The Effects of One Anastomosis Gastric Bypass Surgery on the Gastrointestinal Tract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Pre- and Post-Surgical Follow-Up Evaluations (Baseline and 6 Months)
2.2.1. Medical History
2.2.2. Anthropometric Measurements
2.2.3. Glucose Breath-Test to Assess SIBO
2.2.4. Assessment of Gastrointestinal Symptoms
2.2.5. Biochemical Tests
2.2.6. Quality-of-Life Assessment
2.2.7. Dietary Intake Assessment
2.2.8. Physical Activity
2.2.9. Fecal Sample Collection and Analysis
2.3. Statistical Methods
3. Results
3.1. Characteristics of the Study Participants at Baseline and at 6 Months Post-Surgery
3.2. Comparison between Patients According to Glucose Breath-Test Results at 6 Months Post-Surgery
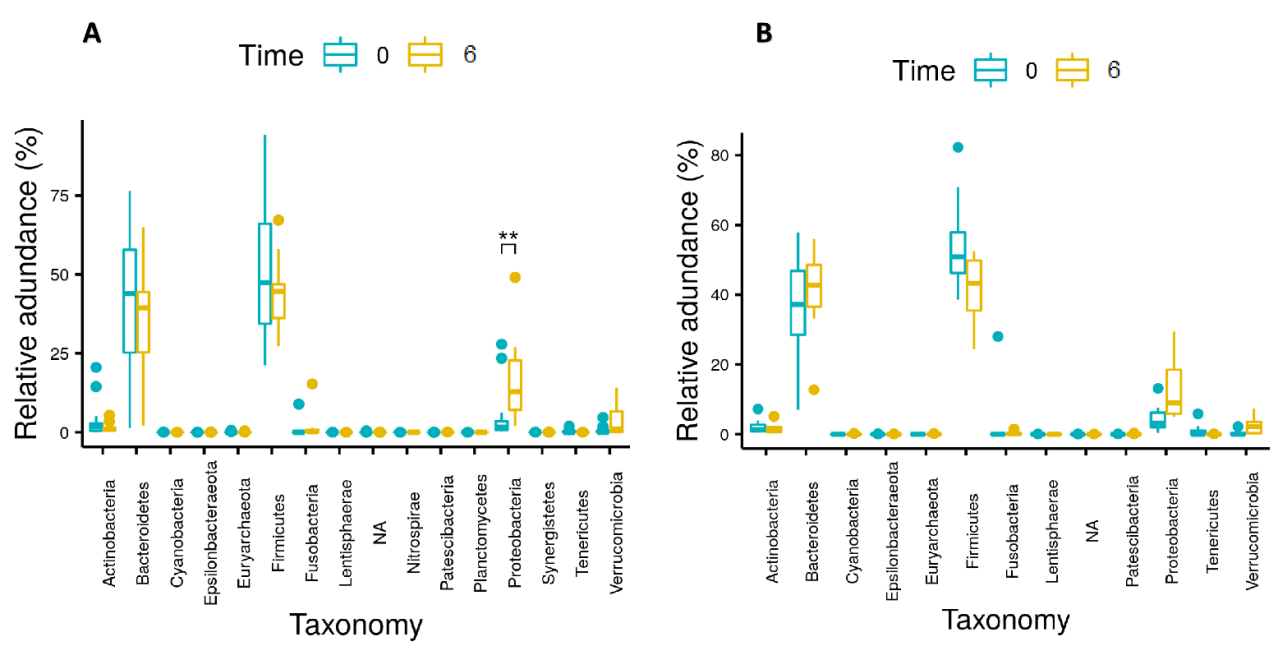
3.3. Gut Microbiota Analysis
3.4. Gut Microbiota Analysis According to Glucose Breath-Test Results at 6 Months Post-Surgery
3.5. Gut Microbiota Analysis of Patients According to FE1 Test at 6 Months Post-Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Higa, K.; Himpens, J.; Buchwald, H.; Scopinaro, N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes. Surg. 2018, 28, 3783–3794. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.C.; Chevallier, J.-M.; Mahawar, K.; Brown, W.; Kow, L.; White, K.P.; Shikora, S. IFSO (International Federation for Surgery of Obesity and Metabolic Disorders) Consensus Conference Statement on One-Anastomosis Gastric Bypass (OAGB-MGB): Results of a Modified Delphi Study. Obes. Surg. 2020, 30, 1625–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deitel, M.; Kular, K.S. Consensus survey on mini-gastric bypass and one-anastomosis gastric bypass. Ann. Bariatr. Metab. Surg. 2018, 1, 1001. [Google Scholar] [CrossRef]
- Kessler, Y.; Adelson, D.; Mardy-Tilbor, L.; Ben-Porat, T.; Szold, A.; Goitein, D.; Sakran, N.; Raziel, A.; Sherf-Dagan, S. Nutritional status following One Anastomosis Gastric Bypass. Clin. Nutr. 2020, 39, 599–605. [Google Scholar] [CrossRef]
- Kassir, R.; Blanc, P.; Lointier, P.; Breton, C.; Debs, T.; Tiffet, O. Laparoscopic Revision of an Omega Loop Gastric Bypass to Treat Afferent Loop Syndrome. Obes. Surg. 2015, 25, 1976–1978. [Google Scholar] [CrossRef]
- Mouillot, T.; Rhyman, N.; Gauthier, C.; Paris, J.; Lang, A.-S.; Lepers-Tassy, S.; Manfredi, S.; Lepage, C.; Leloup, C.; Jacquin-Piques, A.; et al. Study of Small Intestinal Bacterial Overgrowth in a Cohort of Patients with Abdominal Symptoms Who Underwent Bariatric Surgery. Obes. Surg. 2020, 30, 2331–2337. [Google Scholar] [CrossRef]
