Do Micronutrient and Omega-3 Fatty Acid Supplements Affect Human Maternal Immunity during Pregnancy? A Scoping Review
Abstract
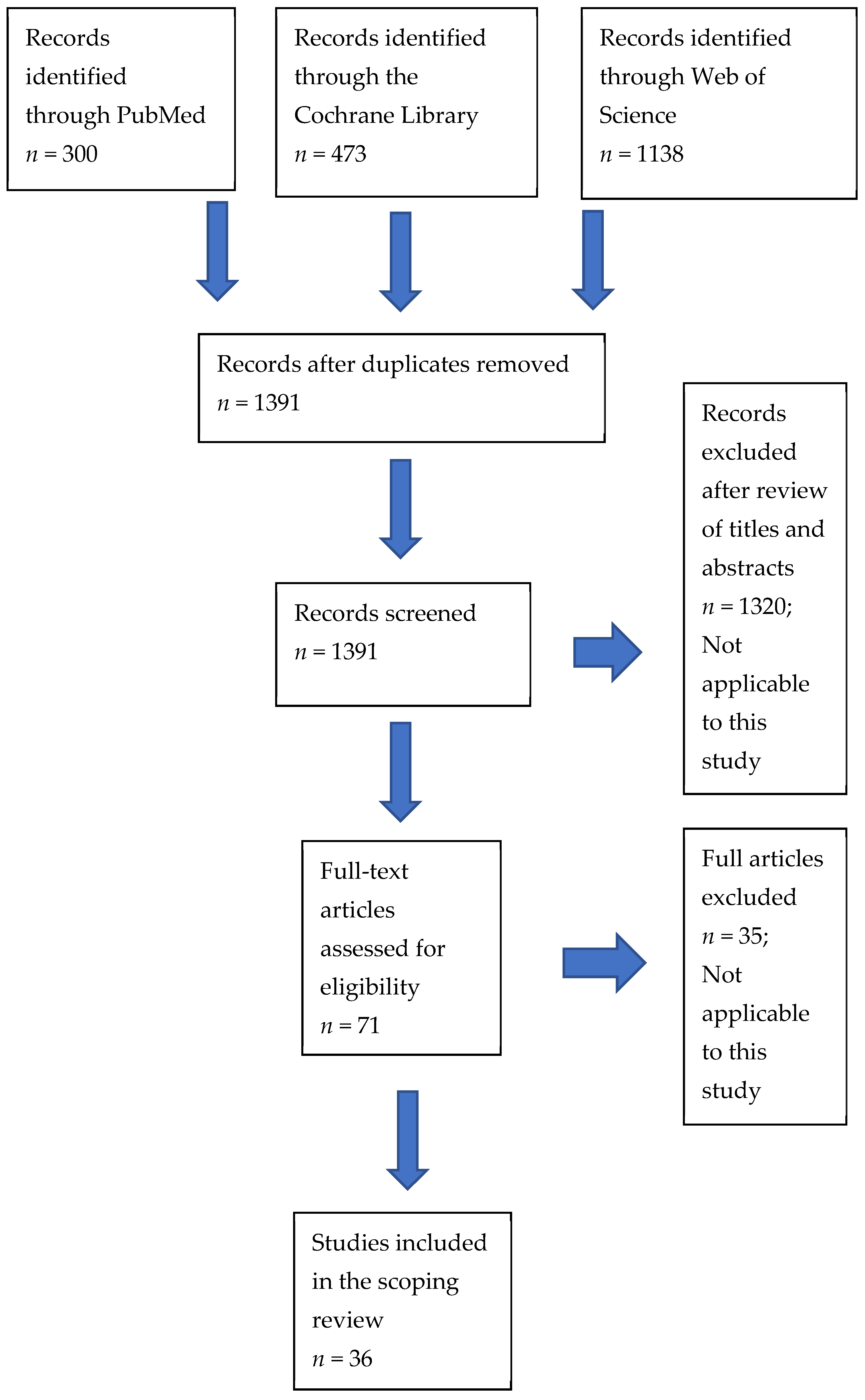
:1. Introduction
2. Materials and Methods
2.1. Information Sources
2.2. Eligibility Criteria
- •
- Articles in English published in established peer-review journals between 1 January 2010 and 26 April 2021;
- •
- Full-length articles describing potential biochemical, epigenomic, and clinical effects of micronutrient and omega-3 long-chain fatty acid supplementation on immunity in pregnant women;
- •
- Longitudinal trials/randomized, controlled trials involving dietary supplementation in pregnant women at any stage of gestation, where comparison is made with their status at enrollment and/or with a control population;
- •
- Participants must have been assessed clinically and/or biochemically at enrollment and at one or more timepoints thereafter;
- •
- Study participants with gestational comorbidities were not excluded from this review.
- •
- Articles focusing on fortification or the effects a particular food rather than supplementation; Foods promoted for well-being typically contain a range of nutritional components, making the identification of benefits associated with individual micronutrients problematic. Similarly, it is difficult to separate the effects of fortification from the elements of the product being added to.
- •
- Animal studies;
- •
- Articles that concentrate on potential benefits for the fetus and newborn (e.g., effects on placental function, colostrum quality, atopy, and neural development);
- •
- Reviews, trial protocols, letters, conference reports, withdrawn manuscripts, and non-English publications.
