Diet and Physical Activity as Determinants of Continuously Measured Glucose Levels in Persons at High Risk of Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
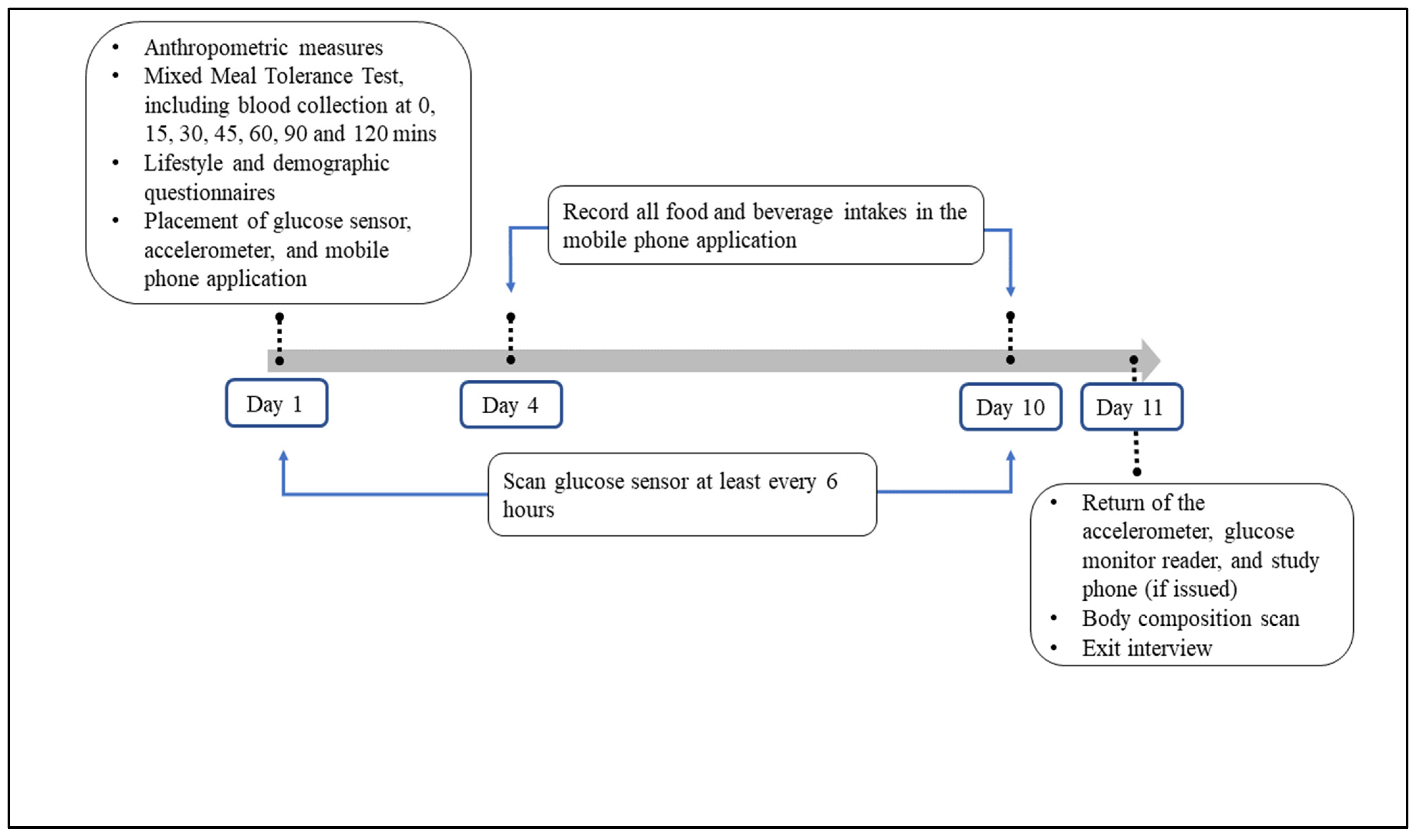
2.2. Study Design
2.2.1. Baseline Assessments
2.2.2. Follow-Up Assessments
2.3. Monitoring of Lifestyle and Glucose Levels during the Free-Living Period
2.3.1. Glucose Monitoring
2.3.2. Assessment of Dietary Intake
2.3.3. Accelerometry Assessment
2.4. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
3.2. Associations of Demographics and Metabolic Measures with Glucose Metrics
3.3. Associations between Lifestyle Behaviors and Glucose Metrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation, 8th ed.; IDF Diabetes Atlas: Brussels, Belgium, 2017; Available online: http://www.diabetesatlas.org (accessed on 18 August 2020).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Chehregosha, H.; Khamseh, M.E.; Malek, M.A. View Beyond HbA1c: Role of Continuous Glucose Monitoring. Diabetes Ther. 2019, 10, 853–863. [Google Scholar] [CrossRef] [Green Version]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, G.; Mi, S.; Tao, H.; Li, Z.; Yang, H.; Zheng, H.; Zhou, Y.; Ma, C. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Ihnat, M.A. ‘Glycaemic variability’: A new therapeutic challenge in diabetes and the critical care setting. Diabet Med. 2010, 27, 862–867. [Google Scholar] [CrossRef]
- Levitan, E.B.; Song, Y.; Ford, E.S.; Liu, S. Is Nondiabetic Hyperglycemia a Risk Factor for Cardiovascular Disease? A Meta-analysis of Prospective Studies. Arch. Intern. Med. 2004, 164, 2147–2155. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating Glucose Is More Deleterious to Endothelial Function and Oxidative Stress Than Mean Glucose in Normal and Type 2 Diabetic Patients. Diabetes 2008, 57, 1349. [Google Scholar] [CrossRef] [Green Version]
- Monnier, L.; Colette, C.; Wojtusciszyn, A.; Dejager, S.; Renard, E.; Molinari, N.; Owens, D.R. Toward Defining the Threshold Between Low and High Glucose Variability in Diabetes. Diabetes Care 2017, 40, 832–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennetsen, S.L.; Feineis, C.S.; Legaard, G.E.; Lyngbæk, M.P.; Karstoft, K.; Ried-Larsen, M. The Impact of Physical Activity on Glycemic Variability Assessed by Continuous Glucose Monitoring in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2020, 11, 486. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toi, N.; Inaba, M.; Kurajoh, M.; Morioka, T.; Hayashi, N.; Hirota, T.; Miyaoka, D.; Emoto, M.; Yamada, S. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J. Clin. Transl. Endocrinol. 2018, 15, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Paing, A.C.; McMillan, K.A.; Kirk, A.F.; Collier, A.; Hewitt, A.; Chastin, S.F. Impact of free-living pattern of sedentary behaviour on intra-day glucose regulation in type 2 diabetes. Eur. J. Appl. Physiol. 2020, 120, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paing, A.C.; McMillan, K.A.; Kirk, A.F.; Collier, A.; Hewitt, A.; Chastin, S.F. The associations of sedentary time and breaks in sedentary time with 24-hour glycaemic control in type 2 diabetes. Prev. Med. Rep. 2018, 12, 94–100. [Google Scholar] [CrossRef]
