Dietary Intake and Status of Vitamin B12 in Slovenian Population
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Dietary Vitamin B12 Intake
2.4. Vitamin B12 and Homocysteine Status
2.5. Data and Statistical Analysis
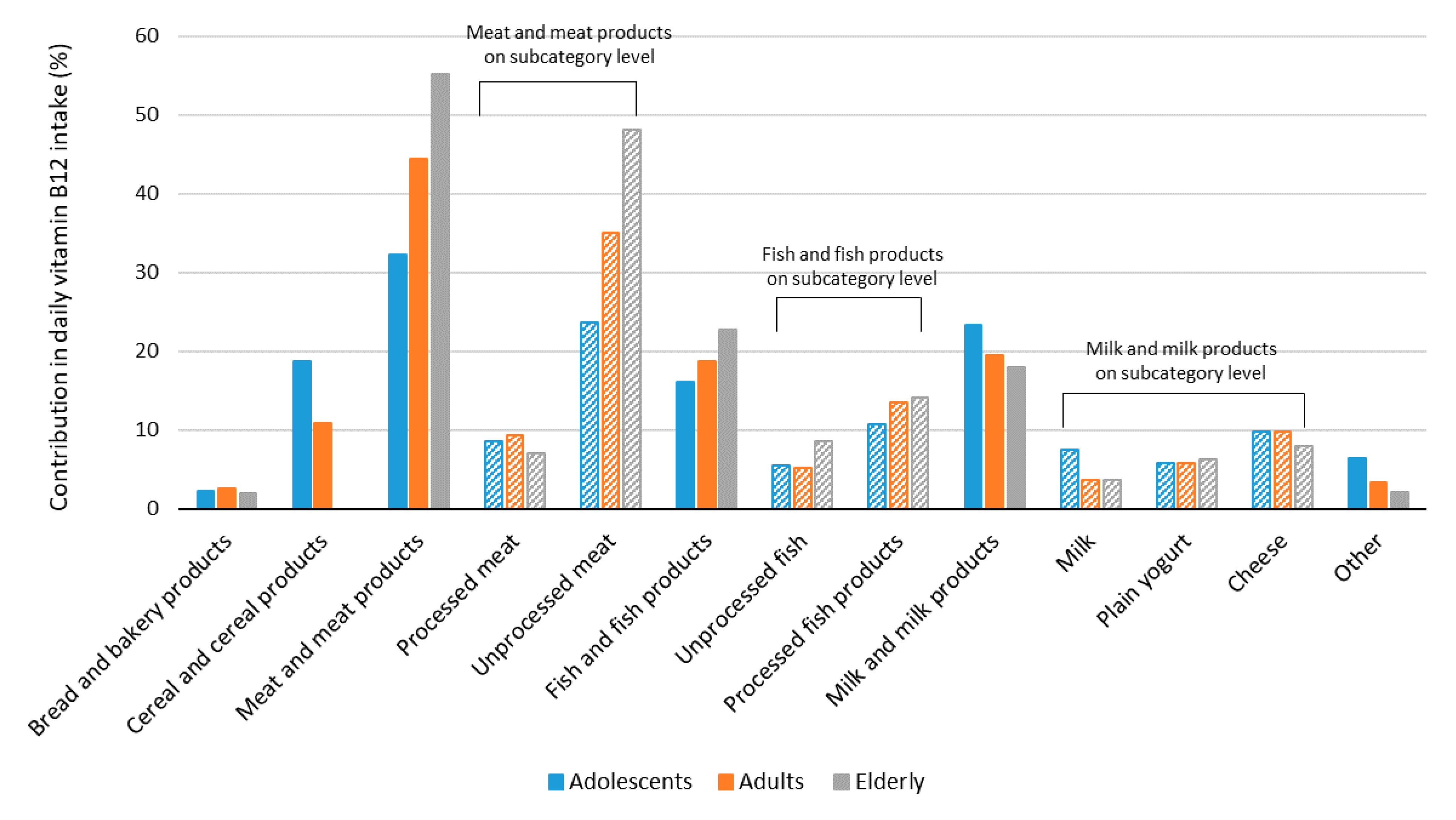
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B(12) deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Castellanos-Sinco, H.B.; Ramos-Peñafiel, C.O.; Santoyo-Sánchez, A.; Collazo-Jaloma, J.; Martínez-Murillo, C.; Montaño-Figueroa, E.; Sinco-Ángeles, A. Megaloblastic anaemia: Folic acid and vitamin B12 metabolism. Rev. Med. Hosp. Gen. México 2015, 78, 135–143. [Google Scholar] [CrossRef][Green Version]
- Lachner, C.; Steinle, N.I.; Regenold, W.T. The Neuropsychiatry of Vitamin B12 Deficiency in Elderly Patients. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 5–15. [Google Scholar] [CrossRef]
- Carmel, R. Current concepts in cobalamin deficiency. Annu. Rev. Med. 2000, 51, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Causes of vitamin B12 and folate deficiency. Food Nutr. Bull. 2008, 29, S20–S34. [Google Scholar] [CrossRef] [PubMed]
- Shane, B.; Stokstad, E.L. Vitamin B12-folate interrelationships. Annu. Rev. Nutr. 1985, 5, 115–141. [Google Scholar] [CrossRef]
- Refsum, H.; Ueland, P.; Nygård, O.; Vollset, S. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998, 49, 31–62. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.S.; Chook, P.; Lolin, Y.I.; Cheung, A.S.; Chan, L.T.; Sun, Y.Y.; Sanderson, J.E.; Metreweli, C.; Celermajer, D.S. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation 1997, 96, 2542–2544. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.N.; Loscalzo, J. Homocysteine and atherothrombosis. NEJM 1998, 338, 1042–1050. [Google Scholar] [CrossRef]
- Clarke, R.; Daly, L.; Robinson, K.; Naughten, E.; Cahalane, S.; Fowler, B.; Graham, I. Hyperhomocysteinemia: An independent risk factor for vascular disease. NEJM 1991, 324, 1149–1155. [Google Scholar] [CrossRef]
- Martens, J.-H.; Barg, H.; Warren, M.A.; Jahn, D. Microbial production of vitamin B 12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- EC. Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJEU 2006, 50, 26–38. [Google Scholar]
- Scott, J. Bioavailability of vitamin B12. Eur. J. Clin. Nutr. 1997, 51, S49–S53. [Google Scholar]
- Squires, M.; Naber, E. Vitamin profiles of eggs as indicators of nutritional status in the laying hen: Vitamin B12 study. Poult. Sci. 1992, 71, 2075–2082. [Google Scholar] [CrossRef]
- Doets, E.L.; Veld, P.H.I.; Szczecińska, A.; Dhonukshe-Rutten, R.A.; Cavelaars, A.E.; vam’t Veer, P.; Brzozowska, A.; de Groot, L.C. Systematic review on daily vitamin B12 losses and bioavailability for deriving recommendations on vitamin B12 intake with the factorial approach. Ann. Nutr. Metab. 2013, 62, 311–322. [Google Scholar] [CrossRef]
- Heyssel, R.M.; Bozian, R.C.; Darby, W.J.; Bell, M.C. Vitamin B12 turnover in man. The assimilation of vitamin B12 from natural foodstuff by man and estimates of minimal daily dietary requirements. Am. J. Clin. Nutr. 1966, 18, 176–184. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A. Vitamin B12 in meat and dairy products. Nutr. Rev. 2015, 73, 106–115. [Google Scholar] [CrossRef]
- Lešková, E.; Kubíková, J.; Kováčiková, E.; Košická, M.; Porubská, J.; Holčíková, K. Vitamin losses: Retention during heat treatment and continual changes expressed by mathematical models. J. Food Compos. Anal. 2006, 19, 252–276. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef]
- Andrès, E.; Loukili, N.H.; Noel, E.; Kaltenbach, G.; Abdelgheni, M.B.; Perrin, A.E.; Noblet-Dick, M.; Maloisel, F.; Schlienger, J.-L.; Blicklé, J.-F. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004, 171, 251–259. [Google Scholar] [CrossRef]