2.3. Risk of Bias
2.4. Synthesis of Results
3. Results
3.1. Vitamin A
3.2. Vitamin B12
3.3. Vitamin C and E
3.4. Vitamin D
3.5. Choline
3.6. Iron and Lactoferrin
3.7. Selenium
3.8. Zinc
3.9. Omega-3 Fatty Acids
3.10. Micronutrients, Malaria, and HIV
3.11. Multiple Micronutrient Supplementation
4. Discussion
4.1. Clinical Benefits of Supplementation
4.2. Omega-3 Fatty Acids
4.3. Genomic Effects
4.4. Timing of Administration
4.5. Comparison with Animal Studies
4.6. Limitations of This Review
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warning, J.C.; McCracken, S.A.; Morris, J.M. A balancing act: Mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011, 141, 715–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racicot, K.; Kwon, J.-Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar] [CrossRef]
- Lin, H.; Mosmann, T.R.; Guilbert, L.; Tuntipopipat, S.; Wegmann, T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 1993, 151, 4562–4573. [Google Scholar] [PubMed]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Schröder-Heurich, B.; Springer, C.J.P.; von Versen-Höynck, F. Vitamin D effects on the immune system from periconception through pregnancy. Nutrients 2020, 12, 1432. [Google Scholar] [CrossRef]
- Chandra, G.; Aggarwal, A.; Kumar, M.; Singh, A.K.; Sharma, V.K.; Upadhyay, R.C. Effect of additional vitamin E and zinc supplementation on immunological changes in peripartum Sahiwal cows. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1166–1175. [Google Scholar] [CrossRef]
- Khatti, A.; Mehrotra, S.; Patel, P.K.; Singh, G.; Maurya, V.P.; Mahla, A.S.; Chaudhari, R.K.; Das, G.K.; Singh, M.; Sarkar, M.; et al. Supplementation of vitamin E, selenium and increased energy allowance mitigates the transition stress and improves postpartum reproductive performance in the crossbred cow. Theriogenology 2017, 104, 142–148. [Google Scholar] [CrossRef]
- De, K.; Pal, S.; Prasad, S.; Dang, A.K. Effect of micronutrient supplementation on the immune function of crossbred dairy cows under semi-arid tropical environment. Trop. Anim. Health Prod. 2014, 46, 203–211. [Google Scholar] [CrossRef]
- Warken, A.C.; Lopes, L.S.; Bottari, N.B.; Glombowsky, P.; Galli, G.M.; Morsch, V.M.; Schetinger, M.R.C.; Da Silva, A.S. Mineral supplementation stimulates the immune system and antioxidant responses of dairy cows and reduces somatic cell counts in milk. An. Acad. Bras. Cienc. 2018, 90, 1649–1658. [Google Scholar] [CrossRef]
- Audet, I.; Girard, C.L.; Lessard, M.; Lo Verso, L.; Beaudoin, F.; Matte, J.J. Homocysteine metabolism, growth performance, and immune responses in suckling and weanling piglets. J. Anim. Sci. 2015, 93, 147–157. [Google Scholar] [CrossRef]
- Berti, C.; Biesalski, H.; Gärtner, R.; Lapillonne, A.; Pietrzik, K.; Poston, L.; Redman, C.; Koletzko, B.; Cetin, I. Micronutrients in pregnancy: Current knowledge and unresolved questions. Clin. Nutr. 2011, 30, 689–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Elmadfa, I.; Meyer, A.L. The role of the status of selected micronutrients in shaping the immune function. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1100–1115. [Google Scholar] [CrossRef]
- Macpherson, A.J.; de Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune function and micronutrient requirements change over the life course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Su, S.; You, T.; Xia, T.; Lin, X.; Chen, Z.; Zhang, L. Serum interleukin-6 level is correlated with the disease activity of systemic lupus erythematosus: A meta-analysis. Clinics 2020, 75, e1801. [Google Scholar] [CrossRef] [PubMed]
- Pieczyńska, J.; Grajeta, H. The role of selenium in human conception and pregnancy. J. Trace. Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef]
- Innis, S.M.; Novak, E.M.; Keller, B.O. Long chain omega-3 fatty acids: Micronutrients in disguise. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H. Scoping the field: Services for carers of people with mental health problems. Health Soc. Care Community 2003, 11, 335–344. [Google Scholar] [CrossRef]
- Walker, S. Do Micronutrient and Omega-3 Fatty Acid Supplements Affect Human Maternal Immunity during Pregnancy? A Scoping Review Protocol. Available online: Osf.io/u3yvr (accessed on 23 October 2021).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.M.; Alam, J.; Khanam, A.; Rashid, M.; Islam, S.; Kabir, Y.; Raqib, R.; Steinhoff, M.C. Vitamin A supplementation during pregnancy enhances pandemic H1N1 vaccine response in mothers, but enhancement of transplacental antibody transfer may depend on when mothers are vaccinated during pregnancy. J. Nutr. 2018, 148, 1968–1975. [Google Scholar] [CrossRef]
- Darling, A.M.; Mugusi, F.M.; Etheredge, A.J.; Gunaratna, N.S.; Abioye, A.I.; Aboud, S.; Duggan, C.; Mongi, R.; Spiegelman, N.; Roberts, U.; et al. Vitamin A and zinc supplementation among pregnant women to prevent placental malaria: A randomized, double-blind, placebo-controlled trial in Tanzania. Am. J. Trop. Med. Hyg. 2017, 96, 826–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.Y.; Ma, A.G.; Yang, F.; Zhang, F.Z.; Luo, Y.B.; Jiang, D.C.; Han, X.X.; Liang, H. A combination of iron and retinol supplementation benefits iron status, IL-2 level and lymphocyte proliferation in anemic pregnant women. Asia Pac. J. Clin. Nutr. 2010, 19, 513–519. [Google Scholar]
- Olofin, I.O.; Spiegelman, D.; Aboud, S.; Duggan, C.; Danaei, G.; Fawzi, W.W. Supplementation with multivitamins and vitamin A and incidence of malaria among HIV-infected Tanzanian women. J. Acquir. Immune Defic. Syndr. 2014, 67, S173–S178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqua, T.J.; Ahmad, S.M.; Ahsan, K.B.; Rashid, M.; Roy, A.; Rahman, S.M.; Shahab-Ferdows, S.; Hampel, D.; Ahmed, T.; Allen, L.H.; et al. Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: A randomized clinical trial in Bangladesh. Eur. J. Nutr. 2016, 55, 281–293. [Google Scholar] [CrossRef]
- Johnston, P.; Powell, L.; McCance, D.; Pogue, K.; McMaster, C.; Gilchrist, S.; Holmes, V.; Young, I.; McGinty, A. Placental protein tyrosine nitration and MAPK in type 1 diabetic pre-eclampsia: Impact of antioxidant vitamin supplementation. J. Diabetes Complicat. 2013, 27, 322–327. [Google Scholar] [CrossRef]
- Bobbitt, K.R.; Peters, R.M.; Li, J.; Rao, S.D.; Woodcroft, K.J.; Cassidy-Bushrow, A.E. Early pregnancy vitamin D and patterns of antenatal inflammation in African-American women. J. Reprod. Immunol. 2015, 107, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, H.; Litonjua, A.A.; McElrath, T.F.; O’Connor, G.; Lee-Parritz, A.; Iverson, R.; Macones, G.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.; et al. Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Investig. 2016, 126, 4702–4715. [Google Scholar] [CrossRef] [Green Version]
- Al-Garawi, A.; Carey, V.J.; Chhabra, D.; Mirzakhani, H.; Morrow, J.; Lasky-Su, J.; Qiu, W.; Laranjo, N.; Litonjua, A.A.; Weiss, S.T. The role of vitamin D in the transcriptional program of human pregnancy. PLoS ONE 2016, 11, e0163832. [Google Scholar] [CrossRef]
- Anderson, C.M.; Gillespie, S.L.; Thiele, D.K.; Ralph, J.L.; Ohm, J.E. Effects of maternal vitamin D supplementation on the maternal and infant epigenome. Breastfeed Med. 2018, 13, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Gharagozloo, M.; Ghahiri, A.; Mehrabian, F.; Maracy, M.R.; Kouhpayeh, S.; Pieper, I.L.; Rezaei, A. Altered Th17/Treg ratio in recurrent miscarriage after treatment with paternal lymphocytes and vitamin D3: A double-blind placebo-controlled study. Iran. J. Immunol. 2015, 12, 252–262. [Google Scholar]
