Fetal Alcohol Spectrum Disorder and Iron Homeostasis
Abstract
1. Introduction
1.1. Prenatal Alcohol Exposure, Maternal Nutrition, and Iron Homeostasis
1.2. Normal Iron Metabolism
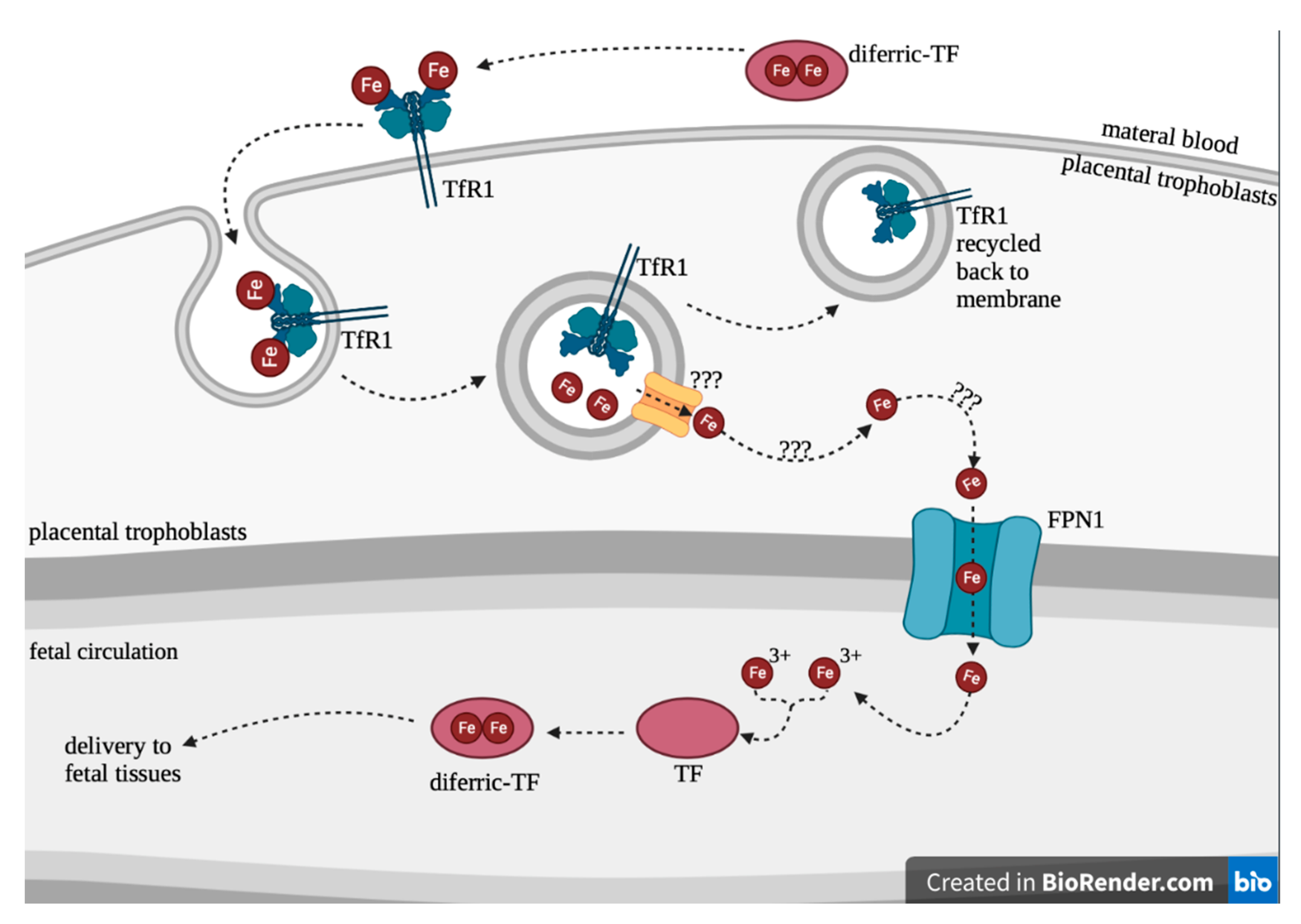
1.3. Placental Iron Transportation and Maternal-Fetal Iron Regulation
1.4. Blood–Brain Barrier Development and Iron Transportation
1.5. Animal Models of FASD
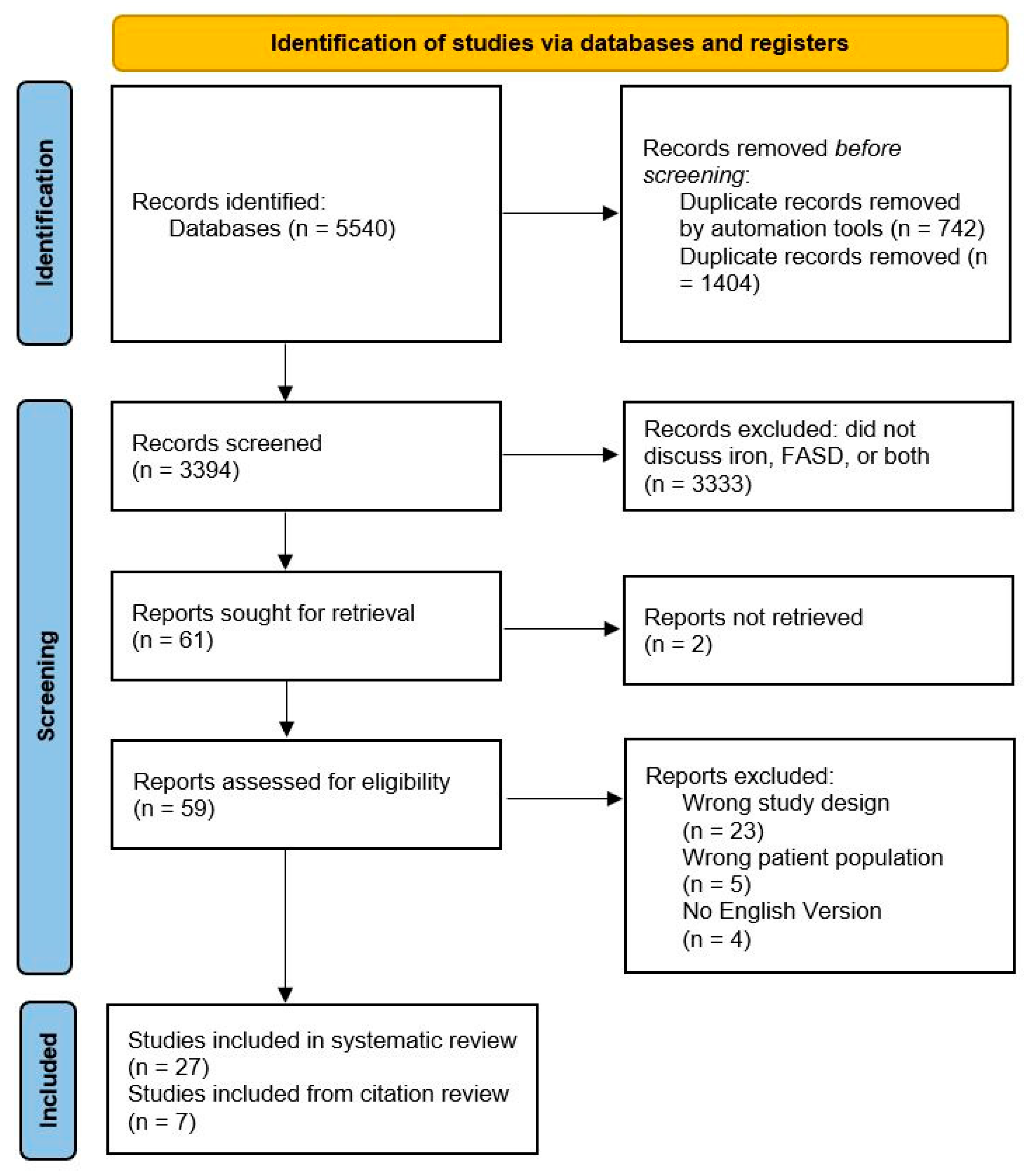
2. Search Methodology
3. Search Results
4. Literature Synthesis
4.1. Animal Studies of Alcohol and Iron in Pregnancy
4.1.1. Neurodevelopment
4.1.2. Iron Regulating Genes, Storage, and Indices
4.1.3. Weight and Growth
4.1.4. Supplementation
4.2. Iron and Alcohol in Pregnancy: Population Studies
4.2.1. Extent of the Problem
4.2.2. Neurodevelopment
4.2.3. Iron Deficiency and Iron Deficiency Anemia
4.2.4. Weight and Growth
4.2.5. Supplementation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gambling, L.; Lang, C.; McArdle, H.J. Fetal Regulation of Iron Transport during Pregnancy. Am. J. Clin. Nutr. 2011, 94, 1903S–1907S. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 2018, 319, 474–482. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef]
- Williams, J.F.; Smith, V.C.; Committee on Substance Abuse. Fetal Alcohol Spectrum Disorders. Pediatrics 2015, 136, e1395–e1406. [Google Scholar] [CrossRef]
- Helfrich, K.K.; Saini, N.; Kling, P.J.; Smith, S.M. Maternal Iron Nutriture as a Critical Modulator of Fetal Alcohol Spectrum Disorder Risk in Alcohol-Exposed Pregnancies. Biochem. Cell Biol. 2018, 96, 204–212. [Google Scholar] [CrossRef]
- Burd, L.; Blair, J.; Dropps, K. Prenatal Alcohol Exposure, Blood Alcohol Concentrations and Alcohol Elimination Rates for the Mother, Fetus and Newborn. J. Perinatol. 2012, 32, 652–659. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Brooke, L.E.; Snell, C.L.; Marais, A.-S.; Hendricks, L.S.; Croxford, J.A.; Viljoen, D.L. Maternal Risk Factors for Fetal Alcohol Syndrome in the Western Cape Province of South Africa: A Population-Based Study. Am. J. Public Health 2005, 95, 1190–1199. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Weisberg, P.; Spong, C. Cigarette Smoking, Alcohol Use and Adverse Pregnancy Outcomes: Implications for Micronutrient Supplementation. J. Nutr. 2003, 133, 1722S–1731S. [Google Scholar] [CrossRef]
- Sebastiani, G.; Borras-Novell, C.; Casanova, M.A.; Tutusaus, M.P.; Martinez, S.F.; Roig, M.D.G.; Garcia-Algar, O. The Effects of Alcohol and Drugs of Abuse on Maternal Nutritional Profile during Pregnancy. Nutrients 2018, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Burd, L.; Roberts, D.; Olson, M.; Odendaal, H. Ethanol and the Placenta: A Review. J. Matern.-Fetal Neonatal Med. 2007, 20, 361–375. [Google Scholar] [CrossRef]
- Keen, C.L.; Uriu-Adams, J.Y.; Skalny, A.; Grabeklis, A.; Grabeklis, S.; Green, K.; Yevtushok, L.; Wertelecki, W.W.; Chambers, C.D. The Plausibility of Maternal Nutritional Status Being a Contributing Factor to the Risk for Fetal Alcohol Spectrum Disorders: The Potential Influence of Zinc Status as an Example. BioFactors 2010, 36, 125–135. [Google Scholar] [CrossRef]