- Conzade, R.; Koenig, W.; Heier, M.; Schneider, A.; Grill, E.; Peters, A.; Thorand, B. Prevalence and predictors of subclinical micronutrient deficiency in german older adults: Results from the population-based KORA-age study. Nutrients 2017, 9, 1276. [Google Scholar] [CrossRef]
- Stover, P.J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. 2010, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ströhle, A.; Hahn, A. Cobalamin: A critical vitamin in the elderly. Prev. Med. 2004, 39, 1256–1266. [Google Scholar] [CrossRef]
- Toh, B.-H.; van Driel, I.R.; Gleeson, P.A. Pernicious anemia. NEJM 1997, 337, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Chapman, L.E.; Darling, A.L.; Brown, J.E. Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. J. 2016, 42, 316–327. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; de Rooij, S.E.; Huijmans, J.G.; Fischer, C.; Hoekstra, J.B. Diagnosis of vitamin B12 deficiency revised. Ned. Tijdschr. Voor Geneeskd. 2005, 149, 2789–2794. [Google Scholar]
- Carmel, R.; Green, R.; Jacobsen, D.W.; Rasmussen, K.; Florea, M.; Azen, C. Serum cobalamin, homocysteine, and methylmalonic acid concentrations in a multiethnic elderly population: Ethnic and sex differences in cobalamin and metabolite abnormalities. Am. J. Clin. Nutr. 1999, 70, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Vitamin B12 deficiency. NEJM 2013, 368, 149–160. [Google Scholar] [CrossRef]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.C.P.G.M.; Eussen, S.J.P.M. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Lysne, V.; Bjørke-Monsen, A.-L.; Behringer, S.; Grünert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci 2016, 3, 27. [Google Scholar] [CrossRef]
- de Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef]
- Green, R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am. J. Clin. Nutr. 2011, 94, 666S–672S. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. CCLM 2015, 53, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Refsum, H.; Stabler, S.P.; Malinow, M.R.; Andersson, A.; Allen, R.H. Total homocysteine in plasma or serum: Methods and clinical applications. Clin. Chem. 1993, 39, 1764–1779. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Nordic Co-Operation. Nordic Nutrition Recommendations 2012: Integrating nutrition and physical activity. Nord. Counc. Minist. Cph. Den. 2014, 627.
- NIJZ. Referenčne vrednosti za energijski vnos ter vnos hranil. Available online: https://www.nijz.si/sites/www.nijz.si/files/uploaded/referencne_vrednosti_2020_3_2.pdf (accessed on 3 February 2021).
- D-A-CH. Reference Values DACH. Available online: https://www.sge-ssn.ch/grundlagen/lebensmittel-und-naehrstoffe/naehrstoffempfehlungen/dachreferenzwerte/ (accessed on 14 July 2021).
- Ströhle, A.; Richter, M.; González-Gross, M.; Neuhäuser-Berthold, M.; Wagner, K.H.; Leschik-Bonnet, E.; Egert, S. The Revised D-A-CH. Reference Values for the Intake of Vitamin B(12): Prevention of Deficiency and Beyond. Mol. Nutr. Food Res. 2019, 63, e1801178. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on dietary reference values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar]
- McLean, E.; de Benoist, B.; Allen, L.H. Review of the Magnitude of Folate and Vitamin B12 Deficiencies Worldwide. Food. Nutr. Bull. 2008, 29, S38–S51. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Folate and Vitamin B 12 Status in the Americas. Nutr. Rev. 2004, 62, S29–S33. [Google Scholar] [CrossRef]
- Arazo-Rusindo, M.C.; Zúñiga, R.N.; Cortés-Segovia, P.; Benavides-Valenzuela, S.; Pérez-Bravo, F.; Castillo-Valenzuela, O.; Mariotti-Celis, M.S. Nutritional Status and Serum Levels of Micronutrients in an Elderly Group Who Participate in the Program for Complementary Food in Older People (PACAM) from the Metropolitan Region, Santiago de Chile. Nutrients 2022, 14, 3. [Google Scholar] [CrossRef]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Dhonukshe-Rutten, R.; De Vries, J.; De Bree, A.; Van Der Put, N.; Van Staveren, W.; De Groot, L. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. Eur. J. Clin. Nutr. 2009, 63, 18–30. [Google Scholar] [CrossRef]