- Quigley, E.M.M. The Spectrum of Small Intestinal Bacterial Overgrowth (SIBO). Curr. Gastroenterol. Rep. 2019, 21, 3. [Google Scholar] [CrossRef]
- Jirapinyo, P.; Makuvire, T.T.; Dong, W.Y.; Chan, W.W.; Thompson, C.C. Impact of Oral-Cecal Transit Time on the Interpretation of Lactulose Breath Tests After RYGB: A Personalized Approach to the Diagnosis of SIBO. Obes. Surg. 2019, 29, 771–775. [Google Scholar] [CrossRef]
- Adike, A.; DiBaise, J.K. Small Intestinal Bacterial Overgrowth: Nutritional Implications, Diagnosis, and Management. Gastroenterol. Clin. N. Am. 2018, 47, 193–208. [Google Scholar] [CrossRef]
- Mattsson, J.; Minaya, M.T.; Monegro, M.; Lebwohl, B.; Lewis, S.K.; Green, P.H.; Stenberg, R. Outcome of breath tests in adult patients with suspected small intestinal bacterial overgrowth. Gastroenterol. Hepatol. Bed Bench 2017, 10, 168–172. [Google Scholar]
- Krajicek, E.J.; Hansel, S.L. Small Intestinal Bacterial Overgrowth: A Primary Care Review. Mayo Clin. Proc. 2016, 91, 1828–1833. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Bjorneklett, A.; Viddal, K.O.; Midtvedt, T.; Nygaard, K. Intestinal and gastric bypass. Changes in intestinal microecology after surgical treatment of morbid obesity in man. Scand. J. Gastroenterol. 1981, 16, 681–687. [Google Scholar] [CrossRef]
- Lakhani, S.V.; Shah, H.N.; Alexander, K.; Finelli, F.C.; Kirkpatrick, J.R.; Koch, T.R. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr. Res. 2008, 28, 293–298. [Google Scholar] [CrossRef]
- Machado, J.D.; Campos, C.S.; Lopes Dah Silva, C.; Marques Suen, V.M.; Barbosa Nonino-Borges, C.; Dos Santos, J.E.; Ceneviva, R.; Marchini, J.S. Intestinal Bacterial Overgrowth After Roux-en-Y Gastric Bypass. Obes. Surg. 2008, 18, 139–143. [Google Scholar] [CrossRef]
- Shah, H.N.; Bal, B.S.; Finelli, F.C.; Koch, T.R. Constipation in patients with thiamine deficiency after Roux-en-Y gastric bypass surgery. Digestion 2013, 88, 119–124. [Google Scholar] [CrossRef]
- Andalib, I.; Shah, H.; Bal, B.S.; Shope, T.R.; Finelli, F.C.; Koch, T.R. Breath Hydrogen as a Biomarker for Glucose Malabsorption after Roux-en-Y Gastric Bypass Surgery. Dis. Markers 2015, 2015, 102760. [Google Scholar] [CrossRef] [Green Version]
- Sabate, J.M.; Coupaye, M.; Ledoux, S.; Castel, B.; Msika, S.; Coffin, B.; Jouet, P. Consequences of Small Intestinal Bacterial Over-growth in Obese Patients Before and After Bariatric Surgery. Obes. Surg. 2017, 27, 599–605. [Google Scholar] [CrossRef]