- Zerofsky, M.S.; Jacoby, B.N.; Pedersen, T.L.; Stephensen, C.B. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial. J. Nutr. 2016, 146, 2388–2397. [Google Scholar] [CrossRef] [Green Version]
- Khatiwada, A.; Wolf, B.J.; Mulligan, J.K.; Shary, J.R.; Hewison, M.; Baatz, J.E.; Newton, D.A.; Hawrylowicz, C.; Hollis, B.W.; Wagner, C.L. Effects of vitamin D supplementation on circulating concentrations of growth factors and immune-mediators in healthy women during pregnancy. Pediatr. Res. 2021, 89, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Samimi, M.; Kashi, M.; Foroozanfard, F.; Karamali, M.; Bahmani, F.; Asemi, Z.; Hamidian, Y.; Talari, H.; Esmaillzadeh, A. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J. Hum. Nutr. Diet. 2016, 29, 505–515. [Google Scholar] [CrossRef]
- Jefferson, K.K.; Parikh, H.I.; Garcia, E.M.; Edwards, D.J.; Serrano, M.G.; Hewison, M.; Shary, J.R.; Powell, A.M.; Hollis, B.W.; Fettweis, J.M.; et al. Relationship between vitamin D status and the vaginal microbiome during pregnancy. J. Perinatol. 2019, 39, 824–836. [Google Scholar] [CrossRef]
- Caudill, M.A.; Obeid, R.; Derbyshire, E.; Bernhard, W.; Lapid, K.; Walker, S.J.; Zeisel, S.H. Building better babies: Should choline supplementation be recommended for pregnant and lactating mothers? Literature overview and expert panel consensus. EGO 2020, 2, 149–161. [Google Scholar]
- Jiang, X.; Bar, H.Y.; Yan, J.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.M.; Wells, M.T.; et al. Pregnancy induces transcriptional activation of the peripheral innate immune system and increases oxidative DNA damage among healthy third trimester pregnant women. PLoS ONE 2012, 7, e46736. [Google Scholar] [CrossRef] [Green Version]
- Mwangi, M.N.; Roth, J.M.; Smit, M.R.; Trijsburg, L.; Mwangi, A.M.; Demir, A.Y.; Wielders, J.P.M.; Mens, P.F.; Verweij, J.J.; Cox, S.E.; et al. Effect of daily antenatal iron supplementation on Plasmodium infection in Kenyan women: A randomized clinical trial. JAMA 2015, 314, 1009–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etheredge, A.J.; Premji, Z.; Gunaratna, N.S.; Abioye, A.I.; Aboud, S.; Duggan, C.; Mongi, R.; Meloney, L.; Spiegelman, N.; Roberts, U.; et al. Iron supplementation in iron-replete and nonanemic pregnant women in Tanzania: A randomized clinical trial. JAMA Pediatr. 2015, 169, 947–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goheen, M.M.; Bah, A.; Wegmüller, R.; Verhoef, H.; Darboe, B.; Danso, E.; Prentice, A.M.; Cerami, C. Host iron status and erythropoietic response to iron supplementation determines susceptibility to the RBC stage of falciparum malaria during pregnancy. Sci. Rep. 2017, 7, 17674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giunta, G.; Giuffrida, L.; Mangano, K.; Fagone, P.; Cianci, A. Influence of lactoferrin in preventing preterm delivery: A pilot study. Mol. Med. Rep. 2012, 5, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Paesano, R.; Pietropaoli, M.; Berlutti, F.; Valenti, P. Bovine lactoferrin in preventing preterm delivery associated with sterile inflammation. Biochem. Cell Biol. 2012, 90, 468–475. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of lactoferrin oral administration in the treatment of anemia and anemia of inflammation in pregnant and non-pregnant women: An interventional study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef] [PubMed]
- Okunade, K.S.; Olowoselu, O.F.; John-Olabode, S.; Hassan, B.O.; Akinsola, O.J.; Nwogu, C.M.; Ugwu, A.O.; Moses, O.E.; Rabiu, K.A.; Ajepe, A.; et al. Effects of selenium supplementation on pregnancy outcomes and disease progression in HIV-infected pregnant women in Lagos: A randomized controlled trial. Int. J. Gynaecol. Obstet. 2021, 153, 533–541. [Google Scholar] [CrossRef]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and micronutrient intake during pregnancy: An overview of recent evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, G.; Isidori, A.M.; Moretti, C.; Di Dato, C.; Greco, E.; Ciolli, P.; Bonomi, M.; Petrone, L.; Fumarola, A.; Campagna, G.; et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine 2019, 66, 542–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.; Pop, V.J.; Bath, S.C.; Vader, H.L.; Redman, C.W.; Rayman, M.P. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur. J. Nutr. 2016, 55, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Shahnazi, M.; Khalili, A.F.; Azimi, S. Effect of zinc supplement on prevention of PPROM and improvement of some pregnancy outcomes in pregnant women with a history of PPROM: A randomized double-blind controlled trial. Iran. Red Crescent Med. J. 2017, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Helmizar, H. Dadih and zinc supplementation during pregnancy benefits pregnancy outcomes and humoral immune response in West Sumatera, Indonesia. Ann. Nutr. Metab. 2019, 75, 332. [Google Scholar]
- Mozurkewich, E.L.; Berman, D.R.; Vahratian, A.; Clinton, C.M.; Romero, V.C.; Chilimigras, J.L.; Vazquez, D.; Qualls, C.; Djuric, Z. Effect of prenatal EPA and DHA on maternal and umbilical cord blood cytokines. BMC Pregnancy Childbirth 2018, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Nishi, D.; Su, K.P.; Usuda, K.; Chang, J.P.-C.; Hamazaki, K.; Ishima, T.; Sano, Y.; Ito, H.; Isaka, K.; Tachibana, Y.; et al. Plasma estradiol levels and antidepressant effects of omega-3 fatty acids in pregnant women. Brain Behav. Immun. 2020, 85, 29–34. [Google Scholar] [CrossRef]
- Haghiac, M.; Yang, X.-H.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Mouzon, S.H.-D. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: A randomized double-blind controlled clinical trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef] [Green Version]
- Horvaticek, M.; Djelmis, J.; Ivanisevic, M.; Oreskovic, S.; Herman, M. Effect of eicosapentaenoic acid and docosahexaenoic acid supplementation on C-peptide preservation in pregnant women with type-1 diabetes: Randomized placebo controlled clinical trial. Eur. J. Clin. Nutr. 2017, 71, 968–972. [Google Scholar] [CrossRef]
- Forsberg, A.; Abrahamsson, T.R.; Nilsson, L.; Ernerudh, J.; Duchén, K.; Jenmalm, M.C. Changes in peripheral immune populations during pregnancy and modulation by probiotics and ω-3 fatty acids. Sci. Rep. 2020, 10, 18723. [Google Scholar] [CrossRef]
- Harper, M.; Li, L.; Zhao, Y.; Klebanoff, M.A.; Thorp, J.M.; Sorokin, Y.; Varner, M.; Wapner, R.; Caritis, S.; Iams, J.D.; et al. Change in mononuclear leukocyte responsiveness in midpregnancy and subsequent preterm birth. Obstet. Gynecol. 2013, 121, 805–811. [Google Scholar] [CrossRef]
- Keelan, J.A.; Mas, E.; D’Vaz, N.; Dunstan, J.A.; Li, S.; Barden, A.E.; Mark, P.J.; Waddell, B.J.; Prescott, S.L.; Mori, T. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 2015, 149, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Chandrasiri, U.P.; Fowkes, F.J.; Richards, J.S.; Langer, C.; Fan, Y.-M.; Taylor, S.M.; Beeson, J.G.; Dewey, K.G.; Maleta, K.; Ashorn, P.; et al. The impact of lipid-based nutrient supplementation on anti-malarial antibodies in pregnant women in a randomized controlled trial. Malar. J. 2015, 14, 193. [Google Scholar] [CrossRef] [Green Version]