- Mijatov, M.A.; Mičetić-Turk, D. Dietary Intake In Adult Female Coeliac Disease Patients In Slovenia: Prehranski Vnos Odraslih Bolnic S Celiakijo V Sloveniji. Zdr Varst 2016, 55, 86–93. [Google Scholar] [CrossRef][Green Version]
- Gregorič, M.; Zdešar Kotnik, K.; Pigac, I.; Gabrijelčič Blenkuš, M. A Web-Based 24-H Dietary Recall Could Be a Valid Tool for the Indicative Assessment of Dietary Intake in Older Adults Living in Slovenia. Nutrients 2019, 11, 2234. [Google Scholar] [CrossRef] [PubMed]
- Jakše, B.; Jakše, B.; Godnov, U.; Pinter, S. Nutritional, Cardiovascular Health and Lifestyle Status of ‘Health Conscious’ Adult Vegans and Non-Vegans from Slovenia: A Cross-Sectional Self-Reported Survey. Int. J. Environ. 2021, 18, 5968. [Google Scholar] [CrossRef]
- Širca Čampa, A.; Hren, I.; Sedmak, M.; Brecelj, J.; Fidler Mis, N.; Krišnik, C.; Koletzko, B. Nutrition of mothers with hypogalactia in Slovenia. J. Pediatr. Gastroenterol. Nutr. 2006, 42, E104. [Google Scholar]
- EFSA. Guidance on the EU Menu methodology. EFSA J. 2014, 12, 3944. [Google Scholar]
- Gregorič, M.; Blaznik, U.; Delfar, N.; Zaletel, M.; Lavtar, D.; Seljak, B.K.; Golja, P.; Kotnik, K.Z.; Pravst, I.; Mis, N.F. Slovenian national food consumption survey in adolescents, adults and elderly. EFSA Supporting Publ. 2019, 16, 1729E. [Google Scholar]
- Blenkuš, M.G.; Gregorič, M.; Tivadar, B.; Koch, V.; Kostanjevec, S.; Turk, V.F.; Žalar, A.; Lavtar, D.; Kuhar, D.; Rozman, U. Prehrambene navade odraslih prebivalcev Slovenije z vidika varovanja zdravja; University of Ljubljana, Faculty of Education: Ljubljana, Slovenija, 2009; p. 183. [Google Scholar]
- Koch, V.; Pokorn, D.; Salobir, K. Prehrambene navade odraslih prebivalcev Slovenije z vidika varovanja zdravja. Ph.D. Thesis, University of Ljubljana, Biotechnical Faculty, Ljubljana, Slovenija, 1997. [Google Scholar]
- Jacques, P.; Sulsky, S.; Sadowski, J.; Phillips, J.; Rush, D.; Willett, W. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am. J. Clin. Nutr. 1993, 57, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.M.; Romero, L.; Stauber, P.; Pareo-Tubbeh, S.L.; Liang, H.C.; Baumgartner, R.N.; Garry, P.J.; Allen, R.H.; Stabler, S.P. Vitamin supplementation and other variables affecting serum homocysteine and methylmalonic acid concentrations in elderly men and women. J. Am. Coll. Nutr. 1996, 15, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Hribar, M.; Hristov, H.; Gregorič, M.; Blaznik, U.; Zaletel, K.; Oblak, A.; Osredkar, J.; Kušar, A.; Žmitek, K.; Rogelj, I.; et al. Nutrihealth Study: Seasonal Variation in Vitamin D Status Among the Slovenian Adult and Elderly Population. Nutrients 2020, 12, 1838. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. 2011, 8, 115. [Google Scholar] [CrossRef]
- De Onis, M.; Onyango, A.; Borghi, E.; Siyam, A.; Blössner, M.; Lutter, C. Worldwide implementation of the WHO child growth standards. Public Health Nutr. 2012, 15, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Vede, T. Izdelava in validacija slikovnega gradiva za določanje vnosa živil; Design and Validation of Food Picture Book; University of Ljubljana, Biotechnical Faculty: Ljubljana, Slovenia, 2016. [Google Scholar]
- Korošec, M.; Golob, T.; Bertoncelj, J.; Stibilj, V.; Seljak, B.K. The Slovenian food composition database. Food Chem. 2013, 140, 495–499. [Google Scholar] [CrossRef]
- Fineli. Finnish food Composition Database; Finnish Public Health Institute, Nutrition Unit Helsinki: Helsinki, Finland, 2004. [Google Scholar]