- Blaychfeld-Magnazi, M.; Reshef, N.; Zornitzki, T.; Madar, Z.; Knobler, H. The effect of a low-carbohydrate high-fat diet and ethnicity on daily glucose profile in type 2 diabetes determined by continuous glucose monitoring. Eur. J. Nutr. 2020, 59, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.A.; Salmon, J.; McNaughton, S.A.; Orellana, L.; Wadley, G.D.; Bruce, C.; Dempsey, P.C.; Lacy, K.E.; Dunstan, D.W. Effects of breaking up sitting on adolescents’ postprandial glucose after consuming meals varying in energy: A cross-over randomised trial. J. Sci. Med. Sport 2018, 21, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Brynes, A.E.; Adamson, J.; Dornhorst, A.; Frost, G.S. The beneficial effect of a diet with low glycaemic index on 24 h glucose profiles in healthy young people as assessed by continuous glucose monitoring. Br. J. Nutr. 2005, 93, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological Momentary Assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Trull, T.J.; Ebner-Priemer, U.W. Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinical assessment and clinical research: Introduction to the special section. Psychol. Assess. 2009, 21, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Potts, C.; Bond, R.; Ryan, A.; Mulvenna, M.; McCauley, C.; Laird, E.; Goode, D. Ecological Momentary Assessment Within a Digital Health Intervention for Reminiscence in Persons with Dementia and Caregivers: User Engagement Study. JMIR Mhealth Uhealth 2020, 8, e17120. [Google Scholar] [CrossRef]
- Tan, K.H.X.; Tan, L.W.L.; Sim, X.; Tai, E.S.; Lee, J.J.M.; Chia, K.S.; van Dam, R.M. Cohort Profile: The Singapore Multi-Ethnic Cohort (MEC) study. Int. J. Epidemiol. 2018, 47, 699. [Google Scholar] [CrossRef] [Green Version]
- Ozbek, M.U.; Erdogan, M.; Karadeniz, M.U.; Cetinkalp, S.; Ozgen, A.G.; Saygili, F.; Yilmaz, C.A.; Tuzun, M. Evaluation of beta cell dysfunction by mixed meal tolerance test and oral L-arginine in patients with newly diagnosed type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2009, 117, 573–576. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487. [Google Scholar] [CrossRef] [Green Version]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumvoll, M.; Mitrakou, A.; Pimenta, W.; Jenssen, T.; Yki-Järvinen, H.A.; Van Haeften, T.; Renn, W.P.; Gerich, J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000, 23, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Retnakaran, R.; Shen, S.; Hanley, A.J.; Vuksan, V.; Hamilton, J.K.; Zinman, B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 2008, 16, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef]
- Chakarova, N.; Dimova, R.; Grozeva, G.; Tankova, T. Assessment of glucose variability in subjects with prediabetes. Diabetes Res. Clin. Pract. 2019, 151, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food Composition System Singapore. 2015. Available online: https://focos.hpb.gov.sg/eservices/ENCF/ (accessed on 18 August 2020).