- Nkhoma, M.; Ashorn, P.; Ashorn, U.; Dewey, K.G.; Gondwe, A.; Mbotwa, J.; Rogerson, S.; Taylor, S.M.; Maleta, K. Providing lipid-based nutrient supplement during pregnancy does not reduce the risk of maternal P falciparum parasitaemia and reproductive tract infections: A randomised controlled trial. BMC Pregnancy Childbirth 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priliani, L.; Oktavianthi, S.; Prado, E.L.; Malik, S.G.; Shankar, A.H. Maternal biomarker patterns for metabolism and inflammation in pregnancy are influenced by multiple micronutrient supplementation and associated with child biomarker patterns and nutritional status at 9–12 years of age. PLoS ONE 2020, 15, e0216848. [Google Scholar] [CrossRef]
- Yadama, A.P.; Mirzakhani, H.; McElrath, T.F.; Litonjua, A.A.; Weiss, S.T. Transcriptome analysis of early pregnancy vitamin D status and spontaneous preterm birth. PLoS ONE 2020, 15, e0227193. [Google Scholar] [CrossRef]
- García-Rodríguez, C.E.; Olza, J.; Aguilera, C.; Mesa, M.; Miles, E.; Noakes, P.; Vlachava, M.; Kremmyda, L.-S.; Diaper, N.D.; Godfrey, K.; et al. Plasma inflammatory and vascular homeostasis biomarkers increase during human pregnancy but are not affected by oily fish intake. J. Nutr. 2012, 142, 1191–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, K.L.; Walsh, C.A.; Brennan, L.; McAuliffe, F.M. Probiotics in pregnancy and maternal outcomes: A systematic review. J. Matern. Fetal Neonatal Med. 2013, 26, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Cherno, M. Feuerbach’s “Man is what He Eats”: A rectification. JHI 1963, 24, 397–406. [Google Scholar] [CrossRef]
| Search Terms |
|---|
| (vitamin D OR vitamin D2 OR vitamin D3 OR cholecalciferol OR ergocalciferol OR calcitriol) AND immun* AND pregnan* |
| choline AND immun* AND pregnan* selenium AND immun* AND pregnan* zinc AND immun* AND pregnan* iron AND immun* AND pregnan* (vitamin B12 OR cobalamin) AND immun* AND pregnan* (vitamin A OR retinol OR beta carotene OR B carotene) AND immun* AND pregnan* (oily fish OR DHA OR docosahexaenoic acid OR EPA OR eicosapentaenoic acid) AND immun* AND pregnan* probiotic AND immun* AND pregnan* iodine AND immun* AND pregnan* (vitamin C OR ascorbic acid) AND immun* AND pregnan* obesity AND immun* AND pregnan* malnutrition AND immun* AND pregnan* |
| Author (Year), Title, Journal | Study Design | Sample and Setting | Objectives and Methodology | Results | Implications |
|---|---|---|---|---|---|
| Mirzakhani et al.; (2016) Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Investig. [32] | Reanalysis of data from the randomized Vitamin D Antenatal Asthma Reduction Trial [VDAART]. Also, a nested case-control subgroup study to investigate peripheral blood gene expression profiles. | Multicenter US study. A total 881 women were randomized to receive vitamin D supplementation (4400 vs. 400 IU/day) initiated early in pregnancy (10–18 weeks). The ITT analysis comprised 4400 IU group: n = 408 (mean age 27.5 ± 5.4, BMI 28.9 ± 7.7). 400 IU group: n = 408 (mean age 27.2 ± 5.6, BMI 29.9 ± 7.6). | The study objectives were to examine the effects of standard- or high-dose supplementation on preeclampsia. An additional element was a nested Case-control study of 157 women looking at peripheral blood vitamin D-associated gene expression at 10 to 18 weeks. The latter including 47 participants who developed preeclampsia. | Outcome data were available for 816, with 67 (8.2%) developing preeclampsia. ITT analysis found no differences between treatment or controls in the incidence of preeclampsia (8.08 vs. 8.33%, respectively; RR 0.97; 95% CI 0.61–1.53). Vitamin D levels ≥30 ng/mL at trial entry and in late pregnancy were associated with a lower risk of preeclampsia (p = 0.04). Transcriptome analysis found differential expression of 348 vitamin D-associated genes (158 upregulated) in blood of women who developed preeclampsia (p < 0.05). | Vitamin D supplementation initiated in weeks 10–18 did not reduce preeclampsia. Higher doses may be of benefit. Differentially expressed vitamin D-associated transcriptomes point to a distinctive immune response during early pregnancy in women developing preeclampsia. |
| Al-Garawi et al.; (2016) The role of vitamin D in the transcriptional program of human pregnancy. PLoS ONE [33] | Nested cohort subgroup study of participants in VDAART (see above). | A total of 30 randomly selected, healthy women (mean age 25.2 ± 5.6 years, mean BMI 32.6 ± 8.8 kg/m2, 10–18 weeks’ gestation), participating a multicenter, randomized, controlled trial of vitamin D supplementation (400 vs. 4400 IU) in pregnancy. | The study objectives were to investigate how gene expression profiles change during pregnancy and the effects of vitamin D supplementation. RNA was isolated from blood samples at enrolment and during the third trimester (32–38 weeks). Differentially expressed genes were identified using significance of analysis of microarrays, and clustered using a weighted gene co-expression network analysis. Gene-set enrichment was performed to identify major biological pathways. | Comparison of profiles between the first and third trimesters identified 5839 significantly differentially expressed genes (FDR < 0.05; 57% down regulated vs. 42% upregulated). Weighted gene co-expression network analysis clustered these transcripts into 14 co-expression modules, of which 2 (green and salmon representing the 61 and 241 probes, respectively) showed significant correlation with maternal vitamin D levels. Pathway analysis demonstrated genes mapped to immune defense pathways and extracellular matrix reorganization, as well as genes enriched in notch signaling and transcription factor networks. | Gene expression profiles of pregnant women change during gestation. Maternal vitamin D levels may influence transcription. Alterations in the maternal transcriptome may contribute to fetal immune imprinting and reduce allergic sensitization in early life. |
| Anderson et al.; (2018) Effects of maternal vitamin D supplementation on the maternal and infant epigenome. Breastfeed Med. [34] | Double-blind, randomized, controlled pilot study. | Single-center Midwestern hospital obstetric practice, USA. Pregnant women recruited between 24- and 28-weeks’ gestation randomized to received vitamin D3 400 IU (n = 6; control group) or 3800 IU (n = 7; intervention group) daily through to 4–6 weeks postpartum. | The objective of this study was to quantify the effects of vitamin D3 supplementation on DNA methylation in pregnant and lactating women and their breastfed infants. Epigenome-wide DNA methylation was quantified in leukocytes collected from mothers at birth and mother-infant dyads at 4–6 weeks postpartum. | High-dose maternal vitamin D supplementation alters DNA methylation in mothers and breastfed infants compared with controls. There were variable gains and losses. At birth, intervention group mothers showed DNA methylation gains at the 76 cystine-guanine (CpG) dinucleotide and losses at 89 CpG dinucleotides. The associated gene clusters map to cell migration/motility and cellular membrane function. At postpartum, the strongest biological relevance in mothers was for cadherin signaling and immune function. | Vitamin D status may affect maternal and infant health during gestation and lactation by influencing the epigenomic landscape. |