- Borbély, Y.; Plebani, A.; Kröll, D.; Ghisla, S.; Nett, P.C. Exocrine Pancreatic Insufficiency after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2016, 12, 790–794. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Valente, R.; Thorell, A.; Rutkowski, W.; Haas, S.L.; Arnelo, U.; Martin, L.; Lohr, J.M. Pancreatic Exocrine Insufficiency after Bariatric Surgery. Nutrients 2017, 9, 1241. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Haupt, M.E.; Geller, D.E.; Hall, J.A.; Quintana Diez, P.M. Less common etiologies of exocrine pancreatic insufficiency. World J. Gastroenterol. 2017, 23, 7059–7076. [Google Scholar] [CrossRef]
- Phillips, M.E.; Hopper, A.D.; Leeds, J.S.; Roberts, K.J.; McGeeney, L.; Duggan, S.N.; Kumar, R. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021, 8, e000643. [Google Scholar] [CrossRef]
- Miroslav, V.; Gregor, K.; Brane, B.; Barbara, R.; Bojan, T.; Sasa, R.; Andreja, K. Is Pancreatic Exocrine Insufficiency a Cause of Malabsorption in Patients after Bariatric Surgery? JOP J. Pancreas Online 2016, 17, 402–405. [Google Scholar]
- Akpinar, M.Y.; Ozturk, D.; Murat, K.; Aksoy, E.K.; Nazligul, Y.; Bulus, H. Sleeve gastrectomy relieves exocrine pancreatic insufficiency in morbidly obese patients: A prospective case-control study. Prz. Gastroenterol. 2019, 14, 268–273. [Google Scholar] [CrossRef]
- Debédat, J.; Clément, K.; Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef]
- Davies, N.K.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: A systematic review. Surg. Obes. Relat. Dis. 2019, 15, 656–665. [Google Scholar] [CrossRef]
- Luijten, J.; Vugts, G.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. The Importance of the Microbiome in Bariatric Surgery: A System-atic Review. Obes. Surg. 2019, 29, 2338–2349. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Angoorani, P.; Hasani-Ranjbar, S.; Siadat, S.D.; Ghasemi, N.; Larijani, B.; Soroush, A.R. Adaptation of human gut microbiota to bariatric surgeries in morbidly obese patients: A systematic review. Microb. Pathog. 2018, 116, 13–21. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Sioka, E.; Chatedaki, C.; Zacharoulis, D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: A Systematic Review and Meta-analysis. Obes. Surg. 2017, 27, 1345–1357. [Google Scholar] [CrossRef]
- Bariatric Surgery Criteria of the Ministry of Health. The Ministry of Health Web Site. Available online: http://www.health.gov.il/hozer/mr33_2013.pdf (accessed on 19 January 2021).