- Young, J.K.; Giesbrecht, H.E.; Eskin, M.N.; Aliani, M.; Suh, M. Nutrition Implications for Fetal Alcohol Spectrum Disorder. Adv. Nutr. 2014, 5, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.D.; Kable, J.A.; Keen, C.L.; Jones, K.L.; Wertelecki, W.; Granovska, I.V.; Pashtepa, A.O.; Chambers, C.D.; the CIFASD. Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Matern. Child Health J. 2015, 19, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Kable, J.A.; Coles, C.D.; Keen, C.L.; Uriu-Adams, J.Y.; Jones, K.L.; Yevtushok, L.; Kulikovsky, Y.; Wertelecki, W.; Pedersen, T.L.; Chambers, C.D.; et al. The Impact of Micronutrient Supplementation in Alcohol-Exposed Pregnancies on Information Processing Skills in Ukrainian Infants. Alcohol 2015, 49, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Naik, V.D.; Lee, J.; Wu, G.; Washburn, S.; Ramadoss, J. Effects of Nutrition and Gestational Alcohol Consumption on Fetal Growth and Development. Nutr. Rev. 2022, 80, 1568–1579. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Krebs, N.F.; Cusick, S.E. The Benefits and Risks of Iron Supplementation in Pregnancy and Childhood. Annu. Rev. Nutr. 2019, 39, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Berti, C.; Mandò, C.; Parisi, F. Placental Iron Transport and Maternal Absorption. Ann. Nutr. Metab. 2011, 59, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Gambling, L.; Czopek, A.; Andersen, H.S.; Holtrop, G.; Srai, S.K.S.; Krejpcio, Z.; McArdle, H.J. Fetal Iron Status Regulates Maternal Iron Metabolism during Pregnancy in the Rat. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1063–R1070. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Wong, S.; Koenig, M.D.; Tussing-Humphreys, L.; Chu, A.; Lelić, M.; Ganz, T.; Nemeth, E. Effects of Maternal Iron Status on Placental and Fetal Iron Homeostasis. J. Clin. Investig. 2019, 130, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Gambling, L.; Danzeisen, R.; Gair, S.; Lea, R.G.; Charania, Z.; Solanky, N.; Joory, K.D.; Srai, S.K.S.; Mcardle, H.J. Effect of Iron Deficiency on Placental Transfer of Iron and Expression of Iron Transport Proteins in Vivo and in Vitro. Biochem. J. 2001, 356, 883–889. [Google Scholar] [CrossRef]
- Milman, N. Iron and Pregnancy—A Delicate Balance. Ann. Hematol. 2006, 85, 559–565. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily Oral Iron Supplementation during Pregnancy. Cochrane Database Syst. Rev. 2015, 12, CD004736. [Google Scholar] [CrossRef]
- Juul, S.E.; Derman, R.J.; Auerbach, M. Perinatal Iron Deficiency: Implications for Mothers and Infants. Neonatology 2019, 115, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.F. Iron Deficiency in Pregnancy, Obstetrics, and Gynecology. Hematol. Oncol. Clin. North Am. 2000, 14, 1061–1077. [Google Scholar] [CrossRef]
- Georgieff, M.K. Long-Term Brain and Behavioral Consequences of Early Iron Deficiency. Nutr. Rev. 2011, 69, S43–S48. [Google Scholar] [CrossRef]
- Radlowski, E.C.; Johnson, R.W. Perinatal Iron Deficiency and Neurocognitive Development. Front. Hum. Neurosci. 2013, 7, 585. [Google Scholar] [CrossRef]
- Olson, H.C.; Streissguth, A.P.; Sampson, P.D.; Barr, H.M.; Bookstein, F.L.; Thiede, K. Association of Prenatal Alcohol Exposure with Behavioral and Learning Problems in Early Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 1187–1194. [Google Scholar] [CrossRef]
