Dehydration Status Aggravates Early Renal Impairment in Children: A Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
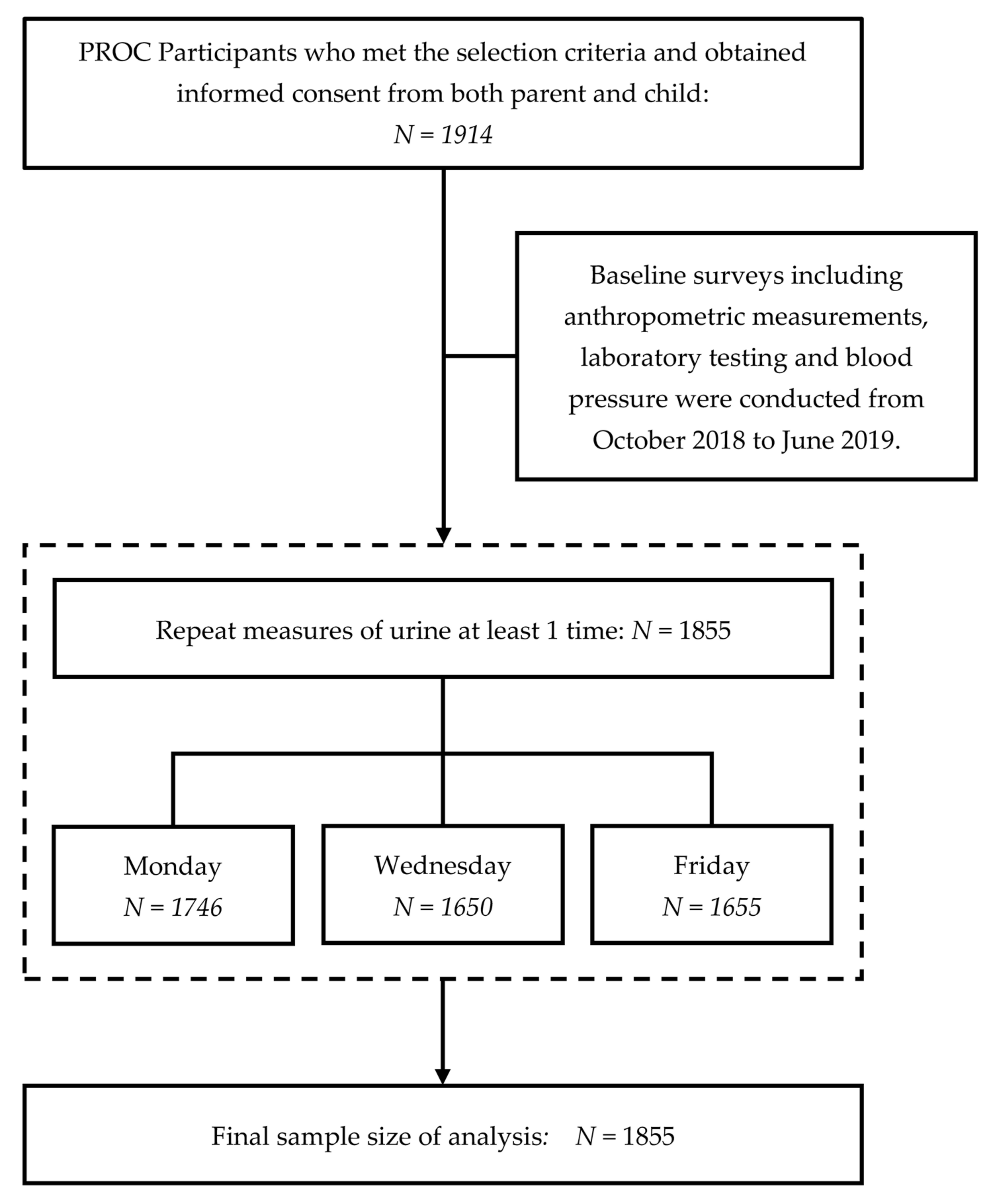
2.1. Study Design and Participants
2.2. Anthropometric Measurements
2.3. Blood Pressure
2.4. Blood Collection and Laboratory Assay
- Serum lipid panels were measured using automatic clinical chemistry analyzer Beckman Coulter AU5800 (Shizuoka, Japan), including blood glucose (BGLU), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
- Hemoglobin A1c (HbA1c) was measured using the automated glycohemoglobin analyzer Tosoh’s HLC-723G8 (Yamaguchi, Japan).