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Pinho, C.P.S.; Diniz, A.D.S.; De Arruda, I.K.G.; Leite, A.; Petribu, M.M.V.; Rodrigues, I.G. Waist circumference measurement sites and their association with visceral and subcutaneous fat and cardiometabolic abnormalities. Arch. Endocrinol. Metab. 2018, 62, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, Y.; Fukumoto, S.; Inaba, M.; Koyama, H.; Shoji, T.; Shoji, S.; Nishizawa, Y. Different Impacts of Neck Circumference and Visceral Obesity on the Severity of Obstructive Sleep Apnea Syndrome. Obes. Silver Spring 2011, 19, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Toolabi, K.; Arefanian, S.; Golzarand, M.; Arefanian, H. Effects of Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) on Weight Loss and Biomarker Parameters in Morbidly Obese Patients: A 12-Month Follow-Up. Obes. Surg. 2011, 21, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; di Stefano, M.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Satta, P.U.; Vernia, P.; et al. Methodology and Indications of H2-Breath Testing in Gastrointestinal Diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29 (Suppl. 1), 1–49. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.Q.; Kwan, L.Y. All Roads Lead to Rome: Update on Rome III Criteria and New Treatment Options. Gastroenterol. Rep. 2007, 1, 56–65. [Google Scholar] [PubMed]
- Oria, H.E.; Moorehead, M.K. Updated Bariatric Analysis and Reporting Outcome System (BAROS). Surg. Obes. Relat. Dis. 2009, 5, 60–66. [Google Scholar] [CrossRef]
- Bergeat, D.; Lechaux, D.; Ghaina, A.; Thibault, R.; Bouygues, V. Postoperative Outcomes of Laparoscopic Bariatric Surgery in Older Obese Patients: A Matched Case-Control Study. Obes. Surg. 2017, 27, 1414–1422. [Google Scholar] [CrossRef]
- Moorehead, M.K.; Ardelt-Gattinger, E.; Lechner, H.; Oria, H.E. The validation of the Moorehead-Ardelt Quality of Life Ques-tionnaire II. Obes. Surg. 2003, 13, 684–692. [Google Scholar] [CrossRef]
- Padwal, R.S.; Majumdar, S.R.; Klarenbach, S.; Birch, D.W.; Karmali, S.; McCargar, L.; Fassbender, K.; Sharma, A.M. Health status, quality of life, and satisfaction of patients awaiting multidisciplinary bariatric care. BMC Health Serv. Res. 2012, 12, 139. [Google Scholar] [CrossRef] [Green Version]
- Shai, I.; Rosner, B.A.; Shahar, D.R.; Vardi, H.; Azrad, A.B.; Kanfi, A.; Schwarzfuchs, D.; Fraser, D. Dietary evaluation and attenuation of relative risk: Multiple comparisons between blood and urinary biomarkers, food frequency, and 24-hour recall question-naires: The DEARR study. J. Nutr. 2005, 135, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Tzameret—Israeli National Nutrient Database 2015; Ministry of Health Public Health Services Nutrition Division: Jerusalem, Israel, 2015.