- Sood, B.; Delaney-Black, V.; Covington, C.; Nordstrom-Klee, B.; Ager, J.; Templin, T.; Janisse, J.; Martier, S.; Sokol, R.J. Prenatal Alcohol Exposure and Childhood Behavior at Age 6 to 7 Years: I. Dose-Response Effect. Pediatrics 2001, 108, E34. [Google Scholar] [CrossRef]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M. Long-Lasting Neural and Behavioral Effects of Iron Deficiency in Infancy. Nutr. Rev. 2006, 64, S34–S91. [Google Scholar] [CrossRef]
- De La Fuente-Ortega, E.; Plaza-Briceno, W.; Vargas-Robert, S.; Haeger, P. Prenatal Ethanol Exposure Misregulates Genes Involved in Iron Homeostasis Promoting a Maladaptation of Iron Dependent Hippocampal Synaptic Transmission and Plasticity. Front. Pharmacol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Rufer, E.S.; Tran, T.D.; Attridge, M.M.; Andrzejewski, M.E.; Flentke, G.R.; Smith, S.M. Adequacy of Maternal Iron Status Protects against Behavioral, Neuroanatomical, and Growth Deficits in Fetal Alcohol Spectrum Disorders. PLoS ONE 2012, 7, e47499. [Google Scholar] [CrossRef] [PubMed]
- Huebner, S.M.; Tran, T.D.; Rufer, E.S.; Crump, P.M.; Smith, S.M. Maternal Iron Deficiency Worsens the Associative Learning Deficits and Hippocampal and Cerebellar Losses in a Rat Model of Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2015, 39, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Shield, K.; Mihic, A.; Chudley, A.E.; Mukherjee, R.A.S.; Bekmuradov, D.; Rehm, J. Comorbidity of Fetal Alcohol Spectrum Disorder: A Systematic Review and Meta-Analysis. Lancet 2016, 387, 978–987. [Google Scholar] [CrossRef]
- Carter, R.C.; Jacobson, S.W.; Molteno, C.D.; Jacobson, J.L. Fetal Alcohol Exposure, Iron-Deficiency Anemia, and Infant Growth. Pediatrics 2007, 120, 559–567. [Google Scholar] [CrossRef]
- Zhang, C. Essential Functions of Iron-Requiring Proteins in DNA Replication, Repair and Cell Cycle Control. Protein Cell 2014, 5, 750–760. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Richardson, D.R.; Lane, D.J.R.; Becker, E.M.; Huang, M.L.-H.; Whitnall, M.; Suryo Rahmanto, Y.; Sheftel, A.D.; Ponka, P. Mitochondrial Iron Trafficking and the Integration of Iron Metabolism between the Mitochondrion and Cytosol. Proc. Natl. Acad. Sci. USA 2010, 107, 10775–10782. [Google Scholar] [CrossRef]
- Eaton, J.W.; Qian, M. Molecular Bases of Cellular Iron Toxicity. Free Radic. Biol. Med. 2002, 32, 833–840. [Google Scholar] [CrossRef]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of Mammalian Iron Homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef]
- Ganz, T. Cellular Iron: Ferroportin Is the Only Way Out. Cell Metab. 2005, 1, 155–157. [Google Scholar] [CrossRef]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The Iron Exporter Ferroportin/Slc40a1 Is Essential for Iron Homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of Cellular Iron Metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Ponka, P.; Lok, C.N. The Transferrin Receptor: Role in Health and Disease. Int. J. Biochem. Cell Biol. 1999, 31, 1111–1137. [Google Scholar] [CrossRef]
