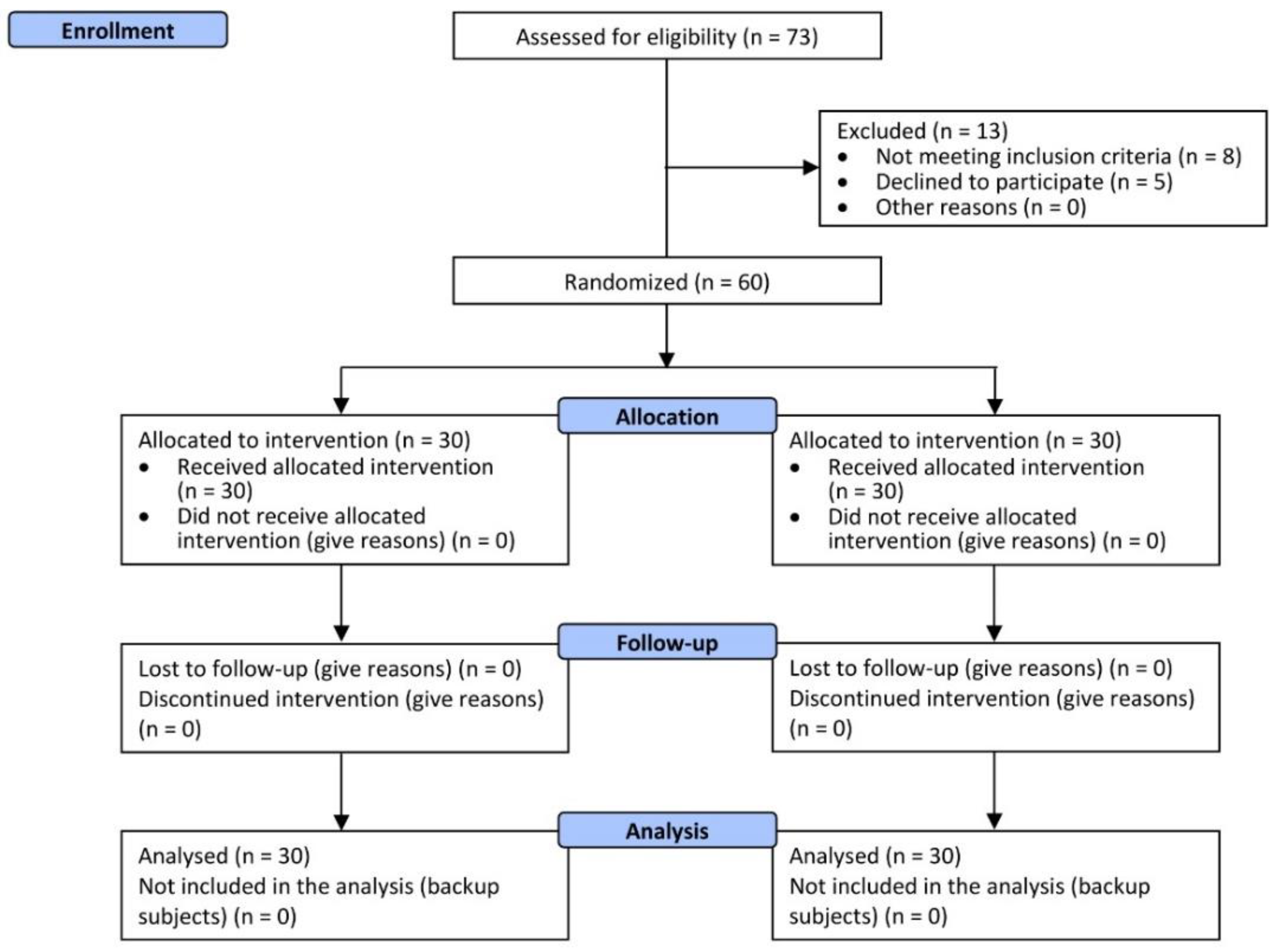
3.1. Participants and Product Tolerability
3.2. Primary Endpoints: The Systemic Antioxidants Pool
3.3. Secondary Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiechio, S.; Zammataro, M.; Barresi, M.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Rapisarda, P. A Standardized Extract Prepared from Red Orange and Lemon Wastes Blocks High-Fat Diet-Induced Hyperglycemia and Hyperlipidemia in Mice. Molecules 2021, 26, 4291. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Frasca, G.; Rizza, L.; Rapisarda, P.; Bonina, F. Antiinflammatory Effects of a Red Orange Extract in Human Keratinocytes Treated with Interferon-Gamma and Histamine. Phytother. Res. 2010, 24, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Legua, P.; Modica, G.; Porras, I.; Conesa, A.; Continella, A. Bioactive Compounds, Antioxidant Activity and Fruit Quality Evaluation of Eleven Blood Orange Cultivars. J. Sci. Food Agric. 2022, 102, 2960–2971. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Lo Cascio, R.; Rapisarda, P.; Dederen, J.C. In Vitro Antioxidant Activity and in Vivo Photoprotective Effect of a Red Orange Extract. Int. J. Cosmet. Sci. 1998, 20, 331–342. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of Their Molecular Mechanisms and Experimental Models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Frasca, G.; Panico, A.M.; Bonina, F.; Messina, R.; Rizza, L.; Musumeci, G.; Rapisarda, P.; Cardile, V. Involvement of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 in the Anti-Inflammatory Effects of a Red Orange Extract in Human Chondrocytes. Nat. Prod. Res. 2010, 24, 1469–1480. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Huff, M.W. Protection from Metabolic Dysregulation, Obesity, and Atherosclerosis by Citrus Flavonoids: Activation of Hepatic PGC1α-Mediated Fatty Acid Oxidation. PPAR Res. 2012, 2012, 857142. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Cardile, V.; Graziano, A.C.E.; Venditti, A. Clinical Evaluation of Moro (Citrus Sinensis (L.) Osbeck) Orange Juice Supplementation for the Weight Management. Nat. Prod. Res. 2015, 29, 2256–2260. [Google Scholar] [CrossRef]
- Bonina, F.P.; Puglia, C.; Frasca, G.; Cimino, F.; Trombetta, D.; Tringali, G.; Roccazzello, A.; Insiriello, E.; Rapisarda, P.; Saija, A. Protective Effects of a Standardised Red Orange Extract on Air Pollution-Induced Oxidative Damage in Traffic Police Officers. Nat. Prod. Res. 2008, 22, 1544–1551. [Google Scholar] [CrossRef]