| Rafiee et al.; (2015) Altered TH17/Treg ratio in recurrent miscarriage after treatment with paternal lymphocytes and vitamin D3: a double-blind placebo-controlled study. Iran J. Immunol. [35] | Double-blinded, placebo-controlled study. | Study conducted at Isfahan University of Medical Sciences, Iran between October 2013, and September 2014. A total of 44 patients with primary recurrent abortion (mean age 27.2 ± 5.0 years) were randomly assigned to the treatment (n = 22) and control (n = 22) groups. | The study objectives were to investigate the effects of vitamin D3 supplementation to enhance immune tolerance in women undergoing lymphocyte immune therapy (LIT) for recurrent miscarriages (RM). Of interest were the effect of vitamin D3 on the imbalance of 2 essential T cells subsets, Th17 and T regulatory (Treg) cells, in RM patients pre- and 3 months post treatment with LIT alone or in combination with vitamin D3. | Vitamin D3 therapy decreased the frequency of Th17 cells in addition to reducing the Th17/Treg ratio in peripheral blood of RM patients compared with the control group (p < 0.05). | RM patients have a higher Th17/Treg ratio in their peripheral blood. Vitamin D3 therapy decreased both the frequency of Th17 cells and the Th17/Treg ratio compared with the control group (p < 0.05) and may be a therapeutic candidate in RM. |
| Zerofsky et al.; (2016) Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized, controlled trial. J. Nutr. [36] | Double-blinded, randomized, controlled study (Kellogg Foundation grant)—see below. | A total of 57 pregnant women (mean age 29.6 ± 4.8 years, median BMI 25.1 kg/m2, IQR 21.3–29.5) at the University of California were randomized to receive either cholecalciferol (400 IU/day) or cholecalciferol 2000 IU/day from <20 weeks’ gestation to delivery. | The objectives of this study were to assess the effects of vitamin D supplementation during pregnancy on vitamin D status and markers of immune function linked with adverse outcomes. | Supplementation with a higher dose significantly increased vitamin D status during pregnancy (p < 0.0001). Women receiving 2000 IU/day had 36% more interleukin-10+ regulatory CD4+ T cells at 36 weeks than did those in the 400-IU/day group (p < 0.007). | Supplementation with 2000 IU/day is more effective at increasing vitamin D status in pregnant women than 400 IU/day and is associated with increased regulatory T cell immunity. This may prevent adverse outcomes caused by excess inflammation. |
| Khatiwada et al., (2021) Effects of vitamin D supplementation on circulating concentrations of growth factors and immune-mediators in healthy women during pregnancy. Pediatr. Res. [37] | Double-blinded, randomized trial at Medical University of South Carolina (Kellogg Foundation grant—see above). | Pregnant women enrolled at 10–14 weeks’ gestation were randomized to 400 or 4400 IU vitamin D3/day. Data on health, safety, circulating 25(OH)D and 9 immune mediators were collected in each trimester. 400 IU group: n = 107 (mean age 28.9 ± 5.29, BMI 29.5 ± 7.95 kg/m2). 4400 IU group: n = 110 (mean age 28.3 ± 4.69, BMI 28.5 ± 6.80 kg/m2). | The study objectives were to investigate the effects of plasma vitamin D metabolite 25(OH)D on plasma immune-mediators during the second and third trimesters of pregnancy, notably whether there exists an association between circulating blood levels and pro-inflammatory and tolerogenic immune-mediator concentrations. | Immune mediators in pregnant women were influenced by baseline (first trimester) plasma 25(OH)D values rather than increased levels secondary to supplementation. Baseline 25(OH)D was associated with baseline TGF-β and with IFN-γ and IL-2 in the second and third trimesters. Baseline IFN-γ, CRP, TGF-β, TNF-α, VEGF, IL-2, and IL-4 were associated with respective immune mediator concentrations in the second and third trimesters, notably at higher vitamin D3 dosage. Immune mediators were not affected by 25(OH)D concentrations in the second and third trimesters. Race was associated with baseline TGF-β, VEGF, and IL-10, and with IL-10 in the second and third trimesters. | Vitamin D supplementation before conception or during early in pregnancy, rather than later pregnancy, may be important when seeking to impact maternal immune-mediator response. |
| Samimi et al.; (2016) The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress, and pregnancy outcomes in pregnant women at high risk of preeclampsia. J. Hum. Nutr. Diet. [38] | Prospective, double-blind, placebo-controlled trial. | A total of 60 primigravida women aged 18-40 years at risk for preeclampsia (as determined biochemical and on ultrasound scanning) attending the Kashan University of Medical Science, Iran. Participants were randomized to either 50,000 IU vitamin D3 every 2 weeks plus 1000 mg/day calcium carbonate supplement or to receive a placebo from 20 to 32 weeks’ gestation. | The study objectives were to examine the effects of vitamin D plus calcium administration on metabolic profiles and pregnancy outcomes among women at risk for pre-eclampsia. Treatment group n = 30 (27.3 ± 3.7 years, BMI 27.4 ± 3.3 kg/m2). Placebo group n = 30 (27.1 ± 5.2 years, BMI 25.6 ± 4.0 kg/m2). A range of biochemical and clinical parameters were investigated. | Taking both vitamin D3 and calcium supplements resulted in a significant reduction in fasting plasma glucose, serum insulin concentrations, insulin resistance, and beta cell function, and a significant rise in insulin sensitivity. Additionally, pregnant women receiving the combination demonstrated increased serum high-density lipoprotein (HDL)-cholesterol and plasma total glutathione concentrations (GSH). | Administration of vitamin D plus calcium for 12 weeks produced beneficial effects on glycemic status, HDL-cholesterol, GSH, and blood pressure among women at risk for preeclampsia. |
| Jefferson et al., (2019) Relationship between vitamin D status and the vaginal microbiome during pregnancy. J. Perinatol. [39] | Prospective randomized study. | A total of 402 healthy pregnant women attending the Medical University of South Carolina from 1 January 2013, to 30 April 2018 were enrolled. 387 were randomized to receive normal (400 IU/day, control group) or a high-dose vitamin D supplement (4400 IU/day; treatment group). In the control group (n = 191), 142 were followed to delivery. In the treatment group (n = 196), 155 were followed to delivery. There were 19 miscarriages and108 subjects dropped out. | The objective of this study was to investigate the association between vitamin D status and the vaginal microbiome in different ethnic American groups during pregnancy. Complete information was available on 236 participants (mean age 29 years, range 18–42, BMI 33.2 kg/m2). These comprised American Indians (n = 2, 1%), Black Americans (n = 83, 35%), Hispanics (n–61, 26%), and White women (n = 90 (38%). There were 112 women in the control group and 124 in the treatment group. Blood samples for 25(OH)D were taken monthly. Vaginal swabs were generally taken at 3 timepoints. | The vaginal microbiome was significantly affected by gestational age and ethnicity. The presence of Megasphaera bacteria correlated negatively (p = 0.0187) with 25(OH)D in Black women. Among controls, White women exhibited a positive correlation between plasma 25(OH)D and profuse L. crispatus. | There is an association between plasma 25(OH)D concentration and certain vaginal bacteria. |
| Author (Year), Title, Journal | Study Design | Sample and Setting | Objectives and Methodology | Results | Implications |
|---|---|---|---|---|---|