- Dunn, L.L.; Suryo Rahmanto, Y.; Richardson, D.R. Iron Uptake and Metabolism in the New Millennium. Trends Cell Biol. 2007, 17, 93–100. [Google Scholar] [CrossRef]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and Characterization of a Mammalian Proton-Coupled Metal-Ion Transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Gruenheid, S.; Canonne-Hergaux, F.; Gauthier, S.; Hackam, D.J.; Grinstein, S.; Gros, P. The Iron Transport Protein NRAMP2 Is an Integral Membrane Glycoprotein That Colocalizes with Transferrin in Recycling Endosomes. J. Exp. Med. 1999, 189, 831–841. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Regulation of Iron Metabolism by Hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef]
- Eisenstein, R.S. Iron Regulatory Proteins and the Molecular Control of Mammalian Iron Metabolism. Annu. Rev. Nutr. 2000, 20, 627–662. [Google Scholar] [CrossRef]
- McArdle, H.J.; Douglas, A.J.; Bowen, B.J.; Morgan, E.H. The Mechanism of Iron Uptake by the Rat Placenta. J. Cell. Physiol. 1985, 124, 446–450. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Haddad-Tóvolli, R.; Dragano, N.R.V.; Ramalho, A.F.S.; Velloso, L.A. Development and Function of the Blood-Brain Barrier in the Context of Metabolic Control. Front. Neurosci. 2017, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Kosman, D.J. Mechanisms and Regulation of Iron Trafficking across the Capillary Endothelial Cells of the Blood-Brain Barrier. Front. Mol. Neurosci. 2015, 8, 31. [Google Scholar] [CrossRef]
- Mao, J.; McKean, D.M.; Warrier, S.; Corbin, J.G.; Niswander, L.; Zohn, I.E. The Iron Exporter Ferroportin 1 Is Essential for Development of the Mouse Embryo, Forebrain Patterning and Neural Tube Closure. Development 2010, 137, 3079–3088. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R.; Li, Y.; Rankin, C.H. Effects of Developmental Exposure to Ethanol on Caenorhabditis Elegans. Alcohol. Clin. Exp. Res. 2008, 32, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Loucks, E.; Ahlgren, S. Assessing Teratogenic Changes in a Zebrafish Model of Fetal Alcohol Exposure. J. Vis. Exp. 2012, 61, 3704. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Andreu-Fernández, V.; Navarro-Tapia, E.; Aras-López, R.; Serra-Delgado, M.; Martínez, L.; García-Algar, O.; Gómez-Roig, M.D. Murine Models for the Study of Fetal Alcohol Spectrum Disorders: An Overview. Front. Pediatr. 2020, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.A.; Walsh, K.; Dunstan, R.; Dunkley, P.R.; Murdoch, R.N. Maternal Hepatic, Endometrial, and Embryonic Levels of Zn, Mg, Cu, and Fe Following Alcohol Consumption during Pregnancy in QS Mice. Res. Commun. Alcohol Subst. Abuse 1995, 16, 207–219. [Google Scholar]
- Gordon, E.F.; Zemel, M.B. Effects of Prenatal Ethanol Exposure on Iron Utilization in the Rat. Nutr. Res. 1984, 4, 469–475. [Google Scholar] [CrossRef]
- Heil, S.H.; Hungund, B.L.; Zheng, Z.H.; Jen, K.L.C.; Subramanian, M.G. Ethanol and Lactation: Effects on Milk Lipids and Serum Constituents. Alcohol 1999, 18, 43–48. [Google Scholar] [CrossRef]
- Helfrich, K.K.; Saini, N.; Kwan, S.T.C.; Rivera, O.C.; Hodges, R.; Smith, S.M. Gestational Iron Supplementation Improves Fetal Outcomes in a Rat Model of Prenatal Alcohol Exposure. Nutrients 2022, 14, 1653. [Google Scholar] [CrossRef]