- Tóth, A.Z.; Szabó, A.; Hegyi, E.; Hegyi, P.; Sahin-Tóth, M. Detection of human elastase isoforms by the ScheBo Pancreatic Elas-tase 1 Test. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G606–G614. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene data-base project: Improved data processing and web-based tools. Nucleic. Acids. Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Timothy Garvey, W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obes. Silver Spring 2020, 28, O1–O58. [Google Scholar]
- Frame-Peterson, L.A.; Megill, R.D.; Carobrese, S.; Schweitzer, M. Nutrient Deficiencies Are Common Prior to Bariatric Surgery. Nutr. Clin. Pract. 2017, 32, 463–469. [Google Scholar] [CrossRef]
- Ozmen, M.M.; Gundogdu, E.; Guldogan, C.E.; Ozmen, F. The Effect of Bariatric Surgery on Exocrine Pancreatic Function. Obes. Surg. 2021, 31, 580–587. [Google Scholar] [CrossRef]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Bülow, R.; Kühn, J.P.; Franke, A.; Heinsen, F.A.; Pietzner, M.; Nauck, M.; Völker, U.; et al. Impaired Exocrine Pancreatic Function Associates with Changes in Intestinal Microbiota Composition and Diversity. Gastroenterology 2019, 156, 1010–1015. [Google Scholar] [CrossRef] [PubMed]




| Variable a | Baseline (n = 32) | 6 Months Post-Surgery (n = 29) | p-Value |
|---|---|---|---|
| Age (years) | 44.5 ± 12.3 | - | - |
| Sex (%female) | 50 | - | - |
| Marital Status (%) | |||
| Married | 75 | - | - |
| Divorced | 6.3 | - | - |
| Single | 18.8 | - | - |
| Co-morbidities (%) | |||
| Diabetes | 9.4 | - | - |
| IFG | 50 | - | - |
| NAFLD | 84.4 | - | - |
| Dyslipidemia | 59.4 | - | - |
| Hypertension | 21.9 | - | - |
| Sleep Apnea | 15.6 | - | - |
| Hypothyroidism | 3.1 | - | - |
| Gastroesophageal Reflux Disease | 21.9 | - | - |
| Orthopedic Problems | 31.3 | - | - |
| Medication (%) | |||
| Drugs for diabetes | 6.3 | 0 | 0.500 |
| Drugs for dyslipidemia | 12.5 | 3.4 | 0.500 |
| Drugs for Hypertension | 18.8 | 10.3 | 0.500 |
| Anti-aggregation drugs | 6.3 | 3.4 | 1.000 |
| Drugs for hypothyroidism | 3.1 | 3.4 | 1.000 |
| Antacids | 9.4 | 31 | 0.109 |
| Anti-depressive drugs | 3.1 | 3.4 | 1.000 |
| Anthropometrics | |||
| Weight (kg) | 120.8 ± 25.2 | 90.9 ± 20.3 | <0.001 |
| Height (meter) | 1.7 ± 0.1 | - | - |
| BMI (kg/m2) | 41.7 ± 6.6 | 31.4 ± 5.6 | <0.001 |
| WC (cm) | 122.8 ± 14.3 | 100.5 ± 13.9 | <0.001 |
| NC (cm) | 38.7 ± 4.5 | 34.8 ± 3.7 | <0.001 |
| %EWL | NR | 67.8 ± 21.2 | - |
| Lifestyle | |||
| Smoking (%yes) | 6.3 | 6.9 | 1.000 |
| Physical activity (%yes) | 56.3 | 79.3 | 0.180 |
| Physical activity (h/week) | 1.2 ± 1.6 | 2.8 ± 2.3 | 0.017 |
| Dietary intake | |||
| Calories (kcal/day) | 2563.0 ± 979.6 | 1626.7 ± 712.8 | <0.001 |
| Protein (g/day) | 136.7 ± 48.8 | 87.3 ± 40.2 | <0.001 |
| Carbohydrates (g/day) | 226.7 ± 85.2 | 150.0 ± 73.7 | <0.001 |