- Food and Nutrient Database. 2016. Available online: http://www.ncc.umn.edu/food-and-nutrient-database/ (accessed on 18 August 2020).
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; van Hees, V.T. GGIR: A Research Community-Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes from Multi-Day Raw Accelerometer Data. J. Meas. Phys. Behav. 2019, 2, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [Green Version]
- Sluik, D.; Buijsse, B.; Muckelbauer, R.; Kaaks, R.; Teucher, B.; Tj, A.; Overvad, K.; Amiano, P.; Ardanaz, E.; Bendinelli, B.; et al. Physical Activity and Mortality in Individuals with Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch. Intern. Med. 2012, 172, 1285–1295. [Google Scholar] [CrossRef]
- Gordon, B.A.; Benson, A.C.; Bird, S.R.; Fraser, S.F. Resistance training improves metabolic health in type 2 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2009, 83, 157–175. [Google Scholar] [CrossRef]
- MacLeod, S.F.; Terada, T.; Chahal, B.S.; Boule, N.G. Exercise lowers postprandial glucose but not fasting glucose in type 2 diabetes: A meta-analysis of studies using continuous glucose monitoring. Diabetes/Metab. Res. Rev. 2013, 29, 593–603. [Google Scholar] [CrossRef]
- Van Dijk, J.-W. and L.J.C. van Loon, Exercise strategies to optimize glycemic control in type 2 diabetes: A continuing glucose monitoring perspective. Diabetes Spectr. A Publ. Am. Diabetes Assoc. 2015, 28, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Ozaslan, B.; Patek, S.D.; Breton, M.D. Impact of Daily Physical Activity as Measured by Commonly Available Wearables on Mealtime Glucose Control in Type 1 Diabetes. Diabetes Technol. Ther. 2020, 22, 742–748. [Google Scholar] [CrossRef]
- Baynes, H.W.; Mideksa, S.; Ambachew, S. The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action. Adipocyte 2018, 7, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Telle-Hansen, V.H.; Gaundal, L.; Myhrstad, M.C.W. Polyunsaturated Fatty Acids and Glycemic Control in Type 2 Diabetes. Nutrients 2019, 11, 1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, G.; Liu, G.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Associations Between Linoleic Acid Intake and Incident Type 2 Diabetes Among, U.S. Men and Women. Diabetes Care 2019, 42, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Huang, Y.Y.; Chen, H.Y.; Hsieh, S.H.; Sun, J.H.; Chen, S.T.; Lin, C.H. Impact of Carbohydrate on Glucose Variability in Patients with Type 1 Diabetes Assessed Through Professional Continuous Glucose Monitoring: A Retrospective Study. Diabetes Ther. 2019, 10, 2289–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Kim, J.H. Glycemic Variability: How Do We Measure It and Why Is It Important? Diabetes Metab. J. 2015, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Salkind, S.J.; Huizenga, R.; Fonda, S.J.; Walker, M.S.; Vigersky, R.A. Glycemic variability in nondiabetic morbidly obese persons: Results of an observational study and review of the literature. J. Diabetes Sci. Technol. 2014, 8, 1042–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberer, F.; Hajnsek, M.; Rumpler, M.; Zenz, S.; Baumann, P.M.; Elsayed, H.; Puffing, A.; Treiber, G.; Pieber, T.R.; Sourij, H.; et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes. Metab. 2017, 19, 1051–1055. [Google Scholar] [CrossRef] [Green Version]
- Olafsdottir, A.F.; Attvall, S.; Sandgren, U.; Dahlqvist, S.; Pivodic, A.; Skrtic, S.; Theodorsson, E.; Lind, M. A Clinical Trial of the Accuracy and Treatment Experience of the Flash Glucose Monitor FreeStyle Libre in Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2017, 19, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Hutchesson, M.J.; Rollo, M.E.; Callister, R.; Collins, C.E. Self-monitoring of dietary intake by young women: Online food records completed on computer or smartphone are as accurate as paper-based food records but more acceptable. J. Acad. Nutr. Diet. 2015, 115, 87–94. [Google Scholar] [CrossRef]
- Board, H.P. (Ed.) Report of the National Nutrition Survey, 2010; Health Promotion Board & Strategic Planning Division: Singapore, 2013.