2.5. Repeat Measurements of Urine Assay
2.6. Hydration Status
2.7. Ethical Consideration
2.8. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
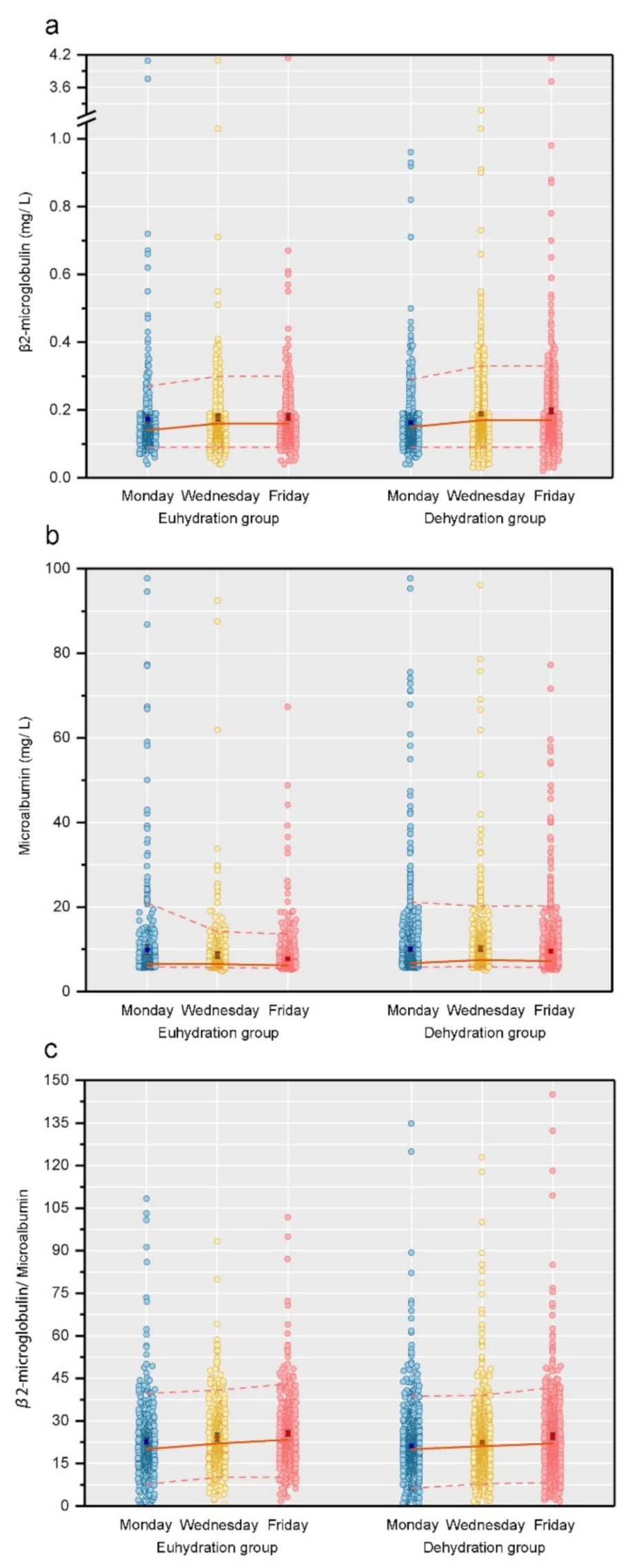
3.2. Three Urine Indicators’ Percentile Change from Monday to Friday
3.3. Temporal Trends of Renal Damage Indicators (Continuous Value) by Hydration Status
3.4. Temporal Trends in the Percentage Change of Renal Damage (Categorical Value) by Hydration Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suh, H.; Kavouras, S.A. Water intake and hydration state in children. Eur. J. Nutr. 2019, 58, 475–496. [Google Scholar] [CrossRef]
- Savoie, F.A.; Kenefick, R.W.; Ely, B.R.; Cheuvront, S.N.; Goulet, E.D. Effect of Hypohydration on Muscle Endurance, Strength, Anaerobic Power and Capacity and Vertical Jumping Ability: A Meta-Analysis. Sports Med. 2015, 45, 1207–1227. [Google Scholar] [CrossRef]
- Watso, J.C.; Farquhar, W.B. Hydration Status and Cardiovascular Function. Nutrients 2019, 11, 1866. [Google Scholar] [CrossRef] [Green Version]
- Wittbrodt, M.T.; Millard-Stafford, M. Dehydration Impairs Cognitive Performance: A Meta-analysis. Med. Sci. Sports Exerc. 2018, 50, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.F.; Sontrop, J.M.; Huang, S.H.; Moist, L.; Bouby, N.; Bankir, L. Hydration and Chronic Kidney Disease Progression: A Critical Review of the Evidence. Am. J. Nephrol. 2016, 43, 281–292. [Google Scholar] [CrossRef]
- D’Anci, K.E.; Constant, F.; Rosenberg, I.H. Hydration and cognitive function in children. Nutr. Rev. 2006, 64, 457–464. [Google Scholar] [CrossRef]
- Bottin, J.H.; Morin, C.; Guelinckx, I.; Perrier, E.T. Hydration in Children: What Do We Know and Why Does it Matter? Ann. Nutr. Metab. 2019, 74 (Suppl 3), 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, M. Emphasise the importance of adequate water intake. Hong Kong Med. J. 2015, 21, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaerjiang, N.; Xiao, H.; Zunong, J.; Shu, W.; Li, M.; Pérez-Escamilla, R.; Hu, Y. Sleep disturbances in children newly enrolled in elementary school are associated with parenting stress in China. Sleep Med. 2021, 88, 247–255. [Google Scholar] [CrossRef]
- Wu, H.Y.; Huang, J.W.; Peng, Y.S.; Hung, K.Y.; Wu, K.D.; Lai, M.S.; Chien, K.L. Microalbuminuria screening for detecting chronic kidney disease in the general population: A systematic review. Ren. Fail. 2013, 35, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah; Kadam, P.; Yachha, M.; Srivastava, G.; Pillai, A.; Pandita, A. Urinary beta-2 microglobulin as an early predictive biomarker of acute kidney injury in neonates with perinatal asphyxia. Eur. J. Pediatr. 2021. [Google Scholar] [CrossRef]