| Mozurkewich et al.; (2018) Effect of prenatal EPA and DHA on maternal and umbilical cord blood cytokines. BMC Pregn. Childbirth [54] | Secondary analysis of a prospective, double-blinded, randomized, controlled trial of fish oil supplementation during pregnancy for prevention of depressive symptoms. | Participants recruited from the antenatal clinics of the University of Michigan Medical Center and St. Joseph Mercy Health System, USA between October 2008 and May 2011. Pregnant women (12–20 weeks’ gestation) at risk of depression based on an Edinburgh Postnatal Scale Score between 9 and 19 or a history of depression who consumed ≤2 portions of fish per week. | The objectives of this study were to investigate the effects of prenatal EPA- and DHA-rich fish oil supplementation on 16 maternal and fetal cytokine production. Participants were assigned to receive daily EPA-rich fish oil (1060 mg EPA, 274 mg DHA), DHA-rich fish oil (900 mg DHA, 180 mg EPA), or placebo. Maternal blood samples were collected at enrollment and after 34–36 weeks’ gestation. Umbilical cord blood was collected at delivery. Follow-up was until 6 weeks post-partum. | Originally, 126 were enrolled and 118 completed the trial. A total of 113 samples were available for cytokine analyses after supplementation (minimum 14 weeks). EPA-rich fish oil supplementation decreased IL-6, IL-15, and TNF-α plasma concentrations compared with placebo, while DHA had no effect. There was no significant difference in IL-1β, IL-2, IL-5, IL-8, IL-12P70, IL-17, INF-γ, or MCP1 between groups. A total of 102 cord blood samples were analyzed. There were no significant differences in cord blood cytokines between groups. | Women with perinatal depressive symptoms related to inflammation may benefit from EPA-rich fish oil supplementation to reduce plasma concentrations of inflammatory cytokines. Future research in depression should focus on the loirole of EPA and clarify the relationship between inflammatory cytokines and depressive symptoms. |
| Nishi et al.; (2020) Plasma estradiol levels and antidepressant effects of omega-3 fatty acids in pregnant women. Brian Behav. Immun. [55] | Double-blinded, parallel-group, randomized, controlled trial (Synchronized Trial on Expectant Mothers with Depressive Symptoms by Omega-3 FAs [SYNCHRO]) | Multicenter trial at Tokyo Medical University, University of Toyama, Chiba University, National Center of Neurology and Psychiatry, and National Center for Child Health and Development, Japan, and China Medical University, Taiwan. A total of 108 pregnant women at 12–24 weeks’ gestation with an Edinburgh Postnatal Depression Scale score ≥9 who eat ≤3 portions of fish/week | The aims of this study were to examine the association between increased estradiol (E2) levels, inflammatory cytokines, and depressive symptoms in pregnant women, and whether these were affected by omega-3 FA supplementation. Participants were randomized to receive omega-3 FA capsules (total 1206 mg EPA, 609 mg DHA/day, n = 49, mean age 32.8 ± 5.3 years) or placebo (n = 51, mean age 32.6 ± 5.3 years) for 12 weeks. Blood samples were taken at baseline and 12-week follow-up. | A total of 100 participants completed blood sampling. Increases in EPA and E2 were significantly associated with a decrease in depressive symptoms in the omega-3 FA group. Unexpectedly, the placebo group showed an association between increase in EPA and increase in depressive symptoms. | EPA supplementation and increased E2 levels during pregnancy may work synergistically to reduce depressive symptoms through a mechanism other than anti-inflammation. EPA may improve depression in non-pregnant people through an anti-inflammatory effect. Future studies should investigate how EPA supplementation and E2 levels in treating depression could be utilized. |
| Haghiac et al.; (2015) Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: a randomized double-blind controlled clinical trial. PLoS ONE [56] | Randomized, double-masked, controlled trial | A total of 72 obese pregnant women at MetroHealth Medical Center, Cleveland, Ohio, USA, were randomized to receive omega-3 FA supplementation (capsules containing EPA, 20:5n-3 plus DHA, 22:6n-3; total 2 g) or placebo twice a day from week 10–16 weeks’ gestation to term. | The objectives of this study were to characterize the effects of omega-3 FA supplementation on inflammatory status in the placenta and adipose tissue of overweight/obese pregnant women and cultured adipose and trophoblast cells. Data was available on 25 omega-3 FA- treated participants (mean age 27 ± 5 years, BMI 33 ± 6) and 24 placebo participants (mean age 27 ± 5 years, BMI 32 ± 6). | After 25 weeks of supplementation, the adipose tissue and placenta showed lower expression of TLR4, IL-6, IL-8, and TNF-α, and there was decreased plasma CRP at delivery. Increased birth weights were observed in the omega-3 FA group. | Omega-3 FA supplementation decreased obesity-associated tissue inflammation in pregnancy. TLR4 appears to have a central role. Additional randomized, controlled, trials are needed to clarify the effect of omega-3 supplementation on birth weight. |
| Horvaticek et al.; (2017) Effect of eicosapentaenoic acid and docosahexaenoic acid supplementation on C-peptide preservation in pregnant women with type-1 diabetes: randomized placebo controlled clinical trial. Eur. J. Clin. Nutr. [57] | Prospective, randomized, placebo-controlled clinical trial. | Conducted at Ministry of Health Referral Center for Diabetes in Pregnancy, Department of Obstetrics and Gynecology, Zagreb University Hospital, Republic of Croatia, 90 pregnant women with type 1 diabetes mellitus (T1DM between 5 and 30 years) at 9 weeks’ gestation were randomized to a standard diabetic diet plus EPA and DHA capsules (total 120 mg EPA, 616 mg DHA/day) or standard diabetic diet plus placebo. | The objectives of this study were to explore the effects of EPA and DHA supplementation on fasting C-(FC) peptide secretion in pregnant women with T1DM. A total of 47 women were randomized to EPA and DHA (mean age 29.8 ± 5.5 years, BMI 23.3 ± 3.3 kg/m2) and 43 to the placebo group (mean age 29.6 ± 4.8 years, BMI 22.9 ± 3.1 kg/m2). Blood samples for FC, fasting blood glucose (FBG), and HbA1c were analyzed during each trimester. | Supplementation with EPA and DHA resulted in a significant increase in FC-peptide during pregnancy. In the placebo group, the rise in FC-peptide was not significant. There were no differences in birth weight and prevalence of fetal macrosomia. C-peptide and FBG concentrations in umbilical vein serum were lower in the treatment group. | EPA and DHA supplementation in pregnancy cause immunological tolerance and stimulate the production of endogenous insulin in women with T1DM. |
| Forsberg et al.; (2020) Changes in peripheral immune populations during pregnancy and modulation by probiotics and ω-3 fatty acids. Sci. Rep. [58] | Prospective, randomized, double-blinded, placebo-controlled, allergy prevention trial (PROOM-3). | Multicenter trial conducted at the Department of Pediatrics and Allergy Center at University Hospital in Linköping and 3 county hospitals in Sweden. A total of 88 pregnant women at 20 weeks’ gestation with clinical symptoms/history of allergic disease, or a child/partner with clinical symptoms/history, were randomized into four groups to receive either L. reuteri oil drops, omega-3 FA (3 capsules containing 640 mg omega-3 FA), a combination, or placebo, twice daily during pregnancy and lactation. | Objectives of this study were to investigated how maternal peripheral immunity is affected by pregnancy and L. reuteri (probiotic) and omega-3 FA supplementation. The reseachers used flow cytometry and a broad panel of immune markers to map peripheral immune cell populations. Groups: L. reuteri oil drops (n = 23, mean age at inclusion 29 years, study product 18.8 weeks). omega-3 FA (n = 21, mean age 30 years, study product 18.6 weeks). L. reuteri + omega-3 FA (n = 22; mean age 29 years, study product 19.6 week). Placebo (n = 22, mean age 29 years, study product 18.5 week). | L. reuteri supplementation from 20 weeks’ gestation decreased activated and resting Treg cells at 4 days post-delivery compared with omega-3 FA and placebo. There were no significant differences between results for the omega-3 FA and placebo groups. Lymphocyte and monocyte populations were not affected by supplementation. | A total of 20 weeks of supplementation with L. reuteri during pregnancy resulted in immunomodulatory effects on activated and resting Treg cells. omega-3 FA supplementation had no effect in this study. |