- Bodner-Montville, J.; Ahuja, J.K.; Ingwersen, L.A.; Haggerty, E.S.; Enns, C.W.; Perloff, B.P. USDA food and nutrient database for dietary studies: Released on the web. J. Food Compos. Anal. 2006, 19, S100–S107. [Google Scholar] [CrossRef]
- Haubrock, J.; Nöthlings, U.; Volatier, J.L.; Dekkers, A.; Ocké, M.; Harttig, U.; Illner, A.K.; Knüppel, S.; Andersen, L.F.; Boeing, H. Estimating usual food intake distributions by using the multiple source method in the EPIC-Potsdam Calibration Study. J. Nutr. 2011, 141, 914–920. [Google Scholar] [CrossRef]
- Pravst, I.; Lavriša, Ž.; Hribar, M.; Hristov, H.; Kvarantan, N.; Seljak, B.K.; Gregorič, M.; Blaznik, U.; Gregorič, N.; Zaletel, K.; et al. Dietary Intake of Folate and Assessment of the Folate Deficiency Prevalence in Slovenia Using Serum Biomarkers. Nutrients 2021, 13, 3860. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., III; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development—Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370. [Google Scholar] [CrossRef]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef]
- Zupanič, N.; Hristov, H.; Gregorič, M.; Blaznik, U.; Delfar, N.; Koroušić Seljak, B.; Ding, E.L.; Fidler Mis, N.; Pravst, I. Total and Free Sugars Consumption in a Slovenian Population Representative Sample. Nutrients 2020, 12, 1729. [Google Scholar] [CrossRef]
- Harttig, U.; Haubrock, J.; Knüppel, S.; Boeing, H. The MSM program: Web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur. J. Clin. Nutr. 2011, 65 (Suppl. S1), S87–S91. [Google Scholar] [CrossRef]
- Kolenikov, S. Calibrating Survey Data using Iterative Proportional Fitting (Raking). Stata J. 2014, 14, 22–59. [Google Scholar] [CrossRef]
- Dunford, E.; Webster, J.; Metzler, A.B.; Czernichow, S.; Ni Mhurchu, C.; Wolmarans, P.; Snowdon, W.; L‘Abbe, M.; Li, N.; Maulik, P.K.; et al. International collaborative project to compare and monitor the nutritional composition of processed foods. Eur. J. Prev. Cardiol. 2012, 19, 1326–1332. [Google Scholar] [CrossRef]
- Rippin, H.L.; Hutchinson, J.; Jewell, J.; Breda, J.J.; Cade, J.E. Adult nutrient intakes from current national dietary surveys of European populations. Nutrients 2017, 9, 1288. [Google Scholar] [CrossRef]
- Vrentzos, G.E.; Papadakis, J.A.; Malliaraki, N.; Zacharis, E.A.; Mazokopakis, E.; Margioris, A.; Ganotakis, E.S.; Kafatos, A. Diet, serum homocysteine levels and ischaemic heart disease in a Mediterranean population. Br. J. Nutr. 2004, 91, 1013–1019. [Google Scholar] [CrossRef]
- Gregoric, M.; Blaznik, U.; Turk, V. Razlicni Vidiki Prehranjevanja Prebivalcev Slovenije (V Starosti Od 3 Mesecev Do 74 Let); NIJZ: Ljubljana, Slovenija, 2020. [Google Scholar]
- Hafner, E.; Pravst, I. Evaluation of the Ability of Nutri-Score to Discriminate the Nutritional Quality of Prepacked Foods Using a Sale-Weighting Approach. Foods 2021, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.B.; Vartanian, L.R.; Wharton, C.M.; Brownell, K.D. Examining the Nutritional Quality of Breakfast Cereals Marketed to Children. J. Am. Diet. Assoc. 2008, 108, 702–705. [Google Scholar] [CrossRef]
- Lavriša, Ž.; Pravst, I. Marketing of Foods to Children through Food Packaging Is Almost Exclusively Linked to Unhealthy Foods. Nutrients 2019, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Sans, P.; Combris, P. World meat consumption patterns: An overview of the last fifty years (1961–2011). Meat Sci. 2015, 109, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Darmon, N.; Ferguson, E.L.; Briend, A. Impact of a Cost Constraint on Nutritionally Adequate Food Choices for French Women: An Analysis by Linear Programming. J. Nutr. Educ. Behav. 2006, 38, 82–90. [Google Scholar] [CrossRef]