- Huebner, S.M.; Blohowiak, S.E.; Kling, P.J.; Smith, S.M. Prenatal Alcohol Exposure Alters Fetal Iron Distribution and Elevates Hepatic Hepcidin in a Rat Model of Fetal Alcohol Spectrum Disorders. J. Nutr. 2016, 146, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Huebner, S.M.; Helfrich, K.K.; Saini, N.; Blohowiak, S.E.; Cheng, A.A.; Kling, P.J.; Smith, S.M. Dietary Iron Fortification Normalizes Fetal Hematology, Hepcidin, and Iron Distribution in a Rat Model of Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2018, 42, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Kwan, S.T.C.; Kezer, C.A.; Helfrich, K.K.; Saini, N.; Huebner, S.M.; Flentke, G.R.; Kling, P.J.; Smith, S.M. Maternal Iron Nutriture Modulates Placental Development in a Rat Model of Fetal Alcohol Spectrum Disorder. Alcohol 2020, 84, 57–66. [Google Scholar] [CrossRef]
- Mendelson, R.A.; Huber, A.M. The Effect of Duration of Alcohol Administration on the Deposition of Trace Elements in the Fetal Rat. Adv. Exp. Med. Biol. 1980, 132, 295–304. [Google Scholar]
- Miller, M.W.; Roskams, A.J.; Connor, J.R. Iron Regulation in the Developing Rat Brain: Effect of in Utero Ethanol Exposure. J. Neurochem. 1995, 65, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Olynyk, J.; Hall, P.; Reed, W.; Williams, P.; Kerr, R.; Mackinnon, M. A Long-Term Study of the Interaction between Iron and Alcohol in an Animal Model of Iron Overload. J. Hepatol. 1995, 22, 671–676. [Google Scholar] [CrossRef]
- Saini, N.; Helfrich, K.K.; Kwan, S.T.C.; Huebner, S.M.; Abazi, J.; Flentke, G.R.; Blohowiak, S.E.; Kling, P.J.; Smith, S.M. Alcohol’s Dysregulation of Maternal-Fetal IL-6 and p-STAT3 Is a Function of Maternal Iron Status. Alcohol. Clin. Exp. Res. 2019, 43, 2332–2343. [Google Scholar] [CrossRef]
- Sanchez, J.; Casas, M.; Rama, R. Effect of Chronic Ethanol Administration on Iron Metabolism in the Rat. Eur. J. Haematol. 1988, 41, 321–325. [Google Scholar] [CrossRef]
- Sozo, F.; Dick, A.M.; Bensley, J.G.; Kenna, K.; Brien, J.F.; Harding, R.; De Matteo, R. Alcohol Exposure during Late Ovine Gestation Alters Fetal Liver Iron Homeostasis without Apparent Dysmorphology. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 304, R1121–R1129. [Google Scholar] [CrossRef]
- Brooten, D.; Peters, M.A.; Glatts, M.; Gaffney, S.E.; Knapp, M.; Cohen, S.; Jordan, C. A Survey of Nutrition, Caffeine, Cigarette and Alcohol Intake in Early Pregnancy in an Urban Clinic Population. J. Nurse. Midwifery 1987, 32, 85–90. [Google Scholar] [CrossRef]
- Carter, R.; Jacobson, J.L.; Molteno, C.D.; Jiang, H.; Meintjes, E.M.; Jacobson, S.W.; Duggan, C. Effects of Heavy Prenatal Alcohol Exposure and Iron Deficiency Anemia on Child Growth and Body Composition through Age 9 Years. Alcohol. Clin. Exp. Res. 2012, 36, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.C.; Senekal, M.; Dodge, N.C.; Bechard, L.J.; Meintjes, E.M.; Molteno, C.D.; Duggan, C.P.; Jacobson, J.L.; Jacobson, S.W. Maternal Alcohol Use and Nutrition During Pregnancy: Diet and Anthropometry. Alcohol. Clin. Exp. Res. 2017, 41, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.C.; Georgieff, M.K.; Ennis, K.M.; Dodge, N.C.; Wainwright, H.; Meintjes, E.M.; Duggan, C.P.; Molteno, C.D.; Jacobson, J.L.; Jacobson, S.W. Prenatal Alcohol-Related Alterations in Maternal, Placental, Neonatal, and Infant Iron Homeostasis. Am. J. Clin. Nutr. 2021, 114, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Hamrick, K.J.; Corbin, K.D.; Hasken, J.M.; Marais, A.S.; Brooke, L.E.; Blankenship, J.; Hoyme, H.E.; Gossage, J.P. Dietary Intake, Nutrition, and Fetal Alcohol Spectrum Disorders in the Western Cape Province of South Africa. Reprod. Toxicol. 2014, 46, 31–39. [Google Scholar] [CrossRef]