- van Veldhuisen, D.J.; Ruilope, L.M.; Maisel, A.S.; Damman, K. Biomarkers of renal injury and function: Diagnostic, prognostic and therapeutic implications in heart failure. Eur. Heart J. 2016, 37, 2577–2585. [Google Scholar] [CrossRef] [Green Version]
- Ji, T.T.; Tan, N.; Lu, H.Y.; Xu, X.Y.; Yu, Y.Y. Early renal injury indicators can help evaluate renal injury in patients with chronic hepatitis B with long-term nucleos(t)ide therapy. World J. Clin. Cases 2020, 8, 6306–6314. [Google Scholar] [CrossRef]
- Barton, K.T.; Kakajiwala, A.; Dietzen, D.J.; Goss, C.W.; Gu, H.; Dharnidharka, V.R. Using the newer Kidney Disease: Improving Global Outcomes criteria, beta-2-microglobulin levels associate with severity of acute kidney injury. Clin. Kidney J. 2018, 11, 797–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magagnotti, C.; Zerbini, G.; Fermo, I.; Carletti, R.M.; Bonfanti, R.; Vallone, F.; Andolfo, A. Identification of nephropathy predictors in urine from children with a recent diagnosis of type 1 diabetes. J. Proteom. 2019, 193, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.O.; Chung, S.; Kim, K.S. Microalbuminuria in children with urinary tract infection. Korean J. Pediatr. 2010, 53, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matjuda, E.N.; Sewani-Rusike, C.R.; Anye, S.N.C.; Engwa, G.A.; Nkeh-Chungag, B.N. Relationship between High Blood Pressure and Microalbuminuria in Children Aged 6–9 Years in a South African Population. Children 2020, 7, 131. [Google Scholar] [CrossRef]
- Gracchi, V.; van den Belt, S.M.; Küpers, L.K.; Corpeleijn, E.; de Zeeuw, D.; Heerspink, H.J. Prevalence and distribution of (micro)albuminuria in toddlers. Nephrol. Dial. Transplant. 2016, 31, 1686–1692. [Google Scholar] [CrossRef] [Green Version]
- Kwak, B.O.; Lee, S.T.; Chung, S.; Kim, K.S. Microalbuminuria in normal Korean children. Yonsei Med. J. 2011, 52, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Tzvi-Behr, S.; Ivgi, H.; Frishberg, Y.; Ben Shalom, E. First-week urine beta-2 microglobulin levels in term healthy neonates. Pediatr. Nephrol. 2021, 36, 1511–1514. [Google Scholar] [CrossRef]
- Li, M.; Shu, W.; Zunong, J.; Amaerjiang, N.; Xiao, H.; Li, D.; Vermund, S.H.; Hu, Y. Predictors of non-alcoholic fatty liver disease in children. Pediatr. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Shatat, I.F.; Qanungo, S.; Hudson, S.; Laken, M.A.; Hailpern, S.M. Changes in Urine Microalbumin-to-Creatinine Ratio in Children with Sickle Cell Disease over Time. Front. Pediatr. 2016, 4, 106. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, L.E.; Pumerantz, A.C.; Fiala, K.A.; Roti, M.W.; Kavouras, S.A.; Casa, D.J.; Maresh, C.M. Human hydration indices: Acute and longitudinal reference values. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 145–153. [Google Scholar] [CrossRef]