- Villamor, E.; Mora-Plazas, M.; Forero, Y.; Lopez-Arana, S.; Baylin, A. Vitamin B-12 Status Is Associated with Socioeconomic Level and Adherence to an Animal Food Dietary Pattern in Colombian School Children. J. Nutr. 2008, 138, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Joubert, L.M.; Manore, M.M. The role of physical activity level and B-vitamin status on blood homocysteine levels. Med. Sci. Sports Exerc. 2008, 40, 1923–1931. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Hou, C.; Sun, F.; Dong, J.; Chu, X.; Guo, Y. Gender differences in risk factors for high plasma homocysteine levels based on a retrospective checkup cohort using a generalized estimating equation analysis. Lipids Health Dis. 2021, 20, 31. [Google Scholar] [CrossRef]
- Clarke, R.; Refsum, H.; Birks, J.; Evans, J.G.; Johnston, C.; Sherliker, P.; Ueland, P.M.; Schneede, J.; McPartlin, J.; Nexo, E. Screening for vitamin B-12 and folate deficiency in older persons. Am. J. Clin. Nutr. 2003, 77, 1241–1247. [Google Scholar] [CrossRef]
- Bao, F.; Cui, M.; Shi, X.; Ju, S.; Cong, H. Distribution characteristics and influencing factors of homocyteine in an apparently healthy examined population. BMC Cardiovasc. Disord. 2021, 21, 429. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef]
- Wong, C. Vitamin B12 deficiency in the elderly: Is it worth screening. Hong Kong Med. J. 2015, 21, 155–164. [Google Scholar] [CrossRef]
- Adams, N.E.; Bowie, A.J.; Simmance, N.; Murray, M.; Crowe, T.C. Recognition by medical and nursing professionals of malnutrition and risk of malnutrition in elderly hospitalised patients. Nutr. Diet. 2008, 65, 144–150. [Google Scholar] [CrossRef]
- Bruins, M.J.; Van Dael, P.; Eggersdorfer, M. The Role of Nutrients in Reducing the Risk for Noncommunicable Diseases during Aging. Nutrients 2019, 11, 85. [Google Scholar] [CrossRef]
- De Groot, C.; Van Den Broek, T.; Van Staveren, W. Energy intake and micronutrient intake in elderly Europeans: Seeking the minimum requirement in the SENECA study. Age Ageing 1999, 28, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Scardella, P.; Piombo, L.; Neri, B.; Asprino, R.; Proietti, A.; Carcaterra, S.; Cava, E.; Cataldi, S.; Cucinotta, D. Malnutrition in elderly: Social and economic determinants. J. Nutr. Health Aging 2013, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Dullemeijer, C.; Souverein, O.W.; Doets, E.L.; van der Voet, H.; van Wijngaarden, J.P.; de Boer, W.J.; Plada, M.; Dhonukshe-Rutten, R.A.; In’t Veld, P.H.; Cavelaars, A.E.; et al. Systematic review with dose-response meta-analyses between vitamin B-12 intake and European Micronutrient Recommendations Aligned’s prioritized biomarkers of vitamin B-12 including randomized controlled trials and observational studies in adults and elderly persons. Am. J. Clin. Nutr. 2012, 97, 390–402. [Google Scholar] [CrossRef]
- Hinds, H.E.; Johnson, A.A.; Webb, M.C.; Graham, A.P. Iron, Folate, and Vitamin B12 Status in the Elderly by Gender and Ethnicity. J. Natl. Med. Assoc. 2011, 103, 870–878. [Google Scholar] [CrossRef]
- Kulnik, D.; Elmadfa, I. Assessment of the nutritional situation of elderly nursing home residents in Vienna. Ann. Nutr. Metab. 2008, 52 (Suppl. S1), 51–53. [Google Scholar] [CrossRef]
- Novaković, R.; Cavelaars, A.; Geelen, A.; Nikolić, M.; Altaba, I.I.; Viñas, B.R.; Ngo, J.; Golsorkhi, M.; Medina, M.W.; Brzozowska, A.; et al. Review Article Socio-economic determinants of micronutrient intake and status in Europe: A systematic review. Public Health Nutr. 2013, 17, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, K.; Svärdsudd, K. Elevated serum levels of methylmalonic acid and homocysteine in elderly people. A population-based intervention study. J. Intern. Med. 1999, 246, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Hermann, S.; Hahn, A. B vitamin status and concentrations of homocysteine and methylmalonic acid in elderly German women. Am. J. Clin. Nutr. 2003, 78, 765–772. [Google Scholar] [CrossRef][Green Version]