| n (%) or Mean (SD) | |
|---|---|
| Baseline Characteristics | |
| Sex | |
| Female | 10 (36%) |
| Male | 18 (64%) |
| Ethnicity | |
| Chinese | 15 (54%) |
| Indian | 7 (25%) |
| Malay | 6 (21%) |
| Age (years) | 46.0 (9.9) |
| Education | |
| Below A’ level or the equivalent | 10 (35.7%) |
| A’ level or the equivalent | 9 (32.1%) |
| University | 9 (32.1%) |
| Body mass index (kg/m2) | 27.5 (1.8) |
| Body fat (kg) | 25.8 (5.7) |
| Fat free mass (kg) | 51.4 (9.4) |
| HbA1c (%) | 5.5 (0.4) |
| Fasting glucose, (mmol/L) | 4.8 (0.3) |
| HOMA2-IR | 1.1 (0.5) |
| Accelerometer measures | |
| Moderate- to vigorous-intensity physical activity (hrs/d) | 1.6 (0.6) |
| Light intensity physical activity (hrs/d) | 4.9 (1.4) |
| Sedentary (hrs/d) | 11.0 (1.7) |
| Sleep (hrs/d) | 5.4 (1.1) |
| Diet intakes | |
| Protein (en%) | 17.6 (4.4) |
| Saturated fat (en%) | 13.0 (1.8) |
| Monounsaturated fat (en%) | 11.5 (1.8) |
| Polyunsaturated fat (en%) | 5.9 (1.2) |
| Carbohydrates (en%) | 50.3 (6.9) |
| Fiber (g/1000 kcal) | 9.7 (3.6) |
| Measures of glucose variation | |
| Mean glucose (mmol/L) | 4.8 (0.5) |
| SD glucose (mmol/L) | 0.9 (0.3) |
| %CV glucose | 19.2 (4.9) |
| %Time-in-range (3.0–7.8 mmol/L) | 95.4 (7.1) |
| %Time above range (>7.8 mmol/L) | 2.3 (3.5) |
| %Time below range (<3.0 mmol/L) | 2.4 (6.5) |
| Mean Glucose | %CV Glucose | %Time-in-Range (3.0–7.8 mmol/L) | ||||
|---|---|---|---|---|---|---|
| Estimate (CI) | p | Estimate (CI) | p | Estimate (CI) | p | |
| Sex | ||||||
| Female | Reference | Reference | Reference | |||
| Male | 0.14 (−0.27, 0.55) | 0.51 | −2.33 (−6.06, 1.40) | 0.22 | 1.12 (−4.13, 6.38) | 0.68 |
| Ethnicity | ||||||
| Chinese | Reference | Reference | Reference | |||
| Indian | −0.14 (−0.68, 0.39) | 0.60 | 0.70 (−3.74, 5.14) | 0.76 | −2.13 (−7.50, 3.24) | 0.44 |
| Malay | −0.27 (−0.85, 0.31) | 0.36 | 1.50 (−3.18, 6.17) | 0.53 | −5.32 (−15.07, 4.43) | 0.28 |
| Age a (years) | −0.05 (−0.27, 0.17) | 0.68 | −0.06 (−1.67, 1.55) | 0.94 | −1.92 (−4.87, 1.04) | 0.20 |
| Education | ||||||
| Below A’ level or the equivalent | Referent | |||||
| A’ level or the equivalent | 0.20 (−0.31, 0.71) | 0.44 | −0.07 (−0.32, 0.18) | 0.59 | −2.26 (−6.70, 2.19) | 0.32 |
| University | 0.44 (0.03, 0.86) | 0.04 | −0.13 (−0.32, 0.07) | 0.20 | −4.38 (−8.10, −0.66) | 0.02 |
| BMI (kg/m2) | 0.12 (0.03, 0.22) | 0.01 | −0.85 (−1.69, −0.00) | 0.049 | 1.66 (0.17, 3.14) | 0.03 |
| Body fat (kg) | 0.03 (0.01, 0.05) | 0.01 | 0.06 (−0.25, 0.37) | 0.69 | −0.12 (−0.45, 0.22) | 0.50 |