- May, P.A.; Hamrick, K.J.; Corbin, K.D.; Hasken, J.M.; Marais, A.-S.; Blankenship, J.; Hoyme, H.E.; Gossage, J.P. Maternal Nutritional Status as a Contributing Factor for the Risk of Fetal Alcohol Spectrum Disorders. Reprod. Toxicol. 2016, 59, 101–108. [Google Scholar] [CrossRef]
- Molteno, C.D.; Jacobson, J.L.; Carter, R.C.; Dodge, N.C.; Jacobson, S.W. Infant Emotional Withdrawal: A Precursor of Affective and Cognitive Disturbance in Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2014, 38, 479–488. [Google Scholar] [CrossRef]
- Nakhid, D.; McMorris, C.A.; Sun, H.; Gibbard, B.; Tortorelli, C.; Lebel, C. Brain Iron and Mental Health Symptoms in Youth with and without Prenatal Alcohol Exposure. Nutrients 2022, 14, 2213. [Google Scholar] [CrossRef]
- Shrestha, S.; Jimenez, E.; Garrison, L.; Pribis, P.; Raisch, D.W.; Stephen, J.M.; Bakhireva, L.N. Dietary Intake Among Opioid- and Alcohol-Using Pregnant Women. Subst. Use Misuse 2018, 53, 260–269. [Google Scholar] [CrossRef]
- Skalny, A.V.; Berezkina, E.S.; Kiyaeva, E.V.; Alidzhanova, I.E.; Grabeklis, A.R.; Tinkov, A.A. The Effect of Alcohol Consumption on Maternal and Cord Blood Electrolyte and Trace Element Levels. Acta Sci. Pol. Technol. Aliment. 2016, 15, 439–445. [Google Scholar] [CrossRef]
- Streissguth, A.P.; Barr, H.M.; Labbe, R.F.; Smith, J.R.; Darby, B.L.; Smith, N.J.; Martin, D.C.; Doan, R.N. Alcohol Use and Iron Status in Pregnant Women. Alcohol. Clin. Exp. Res. 1983, 7, 227–230. [Google Scholar] [CrossRef]
- Connor, J.R. Iron Acquisition and Expression of Iron Regulatory Proteins in the Developing Brain: Manipulation by Ethanol Exposure, Iron Deprivation and Cellular Dysfunction. Dev. Neurosci. 1994, 16, 233–247. [Google Scholar] [CrossRef] [PubMed]
- McArdle, H.J.; Gambling, L.; Kennedy, C. Iron Deficiency during Pregnancy: The Consequences for Placental Function and Fetal Outcome. Proc. Nutr. Soc. 2013, 73, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Benkovic, S.A. Iron Regulation in the Brain: Histochemical, Biochemical, and Molecular Considerations. Ann. Neurol. 1992, 32, S51–S61. [Google Scholar] [CrossRef]
- Wilhelm, C.J.; Guizzetti, M. Fetal Alcohol Spectrum Disorders: An Overview from the Glia Perspective. Front. Integr. Neurosci. 2015, 9, 65. [Google Scholar] [CrossRef]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol Res. Curr. Rev. 2017, 38, 163–171. [Google Scholar]