| Fats (g/day) | 116.8 ± 63.4 | 69.5 ± 38.4 | <0.001 |
| Percent of food intake compared to before surgery | NR | 31.7 ± 14.6 | - |
| No. of dietitian appointments after surgery | NR | 4.3 ± 3.5 | - |
| Participation in support group after surgery (%yes) | NR | 13.8 | - |
| Supplementation (%) | |||
| Multivitamin | 43.8 | 93.1 | <0.001 |
| Calcium | 6.3 | 62.1 | <0.001 |
| Vitamin D | 62.5 | 86.2 | 0.039 |
| Vitamin B12 | 34.4 | 72.4 | 0.007 |
| Iron | 18.8 | 24.1 | 0.375 |
| Folic Acid | 18.8 | 6.9 | 0.250 |
| Biochemical tests ᵇ | |||
| Hemoglobin (g/dL) | 14.0 ± 1.3 | 13.2 ± 1.2 | <0.001 |
| %anemia (<13.5(male), <12(female)) | 12.5 | 28.6 | 0.063 |
| MCV (fL) | 83.3 ± 4.7 | 86.0 ± 5.1 | <0.001 |
| %low values (<80) | 25.0 | 10.7 | 0.063 |
| %high values (>95) | 0 | 0 | NR |
| MCHC (g/dL) | 33.8 ± 0.7 | 33.6 ± 0.7 | 0.632 |
| %low values (<33) | 6.3 | 14.3 | 0.625 |
| %high values (>37) | 0 | 0 | NR |
| Albumin (g/dL) | 4.4 ± 0.3 | 4.2 ± 0.3 | 0.002 |
| %hypoalbuminemia (<3.5) | 0 | 3.6 | 1.000 |
| Total protein (g/dL) | 7.6 ± 0.4 | 7.2 ± 0.4 | <0.001 |
| %low values (<6.3) | 0 | 0 | NR |
| Iron (µg/dL) | 90.3 ± 29.8 | 80.6 ± 25.1 | 0.107 |
| %deficiency (<49 [male], <37 [female]) | 0 | 3.7 | 1.000 |
| Ferritin (ng/mL) | 155.0 ± 138.3 | 154.4 ± 125.3 | 0.564 |
| %deficiency (<22[male], <10[female]) | 0 | 0 | NR |
| Transferrin (mg/dL) | 277.7 ± 37.5 | 238.9 ± 55.7 | 0.001 |
| %low values (<220) | 6.3 | 29.6 | 0.070 |
| %high values (>400) | 0 | 0 | NR |
| Transferrin saturation (%) | 23.8 ± 8.8 | 26.4 ± 14.1 | 0.323 |
| %low values (<20) | 43.8 | 33.3 | 0.289 |
| Folate (ng/mL) | 11.0 ± 5.6 | 12.4 ± 5.7 | 0.285 |
| %deficiency (<2.76) | 0 | 0 | NR |
| Vitamin B12 (pg/mL) | 519.5 ± 233.1 | 534.6 ± 221.9 | 0.725 |
| %deficiency (<239) | 0 | 3.7 | 1.000 |
| Vitamin D (ng/mL) | 25.3 ± 8.6 | 27.5 ± 10.2 | 0.366 |
| %insufficiency (<30) | 78.1 | 63.0 | 0.289 |
| %deficiency (<20) | 25.0 | 18.5 | 1.000 |
| Vitamin A (µg/dL) | - | 42.4 ± 10.1 | - |
| %deficiency (<30) | - | 15.4 | - |
| Quality of life | |||
| VAS QoL | 63.8 ± 18.3 | 81.0 ± 16.0 | <0.001 |
| M-A QoLII score c | 1.1 ± 0.8 | 1.8 ± 0.9 | 0.002 |
| M-A QoLII (%Good/Very good) d | 56.3 | 82.8 | 0.065 |
| GI symptoms (%) | |||
| ROME III score (%positive) e | 9.4 | 20.7 | 0.250 |
| Vomit | 0 | 0 | NR |
| Nausea | 6.3 | 24.1 | 0.125 |
| Regurgitation | 9.4 | 10.3 | 1.000 |
| Hiccups | 9.4 | 31.0 | 0.070 |
| Heartburn | 28.1 | 10.3 | 0.070 |
| Abdominal pain | - | 17.2 | - |
| Flatulence | 12.5 | 58.6 | 0.002 |
| Frequent soft stool | 6.3 | 34.5 | 0.021 |
| No. of feces per day | 1.5 ± 0.8 | 1.7 ± 1.2 | 0.188 |
| ≥3 feces per day | 3.1 | 13.8 | 0.250 |
| Hair loss | 21.9 | 48.3 | 0.016 |
| Glucose breath test (%positive) f,g | 0 | 37.0 | 0.004 |
| PEI (%positive) g,h | 0 | 26.1 i | 0.500 |
| Variable b | SIBO Positive (n = 10) | SIBO Negative (n = 17) | p-Value |