- Ma, G.S. Hydration status and health. Zhonghua Yu Fang Yi Xue Za Zhi 2019, 53, 337–341. [Google Scholar] [CrossRef]
- Kenney, E.L.; Long, M.W.; Cradock, A.L.; Gortmaker, S.L. Prevalence of Inadequate Hydration Among US Children and Disparities by Gender and Race/Ethnicity: National Health and Nutrition Examination Survey, 2009–2012. Am. J. Public Health 2015, 105, e113–e118. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, G.; Du, S.; Zhang, N. The Relationships between Water Intake and Hydration Biomarkers and the Applications for Assessing Adequate Total Water Intake among Young Adults in Hebei, China. Nutrients 2021, 13, 3805. [Google Scholar] [CrossRef]
- Josh, W.; Kristy, H. Young Children’s Understanding of Fluid Intake. Int. J. Nutr. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Howells, K.; Coppinger, T. Teachers’ Perceptions and Understanding of Children's Fluid Intake. Int. J. Environ. Res. Public Health 2020, 17, 4050. [Google Scholar] [CrossRef]
- Michels, N.; Van den Bussche, K.; Vande Walle, J.; De Henauw, S. School Policy on Drinking and Toilets: Weaknesses and Relation With Children's Hydration Status. J. Nutr. Educ. Behav. 2019, 51, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kozioł-Kozakowska, A.; Piórecka, B.; Suder, A.; Jagielski, P. Body Composition and a School Day Hydration State among Polish Children-A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 7181. [Google Scholar] [CrossRef]
- Chang, T.; Ravi, N.; Plegue, M.A.; Sonneville, K.R.; Davis, M.M. Inadequate Hydration, BMI, and Obesity Among US Adults: NHANES 2009-2012. Ann. Fam. Med. 2016, 14, 320–324. [Google Scholar] [CrossRef]
- Arnaoutis, G.; Kavouras, S.A.; Stratakis, N.; Likka, M.; Mitrakou, A.; Papamichael, C.; Sidossis, L.S.; Stamatelopoulos, K. The effect of hypohydration on endothelial function in young healthy adults. Eur. J. Nutr. 2017, 56, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, H.; Hu, S.; He, H.; Shui, H. β2-microglobulin is an independent indicator of acute kidney injury and outcomes in patients with intracerebral hemorrhage. Medicine 2020, 99, e19212. [Google Scholar] [CrossRef]