- Anderson, E.R.; Taylor, M.; Xue, X.; Martin, A.; Moons, D.S.; Omary, M.B.; Shah, Y.M. The Hypoxia-Inducible Factor-C/EBPα Axis Controls Ethanol-Mediated Hepcidin Repression. Mol. Cell. Biol. 2012, 32, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.C.; Senekal, M.; Duggan, C.P.; Dodge, N.C.; Meintjes, E.M.; Molteno, C.D.; Jacobson, J.L.; Jacobson, S.W. Gestational Weight Gain and Dietary Energy, Iron, and Choline Intake Predict Severity of Fetal Alcohol Growth Restriction in a Prospective Birth Cohort. Am. J. Clin. Nutr. 2022, 116, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Werts, R.L.; Van Calcar, S.C.; Wargowski, D.S.; Smith, S.M. Inappropriate Feeding Behaviors and Dietary Intakes in Children with Fetal Alcohol Spectrum Disorder or Probable Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2014, 38, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Fink, B.A.; Fuglestad, A.J.; Eckerle, J.K.; Boys, C.J.; Sandness, K.E.; Radke, J.P.; Miller, N.C.; Lindgren, C.; Brearley, A.M.; et al. Four-Year Follow-up of a Randomized Controlled Trial of Choline for Neurodevelopment in Fetal Alcohol Spectrum Disorder. J. Neurodev. Disord. 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.V.; Kennedy, B.C.; Pisansky, M.T.; Won, K.-J.; Gewirtz, J.C.; Simmons, R.A.; Georgieff, M.K. Prenatal Choline Supplementation Diminishes Early-Life Iron Deficiency-Induced Reprogramming of Molecular Networks Associated with Behavioral Abnormalities in the Adult Rat Hippocampus. J. Nutr. 2016, 146, 484–493. [Google Scholar] [CrossRef] [PubMed]
| Author & Year | Title | Citation | |
|---|---|---|---|
| Basic Science/Animal Model Studies | Amini 1995 | Maternal hepatic, endometrial, and embryonic levels of Zn, Mg, Cu, and Fe following alcohol consumption during pregnancy in QS mice | [57] |
| De La Fuente-Oretega 2019 | Prenatal ethanol exposure misregulates genes involved in iron homeostasis promoting a maladaptation of iron dependent hippocampal synaptic transmission and plasticity | [30] | |
| Gordon 1984 | Effects of prenatal ethanol exposure on iron utilization in the rat | [58] | |
| Heil 1999 | Ethanol and lactation: Effects on milk lipids and serum constituents | [59] | |
| Helfrich 2022 | Gestational Iron Supplementation Improves Fetal Outcomes in a Rat Model of Prenatal Alcohol Exposure | [60] | |
| Huebner 2016 | Prenatal Alcohol Exposure Alters Fetal Iron Distribution and Elevates Hepatic Hepcidin in a Rat Model of Fetal Alcohol Spectrum Disorders | [61] | |
| Huebner 2015 | Maternal iron deficiency worsens the associative learning deficits and hippocampal and cerebellar losses in a rat model of fetal alcohol spectrum disorders | [32] | |
| Huebner 2018 | Dietary Iron Fortification Normalizes Fetal Hematology, Hepcidin, and Iron Distribution in a Rat Model of Prenatal Alcohol Exposure | [62] | |
| Kwan 2020 | Maternal iron nutriture modulates placental development in a rat model of fetal alcohol spectrum disorder | [63] | |
| Mendelson 1980 | The effect of duration of alcohol administration on the deposition of trace elements in the fetal rat | [64] | |
| Miller 1995 | Iron regulation in the developing rat brain: effect of in utero ethanol exposure | [65] | |
| Olynyk 1995 | A long-term study of the interaction between iron and alcohol in an animal model of iron overload | [66] | |
| Rufer 2012 | Adequacy of maternal iron status protects against behavioral, neuroanatomical, and growth deficits in fetal alcohol spectrum disorders | [31] | |
| Saini 2019 | Alcohol’s Dysregulation of Maternal–Fetal IL-6 andp-STAT3 Is a Function of Maternal Iron Status | [67] | |