| Harper et al.; (2013) Change in mononuclear leukocyte responsiveness in midpregnancy and subsequent preterm birth. Obstet. Gynecol. [59] | Ancillary study to a randomized, controlled trial of omega-3 FA supplementation to prevent recurrent preterm birth. | The cohort consisted of 852 women (mean age 27 ± 23–32 years) attending a US Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Unit who participated in the randomized trial. Pregnant women between 16- and 21-weeks’ gestation with prior spontaneous preterm births were randomized to receive omega-3 FA supplementation (2000 mg) or placebo. | The objectives of this study were to explore changes in immune response associated with preterm birth, omega-3 FA supplementation, and a fish diet history. Blood samples were taken at baseline (16–22 weeks) and again at 25–28 weeks’ gestation (follow-up) to examine in vitro maternal peripheral blood mononuclear leukocyte production of interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), in response to stimulation with lipopolysaccharid. | A total of 343 of 852 participants had paired cytokine measurements for either IL-10, TNF-α, or both. Anti-inflammatory IL-10 and proinflammatory TNF-α levels were unaffected by omega-3 FA supplementation or a fish diet. The rate of preterm birth at less than 37 weeks’ gestation was 33.7% for those who ate at least 1 portion of fish/week and 44.4% for those who ate <1 portion of fish/week. | Recurrent preterm birth before 35 weeks was associated with decreased peripheral blood mononuclear leukocyte production of IL-10 in response to a lipopolysaccharide stimulation during the second trimester. This study demonstrated that the variable influences of a fish diet and omega-3 FA supplementation on preterm birth rates were not due to a modulation effect on the maternal immune response. |
| Keelan et al.; (2015) Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators, and their precursors. Reproduction [60] | Placentas were collected from women enrolled in a randomized, placebo-controlled trial of omega-3 FA supplementation from 20 weeks’ gestation. | Conducted at St John of God Hospital and Princess Margaret Hospital, Australia, between 1999 and 2001. A total of 98 pregnant women who ate ≤ than 2 portions of fish/week were randomized to receive omega-3 FA supplementation (3.7 g/day, 56% DHA and 27.7% EPA) or placebo. | The objectives of this study were to examine whether levels of specialized pro-resolving lipid mediators (SPMs) and their precursors varied in placental tissue from women taking omega-3 FA supplementation during pregnancy compared to a control population. | A total of 51 placentas were sampled. Omega-3 FA supplementation increased placental DHA and levels of SPM precursors, but not EPA. Expression of PTGS2, IL-1β, IL-6, and IL-10 was unaffected by omega-3 FA. Conversely, supplementation increased expression of TNF-α 14-fold. | DHA and major SPM precursors levels were increased in the placenta of women who took omega-3 FA supplementation from 20 weeks’ gestation. |
| Author (Year), Title, Journal | Study Design | Sample and Setting | Objectives and Methodology | Results | Implications |
|---|---|---|---|---|---|
| Mwangi et al.; (2015) Effect of daily antenatal iron supplementation on Plasmodium infection in Kenyan women: a randomized clinical trial. JAMA [42] | Randomized clinical trial. | The study involved a total of 470 pregnant rural Kenyan women. Participants were aged 15–45 years with singleton pregnancies and a gestational age of 13–23 weeks and hemoglobin concentration of ≥90 g/L. All women received 5.7 mg iron/day as fortified flour and the usual intermittent malaria preventive throughout the study with sulfadoxine-pyrimethamine. | The objectives of this study were to measure the effect of antenatal iron supplementation on maternal P. falciparum infection risk at birth, iron status, and neonatal outcomes during a malaria epidemic. The intervention group received daily supplementation with 60 mg iron (ferrous fumarate, n = 237; median age 24.0 years, IQR 20.0–28.5, BMI 22.1 ± 2.7 kg/m2) or placebo (n = 233, median age 24.0 years, IQR 20.0–29.0, BMI 21.8 ± 2.6 kg/m2)) from randomization until the first month postpartum. | A total of 40 women were lost to follow-up/excluded. At baseline, 190 of 318 women (59.7%) were iron-deficient. An ITT analysis comparing iron vs. placebo demonstrated a P. falciparum infection risk at birth of 50.9 vs. 52.1% (crude difference, −1.2%; 95% CI −11.8% to 9.5%; p = 0.83) and birth weight of 3202 vs. 3053 g, respectively. (crude difference 150 g; 95% CI 56–244; p = 0.002), respectively. | There were no differences in overall maternal P. falciparum infection risk between women taking iron supplementation or placebo. Iron supplementation led to increased birthweight. There was no evidence that iron supplementation caused serious adverse events. |
| Darling et al.; (2017) Vitamin A and zinc supplementation among pregnant women to prevent placental malaria: a randomized, double-blind, placebo-controlled trial in Tanzania Am. J. Trop. Med. Hyg. [26] | Randomized, double-blinded, placebo-controlled trial with a factorial design. | A total of 2500 HIV-negative primigravid or secundigravid pregnant women in their first trimester in Dar es Salaam, Tanzania. | The objectives of this study were to investigate whether supplementation with vitamin A, zinc, or both starting in the first trimester reduces rick of placental malaria and adverse pregnancy outcomes. A total of 625 participants were allocated to each treatment group: 2500 IU of vitamin A, 25 mg of zinc, both 2500 IU of vitamin A and 25 mg of zinc, or a placebo until delivery. Secondary outcomes included small for gestational age (SGA) births and prematurity. Follow-up was for at least 6 weeks post-delivery. | Placental samples were obtained in 1404 mothers (mean age 22.9 ± 4.4 years, BMI 23.5 ± 4.4 kg/m2), comprising 56% of participants and 62% of all pregnancies ≥28 weeks [n = 2266]). Birth outcomes were obtained for 2434 of 2500 randomized participants. Women who received zinc had a lower risk of histopathology placental malaria compared with those not receiving zinc (RR 0.64; 95% CI 0.44–0.91). PCR-positive malaria, SGA, and prematurity were not affected in either treatment group. | Pregnant women who received zinc had a lower risk of histopathology-positive placental malaria. None of the active treatments influenced PCR-positive malaria, SGA, or prematurity. There were no safety concerns. |