- Holt, P.R. Intestinal Malabsorption in the Elderly. J. Dig. Dis. 2007, 25, 144–150. [Google Scholar] [CrossRef]
- Lewerin, C.; Jacobsson, S.; Lindstedt, G.; Nilsson-Ehle, H. Serum biomarkers for atrophic gastritis and antibodies against Helicobacter pylori in the elderly: Implications for vitamin B12, folic acid and iron status and response to oral vitamin therapy. Scand. J. Gastroenterol. 2008, 43, 1050–1056. [Google Scholar] [CrossRef]
- Selhub, J.; Bagley, L.C.; Miller, J.; Rosenberg, I.H. B vitamins, homocysteine, and neurocognitive function in the elderly. Am. J. Clin. Nutr. 2000, 71, 614S–620S. [Google Scholar] [CrossRef]
- Joo, Y.-E.; Park, H.-K.; Myung, D.-S.; Baik, G.-H.; Shin, J.-E.; Seo, G.-S.; Kim, G.H.; Kim, H.U.; Kim, H.Y.; Cho, S.-I.; et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia: A nationwide multicenter prospective study in Korea. Gut Liver 2013, 7, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Bhatt, H. Atrophic Gastritis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Weck, M.N.; Brenner, H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1083–1094. [Google Scholar] [CrossRef]
- Russell, R.M.; Rasmussen, H.; Lichtenstein, A.H. Modified Food Guide Pyramid for People over Seventy Years of Age. J Nutr 1999, 129, 751–753. [Google Scholar] [CrossRef][Green Version]
- Selhub, J.; Paul, L. Folic acid fortification: Why not vitamin B12 also? BioFactors 2011, 37, 269–271. [Google Scholar] [CrossRef]
- Allen, L.H.; Rosenberg, I.H.; Oakley, G.P.; Omenn, G.S. Considering the case for vitamin B12 fortification of flour. Food Nutr. Bull. 2010, 31, S36–S46. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, T.S.; Norkus, E.P. Approaches to vitamin B12 deficiency. Postgrad. Med. 2001, 110, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Vitamin B(12) deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
| Variable | Adolescents n (%) | Adults n (%) | Elderly n (%) | |
|---|---|---|---|---|
| Overall | n = 468 (100) | n = 364 (100) | n = 416 (100) | |
| Age (mean ± SD) | 13.4 (2.37) | 43.6 (13.81) | 68.7 (2.7) | |
| Residential area | rural | 270 (57.7) | 202 (55.5) | 229 (55.1) |
| intermediate | 76 (16.2) | 56 (15.4) | 71 (17.1) | |
| urban | 122 (26.1) | 106 (29.1) | 116 (27.9) | |
| Sex | male | 238 (50.9) | 173 (47.5) | 213 (51.2) |
| female | 230 (49.1) | 191 (52.5) | 203 (48.8) | |
| Education | no university degree | n.a. | 249 (68.4) | 342 (82.2) |
| university degree | n.a. | 115 (31.6) | 74 (17.8) | |
| Financial status | below average | n.a. | 118 (38.4) | 269 (71.5) |
| above average | n.a. | 189 (61.6) | 107 (28.5) | |
| Employment | employed | n.a. | 226 (62.1) | n.a. |
| unemployed | n.a. | 42 (11.5) | n.a. | |
| student | n.a. | 32 (8.8) | n.a. | |
| retired | n.a. | 64 (17.6) | n.a. | |
| BMI (mean ± SD) | 21.0 (4.2) | 26.7 (5.2) | 28.4 (5.0) | |
| BMI | normal | 301 (64.6) | 148 (40.7) | 108 (26.0) |
| overweight and obese | 167 (35.7) | 216 (59.3) | 308 (74.0) | |
| Smoking status | current, occasional, ex-smoker | 30 (6.4) | 165 (45.3) | 185 (44.5) |
| nonsmoker | 438 (93.6) | 199 (54.7) | 231 (55.5) | |
| IPAQ | low | 108 (23.3) | 127 (35.3) | 137 (33.4) |
| moderate | 141 (30.5) | 108 (30.0) | 133 (32.4) | |
| high | 214 (46.2) | 125 (34.7) | 140 (34.2) | |
| Supplement use | multivitamins | 129 (27.6) | 140 (38.4) | 95 (22.8) |
| use not reported | 339 (72.4) | 224 (61.5) | 321 (77.2) | |
| Diet type | vegetarian/vegan | 12 (2.6) | 8 (2.2) | 3 (0.7) |
| no diet | 456 (97.4) | 356 (97.8) | 413 (99.3) | |
| medical and/or weight loss | 13 (2.8) | 32 (8.8) | 51 (12.3) | |