| Sanchez 1998 | Effect of chronic ethanol administration on iron metabolism in the rat | [68] | |
| Sozo 2013 | Alcohol exposure during late ovine gestation alters fetal liver iron homeostasis without apparent dysmorphology | [69] | |
| Population/Human Studies | Brooten 1987 | A survey of nutrition, caffeine, cigarette and alcohol intake in early pregnancy in an urban clinic population | [70] |
| Carter 2007 | Fetal alcohol exposure, iron-deficiency anemia, and infant growth | [34] | |
| Carter 2012 | Effects of Heavy Prenatal Alcohol Exposure and Iron Deficiency Anemia on Child Growth and Body Composition through Age 9 Years | [71] | |
| Carter 2017 | Maternal Alcohol Use and Nutrition During Pregnancy: Diet and Anthropometry | [72] | |
| Carter 2021 | Prenatal alcohol-related alterations in maternal, placental, neonatal, and infant iron homeostasis | [73] | |
| May 2014 | Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa | [74] | |
| May 2016 | Maternal nutritional status as a contributing factor for the risk of fetal alcohol spectrum disorders | [75] | |
| Molteno 2014 | Infant Emotional Withdrawal: A Precursor of Affective and Cognitive Disturbance in Fetal Alcohol Spectrum Disorders | [76] | |
| Nakhid 2022 | Brain Iron and Mental Health Symptoms in Youth with and without Prenatal Alcohol Exposure | [77] | |
| Shrestha 2018 | Dietary Intake Among Opioid- and Alcohol-Using Pregnant Women | [78] | |
| Skalny 2016 | The effect of alcohol consumption on maternal and cord blood electrolyte and trace element levels | [79] | |
| Streissguth 1983 | Alcohol use and iron status in pregnant women | [80] | |
| Review Articles | Cogswell 2003 | Cigarette smoking, alcohol use and adverse pregnancy outcomes: implications for micronutrient supplementation | [8] |
| Connor 1994 | Iron acquisition and expression of iron regulatory proteins in the developing brain: Manipulation by ethanol exposure, iron deprivation and cellular dysfunction | [81] | |
| Helfrich 2018 | Maternal iron nutriture as a critical modulator of fetal alcohol spectrum disorder risk in alcohol-exposed pregnancies | [5] | |
| McArdle 2014 | Iron deficiency during pregnancy: the consequences for placental function and fetal outcome | [82] | |
| Naik 2022 | Effects of nutrition and gestational alcohol consumption on fetal growth and development | [15] | |
| Sebastiani 2018 | The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradley, R.; Lakpa, K.L.; Burd, M.; Mehta, S.; Katusic, M.Z.; Greenmyer, J.R. Fetal Alcohol Spectrum Disorder and Iron Homeostasis. Nutrients 2022, 14, 4223. https://doi.org/10.3390/nu14204223
Bradley R, Lakpa KL, Burd M, Mehta S, Katusic MZ, Greenmyer JR. Fetal Alcohol Spectrum Disorder and Iron Homeostasis. Nutrients. 2022; 14(20):4223. https://doi.org/10.3390/nu14204223
Chicago/Turabian StyleBradley, Regan, Koffi L. Lakpa, Michael Burd, Sunil Mehta, Maja Z. Katusic, and Jacob R. Greenmyer. 2022. "Fetal Alcohol Spectrum Disorder and Iron Homeostasis" Nutrients 14, no. 20: 4223. https://doi.org/10.3390/nu14204223
APA StyleBradley, R., Lakpa, K. L., Burd, M., Mehta, S., Katusic, M. Z., & Greenmyer, J. R. (2022). Fetal Alcohol Spectrum Disorder and Iron Homeostasis. Nutrients, 14(20), 4223. https://doi.org/10.3390/nu14204223