| Fat free mass (kg) | 0.01 (−0.02, 0.03) | 0.62 | −0.11 (−0.26, 0.05) | 0.17 | 0.15 (−0.03, 0.33) | 0.10 |
| HbA1c (%) | 0.32 (−0.13, 0.78) | 0.16 | −1.21 (−6.88, 4.46) | 0.68 | −3.03 (−10.01, 3.96) | 0.40 |
| Fasting glucose, (mmol/L) | 0.37 (−0.28, 1.02) | 0.27 | −5.03 (−9.78, −0.27) | 0.04 | −2.55 (−12.20, 7.10) | 0.61 |
| HOMA2−IR | 0.35 (−0.03, 0.74) | 0.072 | −0.18 (−4.32, 3.96) | 0.932 | −0.29 (−4.71, 4.14) | 0.898 |
| 2-h glucose, (mmol/L) | 0.17 (−0.04, 0.39) | 0.11 | 0.84 (−1.32, 3.00) | 0.44 | −2.47 (−5.32, 0.37) | 0.09 |
| Glucose iAUC (1000 units) | 2.05 (0.44, 3.65) | 0.01 | 11.56 (−5.52, 28.64) | 0.19 | −7.06 (−29.09, 14.97) | 0.53 |
| Matsuda Index | −0.05 (−0.08, −0.01) | 0.02 | −0.04 (−0.49, 0.42) | 0.87 | 0.21 (−0.28, 0.71) | 0.40 |
| Insulin iAUC (1000 units) | 0.14 (0.08, 0.20) | <0.001 | 0.45 (−0.55, 1.46) | 0.38 | −0.73 (−1.82, 0.36) | 0.19 |
| Insulinogenic index (1000 units) | 8.13 (−7.12, 23.38) | 0.30 | −37.65 (−218.22, 142.92) | 0.68 | −90.05 (−332.14, 152.05) | 0.47 |
| Disposition index | −0.45 (−1.02, 0.12) | 0.12 | 1.75 (−3.63, 7.13) | 0.52 | 2.62 (−4.95, 10.19) | 0.50 |
| Mean Glucose | %CV Glucose | %Time-in-Range (3.0–7.8 mmol/L) | ||||
|---|---|---|---|---|---|---|
| Estimate (CI) | p | Estimate (CI) | p | Estimate (CI) | p | |
| Movement behaviors | ||||||
| Moderate-to-vigorous intensity physical activity (hrs/d) | −0.07 (−0.23, 0.09) | 0.39 | −1.77 (−3.09, −0.46) | 0.008 | 0.94 (−0.93, 2.81) | 0.32 |
| Multivariable-adjusted | 0.05 (−0.15, 0.25) | 0.64 | −3.03 (−4.67, −1.39) | <0.001 | 2.61 (0.38, 4.84) | 0.02 |
| Light intensity physical activity (hrs/d) | −0.05 (−0.15, 0.05) | 0.36 | −0.70 (−1.64, 0.24) | 0.14 | 0.09 (−1.04, 1.22) | 0.88 |
| Multivariable-adjusted | −0.04 (−0.13, 0.05) | 0.35 | −0.42 (−1.32, 0.47) | 0.36 | −0.15 (−1.40, 1.09) | 0.81 |
| Sedentary (hrs/d) | 0.00 (−0.06, 0.06) | 0.96 | 0.56 (0.03, 1.08) | 0.04 | −0.35 (−1.01, 0.31) | 0.31 |
| Multivariable-adjusted | −0.02 (−0.08, 0.04) | 0.54 | 0.42 (−0.17, 1.01) | 0.16 | −0.33 (−1.03, 0.37) | 0.36 |
| Sleep (hrs/d) | 0.01 (−0.09, 0.12) | 0.83 | −0.03 (−1.06, 0.99) | 0.95 | 0.38 (−1.01, 1.76) | 0.60 |
| Multivariable-adjusted | 0.02 (−0.06, 0.11) | 0.63 | −0.27 (−1.06, 0.52) | 0.51 | 0.62 (−0.38, 1.62) | 0.23 |
| Diet measures a | ||||||
| Protein (en%) | −0.00 (−0.09, 0.09) | 0.98 | −0.07 (−0.65, 0.50) | 0.80 | 1.04 (0.22, 1.86) | 0.01 |
| Multivariable-adjusted | −0.03 (−0.09, 0.03) | 0.32 | −0.31 (−0.79, 0.18) | 0.22 | 0.90 (0.25, 1.56) | 0.007 |