| no diet | 455 (97.2) | 332 (91.2) | 465 (87.7) | |
| Participation in the Nutrihealth study * | 125 (34.3) | 155 (37.3) | ||
| Adolescents n (%) | Adults n (%) | Elderly n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | |
| SI.Menu study n (%) | 468 (100) | 238 (50.9) | 230 (49.2) | 364 (100) | 173 (47.5) | 191 (52.5) | 416 (100) | 213 (51.2) | 203 (48.8) |
| Weighted * n (%) | 150,674 (78.2) | 75,580 (50.2) | 73,094 (49.8) | 1,302,132 (78.2) | 670,464 (51.5) | 631,668 (48.5) | 212,793 (12.8) | 100,247.5 (47.1) | 112,545.5 (52.9) |
| Usual daily vitamin B12 intake | |||||||||
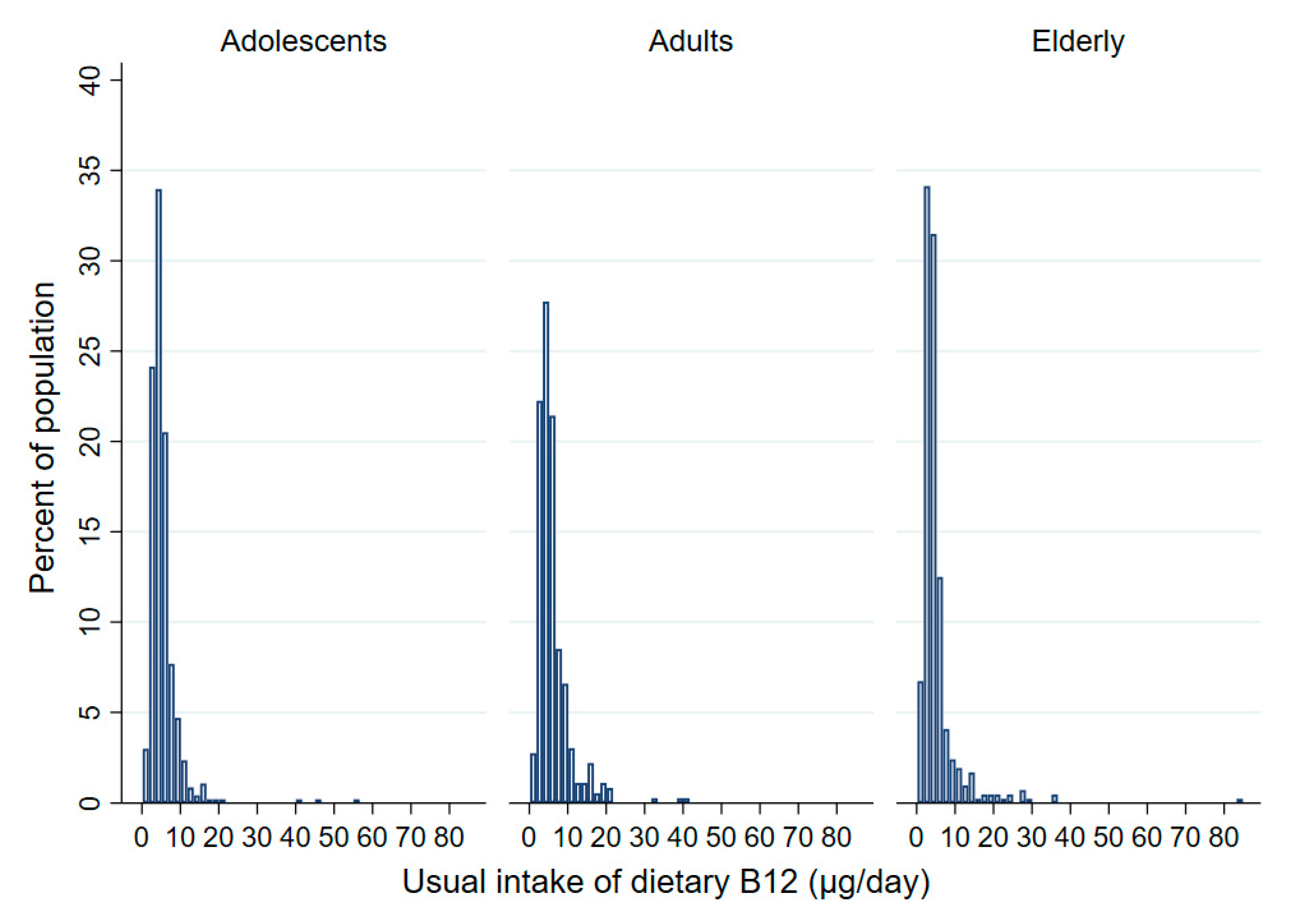
| Mean (95%CI) (µg/day) | 5.4 (5.0–5.8) | 6.0 (5.4–6.5) | 4.7 (4.1–5.4) | 6.2 (5.7–6.8) | 6.9 (6.1–7.8) | 5.5 (4.8–6.2) | 5.0 (4.4–5.6) | 5.7 (4.5–6.8) | 4.4 (3.8–5.0) |
| Q25 (µg/day) | 3.4 | 4.4 | 2.8 | 3.6 | 4.3 | 3.3 | 2.9 | 3.2 | 2.7 |
| Median (µg/day) | 4.7 | 5.3 | 3.9 | 5.0 | 5.4 | 4.4 | 3.6 | 3.8 | 3.4 |
| Q75 (µg/day) | 5.9 | 6.7 | 5.4 | 7.1 | 8.1 | 6.1 | 4.9 | 5.5 | 4.5 |
| Mean (95%CI) (µg/per 1000 kcal/day) | 2.0 (1.9–2.2) | 2.0 (1.8–2.3) | 2.0 (1.8–2.3) | 2.5 (2.3–2.7) | 2.5 (2.2–2.9) | 2.4 (2.1–2.6) | 2.1 (1.9–2.4) | 2.3 (1.8–2.7) | 2.0 (1.7–2.2) |
| Prevalence of inadequate daily vitamin B12 intake (< 4µg) (%) (95% CI) | |||||||||
| <4 µg/day | 37.3 (30.6–44.6) | 24.0 (17.7–31.8) | 51.7 (42.2–61.2) | 31.7 (26.5–37.3) | 20.6 (14.9–27.8) | 42.9 (35.1–51.0) | 58.3 (49.5–66.7) | 53.2 (37.7–68.0) | 63.1 (52.7–72.3) |
| Nutrihealth study n (%) | 125 (100) | 52 (41.6) | 73 (58.4) | 155 (100) | 76 (49.0) | 79 (51.0) | |||
| Serum vitamin B12 (pmol/L) (95% CI) | |||||||||
| Mean (95%CI) | 322.1 | 329.8 | 314.0 | 287.3 | 250.2 | 320.3 | |||
| (294.2–350.1) | (284.7–374.9) | (282.2–345.7) | (258.2–316.2) | (219.1–281.2) | (274.1–366.5) | ||||
| Std. Err. | 14.1 | 22.8 | 16.1 | 14.7 | 15.8 | 23.4 | |||
| Median | 283 | 280 | 283 | 232 | 204 | 232 | |||
| Prevalence of low serum vitamin B12 (%) (95% CI) | |||||||||
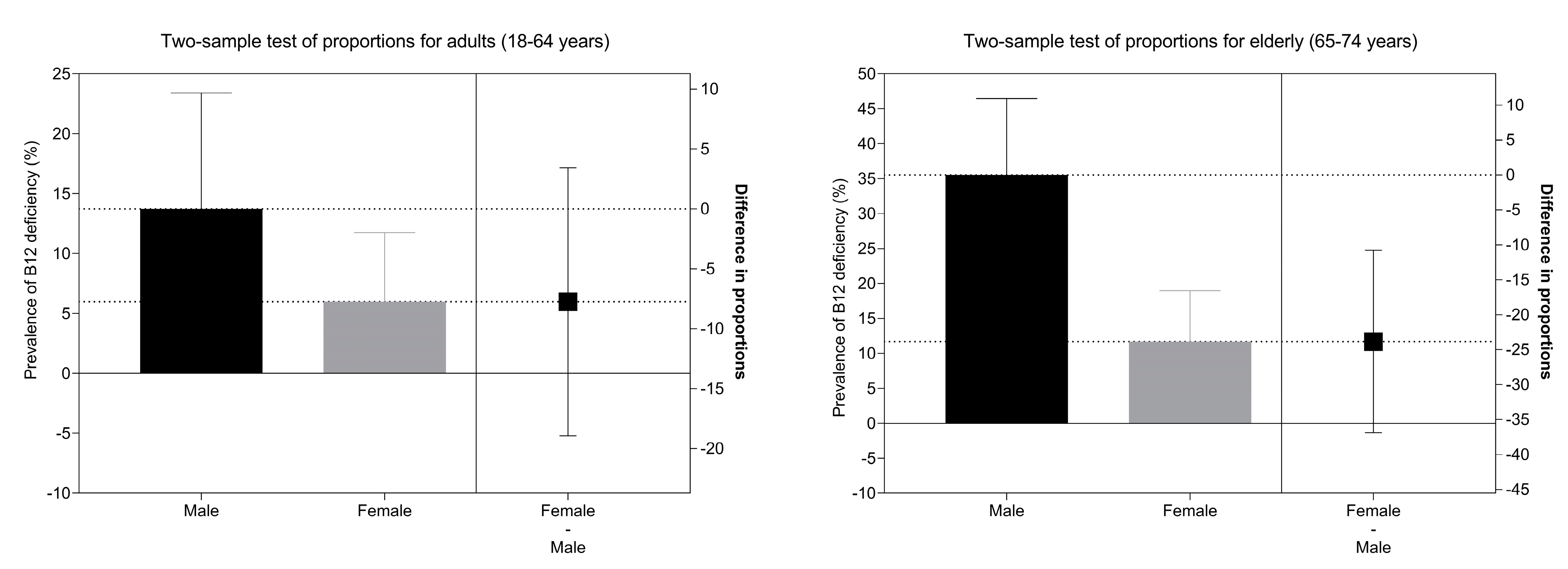
| <148 pmol/L | 3.7 (1.6–8.3) | 2.4 (0.6–9.2) | 5.3 (1.9–13.9) | 10.4 (6.4–16.4) | 11.8 (6.2–21.4) | 9.1 (4.4–18.0) | |||
| <221 pmol/L | 21.1 (14.5–29.7) | 18.9 (10.2–32.2) | 23.6 (14.8–35.5) | 46.0 (38.2–54.1) | 57.9 (46.5–68.5) | 35.1 (25.2–46.4) | |||
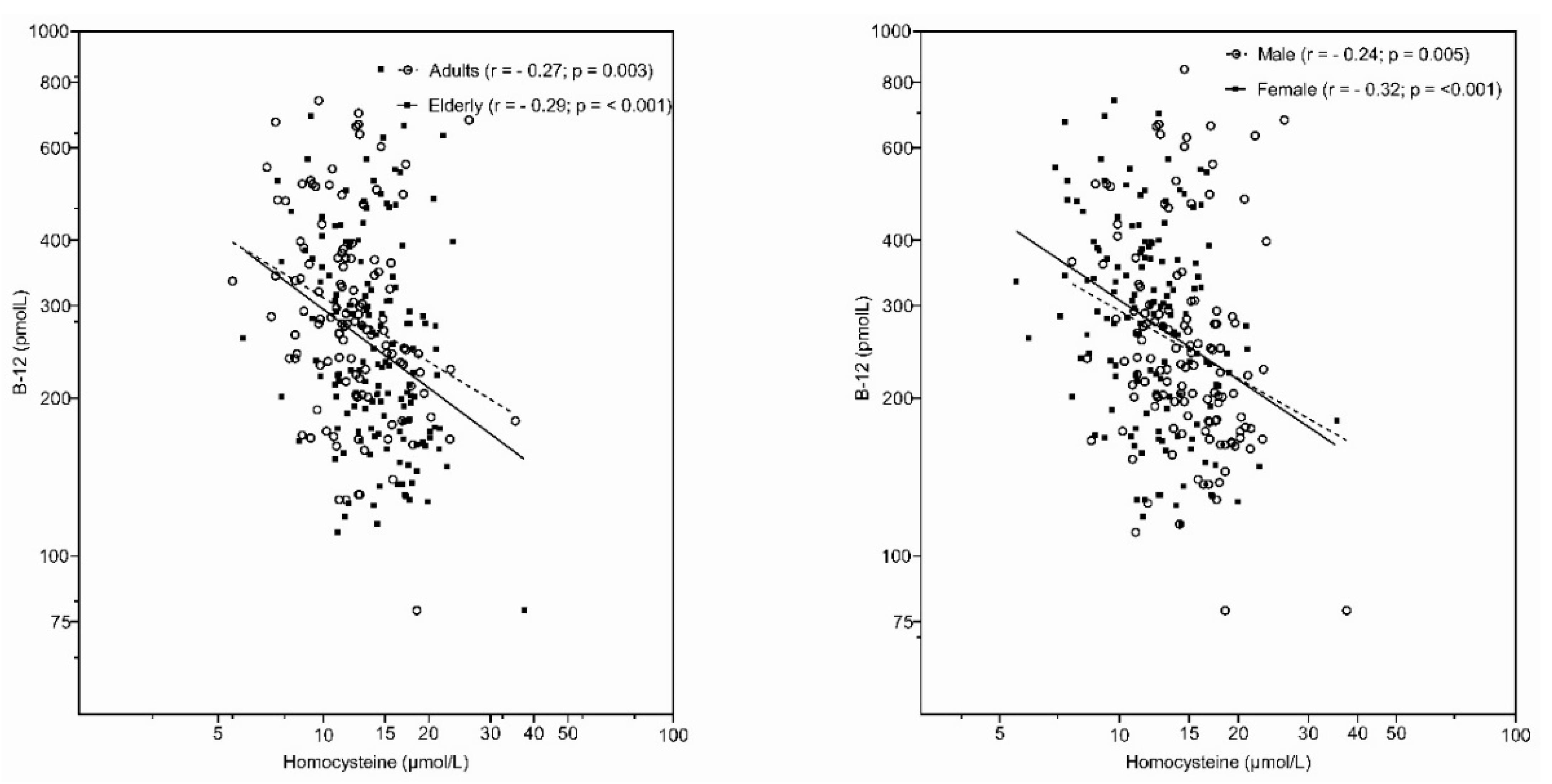
| Serum homocysteine (µmol/L) (95% CI) | |||||||||
| Mean (95% CI) | 12.6 (11.9–13.3) | 13.6 (12.6–14.6) | 14.6 (13.9–15.2) | 14.6 (13.9–15.2) | 16.1 (15.2–17.0) | 13.2 (12.5–13.9) | |||
| Std. Err. | 0.35 | 0.50 | 0.42 | 0.32 | 0.47 | 0.36 | |||
| Median | 12.1 | 12.6 | 15.7 | 14.2 | 10.9 | 13.0 | |||
| Prevalence of high serum homocysteine (µmol/L) (%) (95% CI) | |||||||||
| >10 µmol/L | 75.3 (66.4–82.4) | 88.7 (76.5–95.0) | 61.0 (48.6–72.2) | 88.9 (82.7–93.0) | 96.0 (88.3–98.7) | 82.5 (72.5–89.4) | |||
| >15 µmol/L | 20.5 (13.9–29.1) | 26.4 (15.8–40.8) | 14.2 (8.0–23.9) | 39.9 (32.4–47.8 | 56.6 (45.2–67.3) | 25.0 (16.7–35.7) | |||
| Prevalence of vitamin B12 deficiency (%) (95% CI) using criteria for low serum vitamin B12 (<148 nmol/L) and high serum homocysteine (>15µmol/L) | |||||||||
| 1.2 (0.2–4.7) | 2.3 (0.5–9.1) | / | 7.0 (3.9–12.3) | 7.9 (3.6–16.6) | 6.3 (2.6–14.3) | ||||
| Variable | Adolescents (10–17 Years) | Adults (18–64 Years) | Elderly (65–74 Years) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | Crude OR | Adjusted OR | Prevalence (%) | Crude OR | Adjusted OR | Prevalence (%) | Crude OR | Adjusted OR | ||
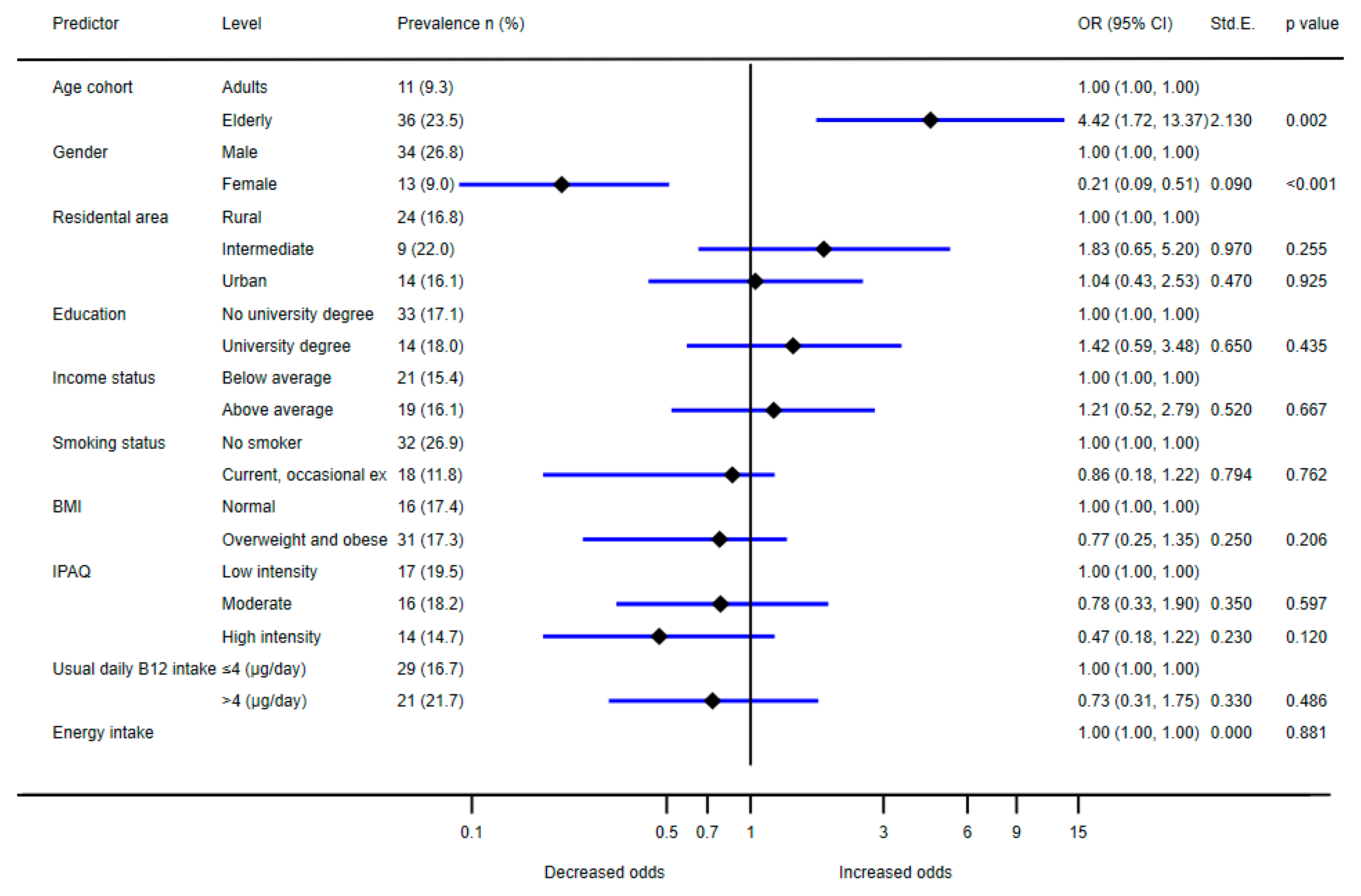
| Overall | 175 (37.4) | 118 (32.4) | 215 (51.7) | |||||||
| Sex | male | 62 (26.1) | 1 | 1 | 40 (23.1) | 1 | 1 | 94 (44.1) | 1 | 1 |
| female | 113 (49.1) | 2.74 (1.83–4.12) | 2.70 (1.80–4.03) | 78 (40.8) | 2.29 (1.42–3.73) | 2.51 (1.45–4.36) | 121 (59.6) | 1.87 (1.24–2.81) | 1.44 (0.90–2.31) | |