- Zeng, X.; Hossain, D.; Bostwick, D.G.; Herrera, G.A.; Zhang, P.L. Urinary β2-Microglobulin Is a Good Indicator of Proximal Tubule Injury: A Correlative Study with Renal Biopsies. J. Biomark. 2014, 2014, 492838. [Google Scholar] [CrossRef] [Green Version]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.-H.; Roumelioti, M.-E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Li, Q. Urine. In Clinical Molecular Diagnostics; Pan, S., Tang, J., Eds.; Springer: Singapore, 2021; pp. 241–252. [Google Scholar] [CrossRef]
- Singh, A.; Satchell, S.C. Microalbuminuria: Causes and implications. Pediatr. Nephrol. 2011, 26, 1957–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.M.; Tikaria, R. Microalbuminuria. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Futrakul, N.; Sridama, V.; Futrakul, P. Microalbuminuria—a biomarker of renal microvascular disease. Ren. Fail. 2009, 31, 140–143. [Google Scholar] [CrossRef]
- Wang, H.W.; Jiang, M.Y. Higher volume of water intake is associated with lower risk of albuminuria and chronic kidney disease. Medicine 2021, 100, e26009. [Google Scholar] [CrossRef]
- Perrier, E.T.; Armstrong, L.E.; Bottin, J.H.; Clark, W.F.; Dolci, A.; Guelinckx, I.; Iroz, A.; Kavouras, S.A.; Lang, F.; Lieberman, H.R.; et al. Hydration for health hypothesis: A narrative review of supporting evidence. Eur. J. Nutr. 2021, 60, 1167–1180. [Google Scholar] [CrossRef]
- Baron, S.; Courbebaisse, M.; Lepicard, E.M.; Friedlander, G. Assessment of hydration status in a large population. Br. J. Nutr. 2015, 113, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Factors | Total | Euhydration | Dehydration | p |
|---|---|---|---|---|
| Sex 1 | 0.033 | |||
| Boy (n (%)) | 927 (50.0) | 331 (46.8) | 596 (51.9) | |
| Girl (n (%)) | 928 (50.0) | 376 (53.2) | 552 (48.1) | |
| Age (year) 2,3 | 6.7 ± 0.30 | 6.7 ± 0.31 | 6.6 ± 0.29 | 0.16 |
| Z-score of height 2 | 0.67 ± 1.40 | 0.53 ± 1.33 | 0.75 ± 1.44 | 0.83 |
| Z-score of weight 2 | 0.61 ± 0.95 | 0.60 ± 0.95 | 0.61 ± 0.96 | <0.001 |
| Z-score of BMI 2 | 0.40 ± 1.53 | 0.21 ± 1.46 | 0.51 ± 1.56 | <0.001 |
| WC (cm) 2 | 56.69 ± 7.81 | 56.05 ± 7.17 | 57.08 ± 8.16 | 0.004 |
| SBP (mm Hg) 2 | 101 ± 8 | 101 ± 8 | 102 ± 9 | 0.049 |
| DBP (mm Hg) 2 | 56 ± 6 | 56 ± 6 | 56 ± 6 | 0.96 |
| BGLU (mmol/L) 2 | 5.07 ± 0.47 | 5.05 ± 0.44 | 5.08 ± 0.48 | 0.16 |
| TC (mmol/L) 2 | 4.53 ± 1.07 | 4.52 ± 0.94 | 4.54 ± 1.15 | 0.57 |
| TG (mmol/L) 2 | 0.69 ± 0.28 | 0.67 ± 0.27 | 0.70 ± 0.29 | 0.009 |
| HDL-C (mmol/L) 2 | 1.62 ± 0.32 | 1.64 ± 0.30 | 1.61 ± 0.33 | 0.08 |
| LDL-C (mmol/L) 2 | 2.50 ± 0.64 | 2.47 ± 0.66 | 2.52 ± 0.63 | 0.09 |
| HbA1c (%) 2 | 5.46 ± 0.26 | 5.47 ± 0.25 | 5.45 ± 0.26 | 0.09 |
| USG | 1.020 (1.015–1.025) | 1.015 (1.010–1.015) | 1.025 (1.020–1.025) | <0.001 |
| β2-MG (mg/L) 4 | ||||
| Monday | 0.14 (0.12–0.18) | 0.14 (0.12–0.18) | 0.15 (0.12–0.18) | 0.52 |