| Saturated fat (en%) | 0.06 (−0.11, 0.22) | 0.52 | 0.37 (−0.79, 1.53) | 0.53 | 0.13 (−2.35, 2.60) | 0.92 |
| Multivariable-adjusted | 0.52 (−0.64, 1.67) | 0.38 | 0.19 (−2.34, 2.71) | 0.89 | ||
| Monounsaturated fat (en%) | 0.01 (−0.17, 0.20) | 0.90 | −0.61 (−1.78, 0.55) | 0.30 | 1.14 (−1.23, 3.51) | 0.35 |
| Multivariable-adjusted | −0.03 (−0.22, 0.16) | 0.78 | −0.44 (−1.89, 1.01) | 0.55 | 0.90 (−1.56, 3.35) | 0.47 |
| Polyunsaturated fat (en%) | −0.01 (−0.19, 0.17) | 0.91 | −1.64 (−3.40, 0.11) | 0.07 | 2.39 (−0.84, 5.62) | 0.15 |
| Multivariable-adjusted | −0.01 (−0.18, 0.17) | 0.94 | −2.23 (−3.51, −0.94) | <0.001 | 3.21 (0.47, 5.94) | 0.02 |
| Carbohydrates (en%) | −0.00 (−0.05, 0.05) | 0.91 | 0.11 (−0.29, 0.50) | 0.60 | −0.58 (−1.26, 0.09) | 0.09 |
| Multivariable-adjusted | 0.01 (−0.03, 0.05) | 0.58 | 0.20 (−0.19, 0.60) | 0.31 | −0.59 (−1.17, −0.02) | 0.04 |
| Fiber (g/1000 kcal) | 0.04 (−0.07, 0.15) | 0.46 | −0.44 (−1.90, 1.01) | 0.55 | 0.39 (−0.78, 1.57) | 0.52 |
| Multivariable-adjusted | 0.003 (−0.10, 0.11) | 0.96 | 0.08 (−1.11, 1.27) | 0.89 | −0.33 (−1.47, 0.80) | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Yao, J.; Chua, X.H.; Chandran, S.R.; Gardner, D.S.L.; Khoo, C.M.; Müller-Riemenschneider, F.; Whitton, C.; van Dam, R.M. Diet and Physical Activity as Determinants of Continuously Measured Glucose Levels in Persons at High Risk of Type 2 Diabetes. Nutrients 2022, 14, 366. https://doi.org/10.3390/nu14020366
Park SH, Yao J, Chua XH, Chandran SR, Gardner DSL, Khoo CM, Müller-Riemenschneider F, Whitton C, van Dam RM. Diet and Physical Activity as Determinants of Continuously Measured Glucose Levels in Persons at High Risk of Type 2 Diabetes. Nutrients. 2022; 14(2):366. https://doi.org/10.3390/nu14020366
Chicago/Turabian StylePark, Su Hyun, Jiali Yao, Xin Hui Chua, Suresh Rama Chandran, Daphne S. L. Gardner, Chin Meng Khoo, Falk Müller-Riemenschneider, Clare Whitton, and Rob M. van Dam. 2022. "Diet and Physical Activity as Determinants of Continuously Measured Glucose Levels in Persons at High Risk of Type 2 Diabetes" Nutrients 14, no. 2: 366. https://doi.org/10.3390/nu14020366
APA StylePark, S. H., Yao, J., Chua, X. H., Chandran, S. R., Gardner, D. S. L., Khoo, C. M., Müller-Riemenschneider, F., Whitton, C., & van Dam, R. M. (2022). Diet and Physical Activity as Determinants of Continuously Measured Glucose Levels in Persons at High Risk of Type 2 Diabetes. Nutrients, 14(2), 366. https://doi.org/10.3390/nu14020366