- Cimino, F.; Cristani, M.; Saija, A.; Bonina, F.P.; Virgili, F. Protective Effects of a Red Orange Extract on UVB-Induced Damage in Human Keratinocytes. Biofactors 2007, 30, 129–138. [Google Scholar] [CrossRef]
- Puglia, C.; Offerta, A.; Saija, A.; Trombetta, D.; Venera, C. Protective Effect of Red Orange Extract Supplementation against UV-Induced Skin Damages: Photoaging and Solar Lentigines. J. Cosmet. Dermatol. 2014, 13, 151–157. [Google Scholar] [CrossRef]
- Nobile, V.; Burioli, A.; Yu, S.; Zhifeng, S.; Cestone, E.; Insolia, V.; Zaccaria, V.; Malfa, G.A. Photoprotective and Antiaging Effects of a Standardized Red Orange (Citrus Sinensis (L.) Osbeck) Extract in Asian and Caucasian Subjects: A Randomized, Double-Blind, Controlled Study. Nutrients 2022, 14, 2241. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The Interactions of Flavonoids within Neuronal Signalling Pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-ΚB Signaling. Nutrients 2019, 11, E648. [Google Scholar] [CrossRef]
- Huang, H.; Hu, C.; Xu, L.; Zhu, X.; Zhao, L.; Min, J. The Effects of Hesperidin on Neuronal Apoptosis and Cognitive Impairment in the Sevoflurane Anesthetized Rat Are Mediated Through the PI3/Akt/PTEN and Nuclear Factor-ΚB (NF-ΚB) Signaling Pathways. Med. Sci. Monit. 2020, 26, e920522. [Google Scholar] [CrossRef]
- Zhang, S.; Tomata, Y.; Sugiyama, K.; Sugawara, Y.; Tsuji, I. Citrus Consumption and Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. Br. J. Nutr. 2017, 117, 1174–1180. [Google Scholar] [CrossRef]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the Brain: Interactions at the Blood-Brain Barrier and Their Physiological Effects on the Central Nervous System. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, H.; Wang, J.; Liu, G.; Cui, S.W.; Kang, J.; Jiang, Y.; Wang, H. Naringenin Prolongs Lifespan and Delays Aging Mediated by IIS and MAPK in Caenorhabditis Elegans. Food Funct. 2021, 12, 12127–12141. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, T.; Chen, L.; Zheng, B.; Liu, R.H. Nobiletin Delays Aging and Enhances Stress Resistance of Caenorhabditis Elegans. Int. J. Mol. Sci. 2020, 21, E341. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 15 June 2022).