| Wednesday | 0.16 (0.13–0.21) | 0.16 (0.13–0.19) | 0.17 (0.14–0.21) | <0.001 |
| Friday | 0.16 (0.13–0.21) | 0.16 (0.13–0.20) | 0.17 (0.13–0.22) | <0.001 |
| MA (mg/L) 4 | ||||
| Monday | 6.60 (6.10–8.50) | 6.50 (6.00–8.20) | 6.70 (6.10–8.70) | <0.001 |
| Wednesday | 7.00 (6.20–9.10) | 6.50 (6.00–8.00) | 7.50 (6.50–9.80) | <0.001 |
| Friday | 6.70 (6.00–8.90) | 6.20 (5.80–7.30) | 7.20 (6.20–9.70) | <0.001 |
| Dependent Variables | Independent Variables | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | ||
| β2-MG | |||||||||
| Euhydration | |||||||||
| Intercept | 0.172 (0.154,0.189) | <0.001 | 0.155 (0.124,0.185) | <0.001 | 0.156 (0.126,0.186) | <0.001 | 0.151 (0.120,0.182) | <0.001 | |
| Monday | ref. | ref. | ref. | ref. | |||||
| Wednesday | 0.007 (−0.014,0.028) | 0.52 | 0.007 (−0.014,0.028) | 0.52 | 0.007 (−0.014,0.028) | 0.52 | 0.007 (−0.014,0.028) | 0.52 | |
| Friday | 0.005 (−0.013,0.023) | 0.58 | 0.005 (−0.013,0.023) | 0.58 | 0.005 (−0.013,0.023) | 0.58 | 0.005 (−0.013,0.023) | 0.58 | |
| Dehydration | |||||||||
| Intercept | 0.163 (0.156,0.169) | <0.001 | 0.167 (0.148,0.185) | <0.001 | 0.166 (0.148,0.183) | <0.001 | 0.166 (0.147,0.184) | <0.001 | |
| Monday | ref. | ref. | ref. | ref. | |||||
| Wednesday | 0.029 (0.018,0.040) | <0.001 | 0.029 (0.018,0.040) | <0.001 | 0.029 (0.018,0.040) | <0.001 | 0.029 (0.018,0.040) | <0.001 | |
| Friday | 0.035 (0.024,0.046) | <0.001 | 0.035 (0.024,0.046) | <0.001 | 0.035 (0.024,0.046) | <0.001 | 0.035 (0.024,0.046) | <0.001 | |
| MA | |||||||||
| Euhydration | |||||||||
| Intercept | 9.502 (8.078,10.926) | <0.001 | 7.631 (5.469,9.794) | <0.001 | 7.623 (5.456,9.790) | <0.001 | 7.747 (5.503,9.990) | <0.001 | |
| Monday | ref. | ref. | ref. | ref. | |||||
| Wednesday | −0.866 (−2.564,0.832) | 0.32 | −0.866 (−2.564,0.832) | 0.32 | −0.866 (−2.564,0.832) | 0.32 | −0.866 (−2.564,0.832) | 0.32 | |
| Friday | −1.822 (−3.211,−0.432) | 0.010 | −1.822 (−3.211,−0.432) | 0.010 | −1.822 (−3.211,−0.432) | 0.010 | −1.822 (−3.211,−0.432) | 0.010 | |
| Dehydration | |||||||||
| Intercept | 10.333 (9.249,11.417) | <0.001 | 6.531 (4.766,8.296) | <0.001 | 6.468 (4.731,8.205) | <0.001 | 6.311 (4.513,8.110) | <0.001 | |
| Monday | ref. | ref. | ref. | ref. | |||||
| Wednesday | −0.258 (−1.430,0.915) | 0.67 | −0.258 (−1.430,0.915) | 0.67 | −0.258 (−1.430,0.915) | 0.67 | −0.258 (−1.430,0.915) | 0.67 | |
| Friday | −0.727 (−1.779,0.325) | 0.18 | −0.727 (−1.779,0.325) | 0.18 | −0.727 (−1.779,0.325) | 0.18 | −0.727 (−1.779,0.325) | 0.18 | |
| β2-MG/MA | |||||||||
| Euhydration | |||||||||
| Intercept | 22.562 (20.670,24.454) | <0.001 | 24.367 (20.596,28.138) | <0.001 | 24.367 (20.626,28.108) | <0.001 | 23.792 (19.896,27.687) | <0.001 | |