|---|---|---|---|
| Age (years) | 41.0 ± 9.9 | 48.3 ± 12.8 | 0.133 |
| Sex (%female) | 80 | 41.2 | 0.107 |
| Medication (%) | |||
| Drugs for diabetes | 0 | 0 | NR |
| Drugs for dyslipidemia | 0 | 5.9 | 1.000 |
| Drugs for Hypertension | 0 | 17.6 | 0.274 |
| Anti-aggregates | 0 | 5.9 | 1.000 |
| Drugs for hypothyroidism | 0 | 5.9 | 1.000 |
| Anti-acids | 20 | 35.3 | 0.666 |
| Drugs for depression | 10 | 0 | 0.370 |
| Bypass length (cm) | 175.0 ± 26.4 | 175.3 ± 19.7 | 0.976 |
| Anthropometric measurements | |||
| Weight (kg) | 87.2 ± 15.2 | 93.7 ± 23.8 | 0.449 |
| BMI (kg/m2) | 32.0 ± 5.4 | 31.2 ± 6.1 | 0.755 |
| WC (cm) | 97.2 ± 9.3 | 102.3 ± 16.2 | 0.370 |
| NC (cm) | 33.3 ± 3.2 | 35.5 ± 4.0 | 0.158 |
| %EWL | 64.8 ± 19.6 | 69.4 ± 22.5 | 0.600 |
| Lifestyle | |||
| Smoking (%yes) | 10 | 5.9 | 1.000 |
| Physical Activity (%yes) | 80 | 82.4 | 1.000 |
| Physical Activity (h/week) | 2.7 ± 2.3 | 2.9 ± 2.4 | 0.766 |
| Dietary intake | |||
| Calories (kcal/day) | 1192.9 ± 471.6 | 1908.4 ± 704.1 | 0.009 |
| Protein (g/day) | 65.1 ± 26.9 | 101.3 ± 42.2 | 0.023 |
| Carbohydrates (g/day) | 113.6 ± 52.9 | 175.1 ± 77.3 | 0.036 |
| Fats (g/day) | 49.2 ± 19.3 | 82.1 ± 41.8 | 0.028 |
| Percent of food intake compared to before surgery | 33.5 ± 13.1 | 28.8 ± 13.1 | 0.414 |
| No. of dietitian appointments after surgery | 3.5 ± 1.8 | 4.6 ± 4.4 | 0.675 |
| Participation in support group after surgery (%) | 10 | 17.6 | 1.000 |
| Supplementation (%) | |||
| Multivitamin | 90 | 94.1 | 1.000 |
| Calcium | 60 | 64.7 | 1.000 |
| Vitamin D | 70 | 94.1 | 0.128 |
| Vitamin B12 | 60 | 82.4 | 0.365 |
| Iron | 10 | 29.4 | 0.363 |
| Folic Acid | 0 | 11.8 | 0.516 |
| Biochemical tests | |||
| Hemoglobin (g/dL) | 13.2 ± 0.4 | 13.2 ± 1.5 | 0.928 |
| %anemia (<13.5(male), <12(female)) | 20 | 35.3 | 0.666 |
| MCV (fL) | 88.1 ± 3.2 | 84.9 ± 5.8 | 0.116 |
| %low values (<80) | 0 | 17.6 | 0.274 |
| %high values (>95) | 0 | 0 | NR |
| MCHC (g/dL) | 33.7 ± 0.7 | 33.7 ± 0.7 | 0.891 |
| %low values (<33) | 10 | 11.8 | 1.000 |
| %high values (>37) | 0 | 0 | NR |
| Albumin (g/dL) | 4.2 ± 0.4 | 4.2 ± 0.3 | 0.967 |
| %hypoalbuminemia (<3.5) | 10 | 0 | 0.370 |
| Total protein (g/dL) | 7.2 ± 0.4 | 7.2 ± 0.4 | 0.994 |
| %low values (<6.3) | 0 | 0 | NR |
| Iron (µg/dL) | 75.9 ± 13.4 | 83.3 ± 30.0 | 0.470 |
| %deficiency (<49(male), <37(female)) | 0 | 5.9 | 1.000 |
| Ferritin (ng/mL) | 98.9 ± 80.4 | 187.0 ± 137.2 | 0.077 |
| %deficiency (<22(male), <10(female)) | 0 | 0 | NR |
| Transferrin (mg/dL) | 249.8 ± 60.3 | 232.5 ± 53.7 | 0.477 |
| %low values (<220) | 20 | 35.3 | 0.666 |
| %high values (>400) | 0 | 0 | NR |
| Transferrin saturation (%) | 23.1 ± 6.5 | 28.3 ± 17.0 | 0.364 |
| %low values (<20) | 40 | 29.4 | 0.683 |
| Folate (ng/mL) | 8.4 ± 3.6 | 14.7 ± 5.5 | 0.003 |