| Place of living | rural | 99 (36.7) | 1 | 1 | 68 (33.7) | 1 | 1 | 115 (50.2) | 1 | 1 |
| intermediate | 24 (31.6) | 0.80 (0.44–1.41) | 0.78 (0.44–1.38) | 22 (39.3) | 1.27 (0.66–2.44) | 1.27 (0.63–2.59) | 41 (57.8) | 1.35 (0.77–2.41) | 1.34 (0.74–2.43) | |
| urban | 52 (42.6) | 1.28 (0.81–2.03) | 1.20 (0.76–1.92) | 28 (26.4) | 0.71 (0.40–1.22) | 0.73 (0.40–1.33) | 59 (50.9) | 1.03 (0.64–1.64) | 1.02 (0.61–1.70) | |
| Education | no university degree | n.a. | n.a. | 78 (31.3) | 1 | 1 | 180 (52.6) | 1 | 1 | |
| university degree | 40 (34.8) | 1.17 (0.71–1.91) | 1.62 (0.88–3.00) | 35 (47.3) | 0.81 (0.47–1.37) | 0.94 (0.51–1.72) | ||||
| Financial status | below average | n.a. | n.a. | 40 (33.9) | 1 | 1 | 148 (55.0) | 1 | 1 | |
| above average | 57 (30.2) | 0.84 (0.50–1.42) | 0.94 (0.51–1.71) | 48 (44.9) | 0.67 (0.41–1.07) | 0.65 (0.40–1.08) | ||||
| BMI | normal | 110 (35.5) | 1 | 1 | 48 (32.4) | 1 | 1 | 57 (52.8) | 1 | 1 |
| overweight and obese | 65 (38.9) | 1.10 (0.73–1.66) | 1.13 (0.75–1.72) | 70 (32.4) | 1.00 (0.62–1.60) | 1–03 (0.59–1.80) | 158 (51.3) | 0.94 (0.59–1.50) | 1.00 (0.61–1.65) | |
| IPAQ | low intensity | 27 (25.0) | 1 | 1 | 36 (28.4) | 1 | 1 | 64 (46.7) | 1 | 1 |
| moderate | 68 (48.2) | 2.79 (1.57–5.03) | 2.48 (1.41–4.38) | 33 (30.6) | 1.11 (0.61–2.02) | 1.28 (0.66–2.46) | 83 (62.4) | 1.89 (1.13–3.17) | 1.86 (1.10–3.14) | |
| high intensity | 76 (35.5) | 1.65 (0.96–2.89) | 1.64 (0.96–2.80) | 47 (37.6) | 1.52 (0.87–2.68) | 2.13 (1.12–4.03) | 64 (45.7) | 0.96 (0.58–1.58) | 1.02 (0.61–1.71) | |
| Employment | employed | n.a. | n.a. | 65 (28.8) | 1 | 1 | n.a. | n.a. | ||
| unemployed | 15 (35.7) | 1.37 (0.64–2.88) | 1.71 (0.72–4.05) | |||||||
| student | 8 (25.0) | 0.83 (0.30–2.02) | 1.13 (0.38–3.34) | |||||||
| retired | 30 (46.9) | 2.19 (1.18–4.01) | 2.80 (1.32–5.94) | |||||||
| Smoking status | nonsmoker | 164 (37.4) | 1 | 1 | 64 (32.2) | 1 | 1 | 133 (57.6) | 1 | 1 |
| current/ex-smoker | 11 (36.7) | 0.97 (0.41–2.20) | 0.96 (0.42–2.20) | 54 (32.7) | 1.03 (0.64–1.63) | 1.02 (0.59–1.76) | 82 (44.3) | 0.59 (0.39–0.88) | 0.64 (0.40–1.02) | |
| Medical diet | no special diet | 169 (37.1) | 1 | 1 | 105 (31.6) | 1 | 1 | 184 (50.4) | 1 | 1 |
| medical/weight loss | 6 (46.2) | 1.45 (0.40–5.13) | 1.61 (0.49–5.26) | 13 (40.6) | 1.48 (0.64–3.29) | 1.28 (0.53–3.08) | 31 (60.8) | 1.52 (0.81–2.93) | 1.34 (0.70–2.56) | |
| Behavioral diet | no diet | 166 (36.4) | 1 | n.a. | 113 (31.7) | 1 | n.a. | 212 (51.3) | n.a. | n.a. |
| vegetarian/vegan | 9 (75.0) | 5.24 (1.28–30.40) | n.a. | 5 (62.5) | 3.58 (0.68–23.39) | n.a. | 3 (100.0) | n.a. | n.a. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavriša, Ž.; Hristov, H.; Hribar, M.; Žmitek, K.; Kušar, A.; Koroušić Seljak, B.; Gregorič, M.; Blaznik, U.; Gregorič, N.; Zaletel, K.; et al. Dietary Intake and Status of Vitamin B12 in Slovenian Population. Nutrients 2022, 14, 334. https://doi.org/10.3390/nu14020334
Lavriša Ž, Hristov H, Hribar M, Žmitek K, Kušar A, Koroušić Seljak B, Gregorič M, Blaznik U, Gregorič N, Zaletel K, et al. Dietary Intake and Status of Vitamin B12 in Slovenian Population. Nutrients. 2022; 14(2):334. https://doi.org/10.3390/nu14020334
Chicago/Turabian StyleLavriša, Živa, Hristo Hristov, Maša Hribar, Katja Žmitek, Anita Kušar, Barbara Koroušić Seljak, Matej Gregorič, Urška Blaznik, Nadan Gregorič, Katja Zaletel, and et al. 2022. "Dietary Intake and Status of Vitamin B12 in Slovenian Population" Nutrients 14, no. 2: 334. https://doi.org/10.3390/nu14020334
APA StyleLavriša, Ž., Hristov, H., Hribar, M., Žmitek, K., Kušar, A., Koroušić Seljak, B., Gregorič, M., Blaznik, U., Gregorič, N., Zaletel, K., Oblak, A., Osredkar, J., & Pravst, I. (2022). Dietary Intake and Status of Vitamin B12 in Slovenian Population. Nutrients, 14(2), 334. https://doi.org/10.3390/nu14020334