| Monday | ref. | ref. | ref. | ref. | |||||
| Wednesday | 1.928 (−0.622,4.478) | 0.14 | 1.928 (−0.622,4.478) | 0.14 | 1.928 (−0.622,4.478) | 0.14 | 1.928 (−0.622,4.478) | 0.14 | |
| Friday | 2.963 (0.916,5.009) | 0.005 | 2.963 (0.916,5.009) | 0.005 | 2.963 (0.916,5.009) | 0.005 | 2.963 (0.916,5.009) | 0.005 | |
| Dehydration | |||||||||
| Intercept | 21.008 (20.204,21.812) | <0.001 | 24.704 (22.161,27.247) | <0.001 | 24.569 (22.113,27.024) | <0.001 | 24.875 (22.373,27.377) | <0.001 | |
| Monday | ref. | ref. | ref. | ref. | |||||
| Wednesday | 1.687 (0.562,2.813) | 0.003 | 1.687 (0.562,2.813) | 0.003 | 1.687 (0.562,2.813) | 0.003 | 1.687 (0.562,2.813) | 0.003 | |
| Friday | 3.559 (2.188,4.931) | <0.001 | 3.559 (2.188,4.931) | <0.001 | 3.559 (2.188,4.931) | <0.001 | 3.559 (2.188,4.931) | <0.001 | |
| Indicator | Euhydration | Dehydration | χ2 | p |
|---|---|---|---|---|
| Elevated β2-MG | ||||
| Monday | 111 (15.7) | 188 (16.4) | 0.15 | 0.70 |
| Wednesday | 149 (21.1) | 327 (28.5) | 12.59 | <0.001 |
| Friday | 161 (22.8) | 373 (32.5) | 20.16 | <0.001 |
| Elevated MA | ||||
| Monday | 37 (5.2) | 62 (5.4) | 0.02 | 0.88 |
| Wednesday | 19 (2.7) | 60 (5.2) | 6.92 | 0.001 |
| Friday | 13 (1.8) | 59 (5.3) | 12.78 | <0.001 |
| Elevated β2-MG/MA | ||||
| Monday | 34 (4.8) | 46 (4.0) | 0.68 | 0.41 |
| Wednesday | 41 (5.8) | 47 (4.1) | 2.82 | 0.09 |
| Friday | 54 (7.6) | 74 (6.5) | 0.97 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaerjiang, N.; Li, M.; Xiao, H.; Zunong, J.; Li, Z.; Huang, D.; Vermund, S.H.; Pérez-Escamilla, R.; Jiang, X.; Hu, Y. Dehydration Status Aggravates Early Renal Impairment in Children: A Longitudinal Study. Nutrients 2022, 14, 335. https://doi.org/10.3390/nu14020335
Amaerjiang N, Li M, Xiao H, Zunong J, Li Z, Huang D, Vermund SH, Pérez-Escamilla R, Jiang X, Hu Y. Dehydration Status Aggravates Early Renal Impairment in Children: A Longitudinal Study. Nutrients. 2022; 14(2):335. https://doi.org/10.3390/nu14020335
Chicago/Turabian StyleAmaerjiang, Nubiya, Menglong Li, Huidi Xiao, Jiawulan Zunong, Ziang Li, Dayong Huang, Sten H. Vermund, Rafael Pérez-Escamilla, Xiaofeng Jiang, and Yifei Hu. 2022. "Dehydration Status Aggravates Early Renal Impairment in Children: A Longitudinal Study" Nutrients 14, no. 2: 335. https://doi.org/10.3390/nu14020335
APA StyleAmaerjiang, N., Li, M., Xiao, H., Zunong, J., Li, Z., Huang, D., Vermund, S. H., Pérez-Escamilla, R., Jiang, X., & Hu, Y. (2022). Dehydration Status Aggravates Early Renal Impairment in Children: A Longitudinal Study. Nutrients, 14(2), 335. https://doi.org/10.3390/nu14020335