| %deficiency (<2.76) | 0 | 0 | NR |
| Vitamin B12 (pg/mL) | 442.1 ± 212.8 | 589.1 ± 214.6 | 0.097 |
| %deficiency (<239) | 10 | 0 | 0.370 |
| Vitamin D (ng/mL) | 25.5 ± 10.2 | 28.7 ± 10.4 | 0.444 |
| %insufficiency (<30) | 60 | 64.7 | 1.000 |
| %deficiency (<20) | 30 | 11.8 | 0.326 |
| Vitamin A (µg/dL) | 38.5 ± 12.9 | 44.9 ± 7.3 | 0.171 |
| %deficiency (<30) | 40 | 0 | 0.014 |
| Quality of life | |||
| VAS QoL | 77.5 ± 19.5 | 84.4 ± 9.2 | 0.317 |
| M-A QoLII score c | 1.7 ± 0.7 | 2.0 ± 0.7 | 0.265 |
| M-A QoLII (%Good/Very good) d | 80 | 88.2 | 0.613 |
| GI symptoms (%) | |||
| ROME III score (%positive) e | 10 | 23.5 | 0.621 |
| Vomit | 0 | 0 | NR |
| Nausea | 20 | 23.5 | 1.000 |
| Regurgitation | 30 | 0 | 0.041 |
| Hiccups | 40 | 23.5 | 0.415 |
| Heartburn | 10 | 11.8 | 1.000 |
| Abdominal pain | 30 | 11.8 | 0.326 |
| Flatulence | 50 | 64.7 | 0.453 |
| Frequent soft stool | 50 | 17.6 | 0.102 |
| No. of feces per day | 1.6 ± 0.7 | 1.8 ± 1.4 | 0.598 |
| ≥3 feces per day | 10 | 17.6 | 1.000 |
| Hair loss | 70 | 35.3 | 0.120 |
| PEI (%positive) f | 22.2 | 23.1 | 1.000 |
| Phyla | Relative Abundance at Baseline (%) | Relative Abundance at 6 Months Post-Surgery (%) | p-Value (FDR Adjusted) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | ||
| Firmicutes | 52.28 | 27.54 | 86.38 | 42.47 | 24.35 | 67.20 | 0.021 |
| Fusobacteria | 1.320 | 0 | 27.99 | 0.87 | 0 | 15.32 | 0.021 |
| Proteobacteria | 4.533 | 0.288 | 27.88 | 14.17 | 2.046 | 49.07 | <0.001 |
| Tenericutes | 0.505 | 0 | 5.802 | 0.003 | 0 | 0.061 | 0.021 |
| Verrucomicrobia | 0.446 | 0 | 4.659 | 3.326 | 0 | 14.07 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaniel, O.; Sherf-Dagan, S.; Szold, A.; Langer, P.; Khalfin, B.; Kessler, Y.; Raziel, A.; Sakran, N.; Motro, Y.; Goitein, D.; et al. The Effects of One Anastomosis Gastric Bypass Surgery on the Gastrointestinal Tract. Nutrients 2022, 14, 304. https://doi.org/10.3390/nu14020304
Kaniel O, Sherf-Dagan S, Szold A, Langer P, Khalfin B, Kessler Y, Raziel A, Sakran N, Motro Y, Goitein D, et al. The Effects of One Anastomosis Gastric Bypass Surgery on the Gastrointestinal Tract. Nutrients. 2022; 14(2):304. https://doi.org/10.3390/nu14020304
Chicago/Turabian StyleKaniel, Osnat, Shiri Sherf-Dagan, Amir Szold, Peter Langer, Boris Khalfin, Yafit Kessler, Asnat Raziel, Nasser Sakran, Yair Motro, David Goitein, and et al. 2022. "The Effects of One Anastomosis Gastric Bypass Surgery on the Gastrointestinal Tract" Nutrients 14, no. 2: 304. https://doi.org/10.3390/nu14020304
APA StyleKaniel, O., Sherf-Dagan, S., Szold, A., Langer, P., Khalfin, B., Kessler, Y., Raziel, A., Sakran, N., Motro, Y., Goitein, D., & Moran-Gilad, J. (2022). The Effects of One Anastomosis Gastric Bypass Surgery on the Gastrointestinal Tract. Nutrients, 14(2), 304. https://doi.org/10.3390/nu14020304