- Krabbe, K.S.; Pedersen, M.; Bruunsgaard, H. Inflammatory Mediators in the Elderly. Exp. Gerontol. 2004, 39, 687–699. [Google Scholar] [CrossRef]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic Low-Grade Inflammation and Age-Related Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient Synthesis of Glutathione Underlies Oxidative Stress in Aging and Can Be Corrected by Dietary Cysteine and Glycine Supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef]
- Cysteine Disulfides (Cys-ss-X) as Sensitive Plasma Biomarkers of Oxidative Stress—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30643157/ (accessed on 12 September 2022).
- Rao, P.M.; Kelly, D.M.; Jones, T.H. Testosterone and Insulin Resistance in the Metabolic Syndrome and T2DM in Men. Nat. Rev. Endocrinol. 2013, 9, 479–493. [Google Scholar] [CrossRef]
- Doshi, S.B.; Agarwal, A. The Role of Oxidative Stress in Menopause. J. Midlife Health 2013, 4, 140–146. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Farquhar, C.; Roberts, H.; Lethaby, A.; Lee, J. Long-Term Hormone Therapy for Perimenopausal and Postmenopausal Women. Cochrane Database Syst. Rev. 2017, 1, CD004143. [Google Scholar] [CrossRef]
- Gurney, E.P.; Nachtigall, M.J.; Nachtigall, L.E.; Naftolin, F. The Women’s Health Initiative Trial and Related Studies: 10 Years Later: A Clinician’s View. J. Steroid Biochem. Mol. Biol. 2014, 142, 4–11. [Google Scholar] [CrossRef]
- Hill, D.A.; Crider, M.; Hill, S.R. Hormone Therapy and Other Treatments for Symptoms of Menopause. Am. Fam. Physician 2016, 94, 884–889. [Google Scholar]
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone Therapy Position Statement of The North American Menopause Society. Menopause 2017, 24, 728–753. [Google Scholar] [CrossRef]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal Hormone Therapy and Health Outcomes during the Intervention and Extended Poststopping Phases of the Women’s Health Initiative Randomized Trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef]
- Krebs, E.E.; Ensrud, K.E.; MacDonald, R.; Wilt, T.J. Phytoestrogens for Treatment of Menopausal Symptoms: A Systematic Review. Obstet. Gynecol. 2004, 104, 824–836. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, E2649. [Google Scholar] [CrossRef]
- De Franciscis, P.; Colacurci, N.; Riemma, G.; Conte, A.; Pittana, E.; Guida, M.; Schiattarella, A. A Nutraceutical Approach to Menopausal Complaints. Medicina 2019, 55, 544. [Google Scholar] [CrossRef]
- Morini, F.; Dusatti, F.; Bonina, F.P.; Saija, A.; Ferro, M. Iron-Induced Lipid Peroxidation in Human Skin-Derived Cell Lines: Protection by a Red Orange Extract. Altern. Lab. Anim. 2000, 28, 427–433. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; Acquaviva, R.; La Mantia, A.; Di Giacomo, C. Phytocomplex of a Standardized Extract from Red Orange (Citrus Sinensis, L. Osbeck) against Photoaging. Cells 2022, 11, 1447. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; Lauro, M.R.; Cerrato, M.; Aquino, R.P. Development of Health Products from Natural Sources. Curr. Med. Chem. 2019, 26, 4606–4630. [Google Scholar] [CrossRef]