| Goheen et al.; (2017) Host iron status and erythropoietic response to iron supplementation determines susceptibility to the RBC stage of falciparum malaria during pregnancy. Sci. Rep. [44] | Observational cohort study. | Pregnant women (18–45 years old between 14 and 22 weeks’ gestation) from the Kiang West and Jarra East regions of rural Gambia. Participants were recruited between June 2014 and March 2016 from the reference arm of a randomized trial testing the efficacy and safety of a hepcidin-guided screen-and-treat strategy for combatting anemia. | The objective of this study was to investigate whether iron supplementation increased the risk of P. falciparum by means of an in-vitro study of RBCs from pregnant women during their second and third trimesters. RBCs were collected and assayed before (n = 327) and 14 days (n = 82), 49 days (n = 112), and 84 days (n = 115) after iron supplementation (60 mg iron as ferrous fumarate daily). | P. falciparum erythrocytic stage growth in vitro is reduced in anemic pregnant women at baseline, but increased during supplementation. The elevated growth rates paralleled increases in circulating CD71-positive reticulocytes and other markers of young RBCs. | In vitro, P. falciparum growth in response to iron supplementation is associated with elevated erythropoiesis, an essential step in erythroid recovery. These results support WHO recommendations regarding iron supplementation. |
| Chandrasiri et al.; (2015) The impact of lipid-based nutrient supplementation on anti-malarial antibodies in pregnant women in a randomized controlled trial. Malar. J. [61] | Single-blinded, randomized, controlled trial. | A total of 1009 pregnant Malawian women (median age 24, IQR 20–28, mean gestation 16.5 ± 2.20 weeks; median BMI 21.6, IQR 20.3–23.5 kg/m2) enrolled in the high-energy, micronutrient fortified lipid-based nutrient supplements (iLiNS-DYAD) trial. The source/composition of the lipid-based nutrient (LNS) is unclear. All participants received two doses of sulphadoxine-pyrimethamine (SP) malaria intermittent preventative treatment at enrolment and at 28–34 gestation weeks. | The objective of this study was to investigate whether different nutrient supplements offered to pregnant women reduced their susceptibility to malaria by improving immunity as judged by changes in antibody levels. Antibodies to antigens expressed by a placental-binding parasite isolate, a non-placental binding parasite isolate, merozoites, and schizonts were measured at enrollment (before 20 gestation weeks) and at 36 weeks in women receiving a daily lipid-based nutrient supplement, multiple micronutrients, or iron and folic acid. | Antibodies to placental-binding isolates significantly increased while antibodies to most merozoite antigens declined between timepoints. The type of supplementation did not affect antibody levels at 36 weeks’ gestation or their rate of change. There was a negative association between maternal BMI and antibodies to placental-binding antigens (coefficient [95% CI] −1.04 [−1.84, −0.24]). | Nutrient supplementation did not affect antimalarial antibody responses. Women with higher socioeconomic status had significantly lower IgG and opsonizing antibodies to placental-binding antigens that were not influenced by supplementation type. |
| Nkoma et al.; (2017) Providing lipid-based nutrient supplement during pregnancy does not reduce the risk of maternal P falciparum parasitemia and reproductive tract infections: a randomised controlled trial. BMC Pregnancy Childbirth [62] | Randomized, controlled trial. | Substudy of 1391 pregnant Malawian women enrolled in the iLiNS-DYAD trial between 2011 and 2013. See Chandrasiri et al. [61] above. The source/composition of the LNS is unclear. | The objectives of this study were to investigate the impact of daily lipid-based nutrient SQ-LNS (n = 462, mean age 25 ± 6, BMI 22.2 ± 3.0 kg/m2), multiple micronutrients (MMN, n = 466 mean age 25 ± 6 years, BMI 22.2 ± 2.9 kg/m2), or iron and folic acid (n = 463, mean age 25 ± 6, BMI 22.1 ± 2.6 kg/m2) from <20 weeks’ gestation on occurrence of P. falciparum parasitemia during pregnancy, and trichomoniasis, vaginal candidiasis, and UTI after delivery. Assessment was at 32 (RDT) and 36 weeks’ gestation (PCR), at delivery (RDT and PCR), and 1 week post-delivery (microscopy and urine analysis). | The prevalence of P. falciparum parasitemia was 10.7% at 32 weeks’ gestation, 9% at 36 weeks’ gestation, and 8.3% at delivery. The value for PCR testing at delivery was 20.2%. After delivery, the prevalence of trichomoniasis was 10.5%, vaginal candidiasis 0.5%, and UTI 3.1%. There were no differences between intervention groups in the prevalence of these infections. | SQ-LNS did not influence the occurrence of maternal P. falciparum parasitemia, trichomoniasis, vaginal candidiasis, or UTI. |
| Olofin et al.; (2014) Supplementation with multivitamins and vitamin A and incidence of malaria among HIV-infected Tanzanian women. J. Acquir. Immune. Defic. Syndr. [28] | Randomized, controlled trial with modifications. | A total of 1078 HIV-infected pregnant women (mostly second trimester) in Dar es Salaam, Tanzania, from April 1995 until August 2003, with modifications in 1998 and 2000. Malaria was defined by a presumptive clinical diagnosis and/or examination of blood smears for the malaria parasite. Women received malaria prophylaxis during pregnancy. Participants were followed up monthly during the prenatal and postpartum periods. | The object was to examine whether daily multivitamin supplementation (vitamin B complex, C, and E) or vitamin A supplementation altered malaria incidence in HIV-infected pregnant women. Four groups: multivitamins (20 mg vitamins B1, 20 mg B2, 25 mg B6, 100 mg niacin, 50 mg B12, 500 mg C, 30 mg E, and 800 mg folic acid), vitamin A alone (30 mg b-carotene with 5000 IU preformed vitamin A), both multivitamins and vitamin A, or placebo. | Median follow-up was 41.0 months (or to next pregnancy, loss to follow-up, or death). Multivitamin supplementation compared with no multivitamin ± s significantly lowered the risk of clinically diagnosed clinical malaria (RR 0.78; 95% CI 0.67–0.92). Vitamin A supplementation did not change malaria incidence. Multivitamins increased the risk of any malaria parasitemia (RR 1.24; 95% CI 1.02–1.50). | Multivitamin supplements protected against development of symptomatic malaria among pregnant, HIV-positive women. The clinical significance of increased malaria parasitemia among supplemented women is unknown. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rees, G.; Brough, L.; Orsatti, G.M.; Lodge, A.; Walker, S. Do Micronutrient and Omega-3 Fatty Acid Supplements Affect Human Maternal Immunity during Pregnancy? A Scoping Review. Nutrients 2022, 14, 367. https://doi.org/10.3390/nu14020367
Rees G, Brough L, Orsatti GM, Lodge A, Walker S. Do Micronutrient and Omega-3 Fatty Acid Supplements Affect Human Maternal Immunity during Pregnancy? A Scoping Review. Nutrients. 2022; 14(2):367. https://doi.org/10.3390/nu14020367
Chicago/Turabian StyleRees, Gail, Louise Brough, Gustavo Moya Orsatti, Anna Lodge, and Steven Walker. 2022. "Do Micronutrient and Omega-3 Fatty Acid Supplements Affect Human Maternal Immunity during Pregnancy? A Scoping Review" Nutrients 14, no. 2: 367. https://doi.org/10.3390/nu14020367
APA StyleRees, G., Brough, L., Orsatti, G. M., Lodge, A., & Walker, S. (2022). Do Micronutrient and Omega-3 Fatty Acid Supplements Affect Human Maternal Immunity during Pregnancy? A Scoping Review. Nutrients, 14(2), 367. https://doi.org/10.3390/nu14020367