| Active | Placebo | |||||
|---|---|---|---|---|---|---|
| T0 | T2 | T8 | T0 | T2 | T8 | |
| 1. Physical functioning | 85.7 | 84.5 | 88.3 | 83.0 | 81.3 | 89.8 p = 0.0143 |
| 2. Role limit. (physical health) | 64.2 | 76.7 p = 0.0211 | 83.3 p = 0.0021, ‡ | 69.2 | 78.3 | 70.0 |
| 3. Role limit. (emot. probl.) | 54.4 | 78.9 p = 0.0015 | 78.9 p = 0.0190 | 65.6 | 68.9 | 78.9 p = 0.0498 |
| 4. Energy/fatigue | 59.5 | 62.7 p = 0.0522 | 63.3 | 61.7 | 61.5 | 63.7 |
| 5. Emotional well-being | 35.3 | 31.3 p = 0.0455 | 28.3 p = 0.0136 | 30.7 | 32.5 | 26.1 p = 0.0404 |
| 6. Social functioning | 53.8 | 53.3 | 54.2 ‡ | 59.6 | 58.8 | 60.0 |
| 7. Pain | 68.5 | 71.3 | 74.2 | 74.5 | 72.9 | 75.9 |
| 8. General health | 45.8 | 49.8 p = 0.0010 | 52.7 p = 0.0030 | 46.3 | 48.5 | 50.3 p = 0.0874 |
| 9. Physical functioning | 85.7 | 84.5 | 88.3 | 83.0 | 81.3 | 89.8 p = 0.0143 |
| Active | Placebo | |||||
|---|---|---|---|---|---|---|
| T0 | T2 | T8 | T0 | T2 | T8 | |
| 1. Hot flashes and sweating | 2.6 | 1.9 p = 0.0052 | 1.7 p = 0.0033 | 2.1 | 1.9 | 1.6 |
| 2. Heart problems | 1.7 | 1.4 | 0.9 p = 0.0230 | 1.8 | 1.5 | 1.4 |
| 3. Sleep problems | 2.2 | 1.5 p = 0.0292 | 1.3 p = 0.0033 | 2.1 | 1.7 p = 0.0339 | 1.8 |
| 4. Feeling unhappy | 1.8 | 1.5 | 1.1 p = 0.0261 | 1.7 | 1.7 | 1.6 |
| 5. Nervousness | 1.9 | 1.5 | 1.0 p = 0.0020, ‡ | 1.9 | 1.7 | 1.6 |
| 6. Anxiety | 1.7 | 1.3 p = 0.0559 | 1.1 p = 0.0087 | 1.8 | 1.7 | 1.5 |
| 7. Phys. and mental fatigue | 2.3 | 1.7 p = 0.0085 | 1.3 p = 0.0026 | 2.2 | 1.9 | 1.9 |
| 8. Sexual problems | 1.9 | 1.2 p = 0.0110 | 0.7 p = 0.0010, ‡ | 1.4 | 1.3 | 1.4 |
| 9. Urinary problems | 1.6 | 1.3 | 1.0 p = 0.0290 | 1.6 | 1.4 | 1.5 |
| 10. Vaginal dryness | 1.8 | 1.0 p = 0.0163 | 0.7 p = 0.0009, ‡ | 1.8 | 1.4 | 1.5 |
| 11. Joint and muscle problems | 2.0 | 1.7 | 1.5 p = 0.0310 | 1.8 | 1.7 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobile, V.; Pisati, M.; Cestone, E.; Insolia, V.; Zaccaria, V.; Malfa, G.A. Antioxidant Efficacy of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Elderly Subjects: A Randomized, Double Blind, Controlled Study. Nutrients 2022, 14, 4235. https://doi.org/10.3390/nu14204235
Nobile V, Pisati M, Cestone E, Insolia V, Zaccaria V, Malfa GA. Antioxidant Efficacy of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Elderly Subjects: A Randomized, Double Blind, Controlled Study. Nutrients. 2022; 14(20):4235. https://doi.org/10.3390/nu14204235
Chicago/Turabian StyleNobile, Vincenzo, Marta Pisati, Enza Cestone, Violetta Insolia, Vincenzo Zaccaria, and Giuseppe Antonio Malfa. 2022. "Antioxidant Efficacy of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Elderly Subjects: A Randomized, Double Blind, Controlled Study" Nutrients 14, no. 20: 4235. https://doi.org/10.3390/nu14204235
APA StyleNobile, V., Pisati, M., Cestone, E., Insolia, V., Zaccaria, V., & Malfa, G. A. (2022). Antioxidant Efficacy of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Elderly Subjects: A Randomized, Double Blind, Controlled Study. Nutrients, 14(20), 4235. https://doi.org/10.3390/nu14204235








