Dietary Alpha-Linolenic Acid Supports High Retinal DHA Levels
Abstract
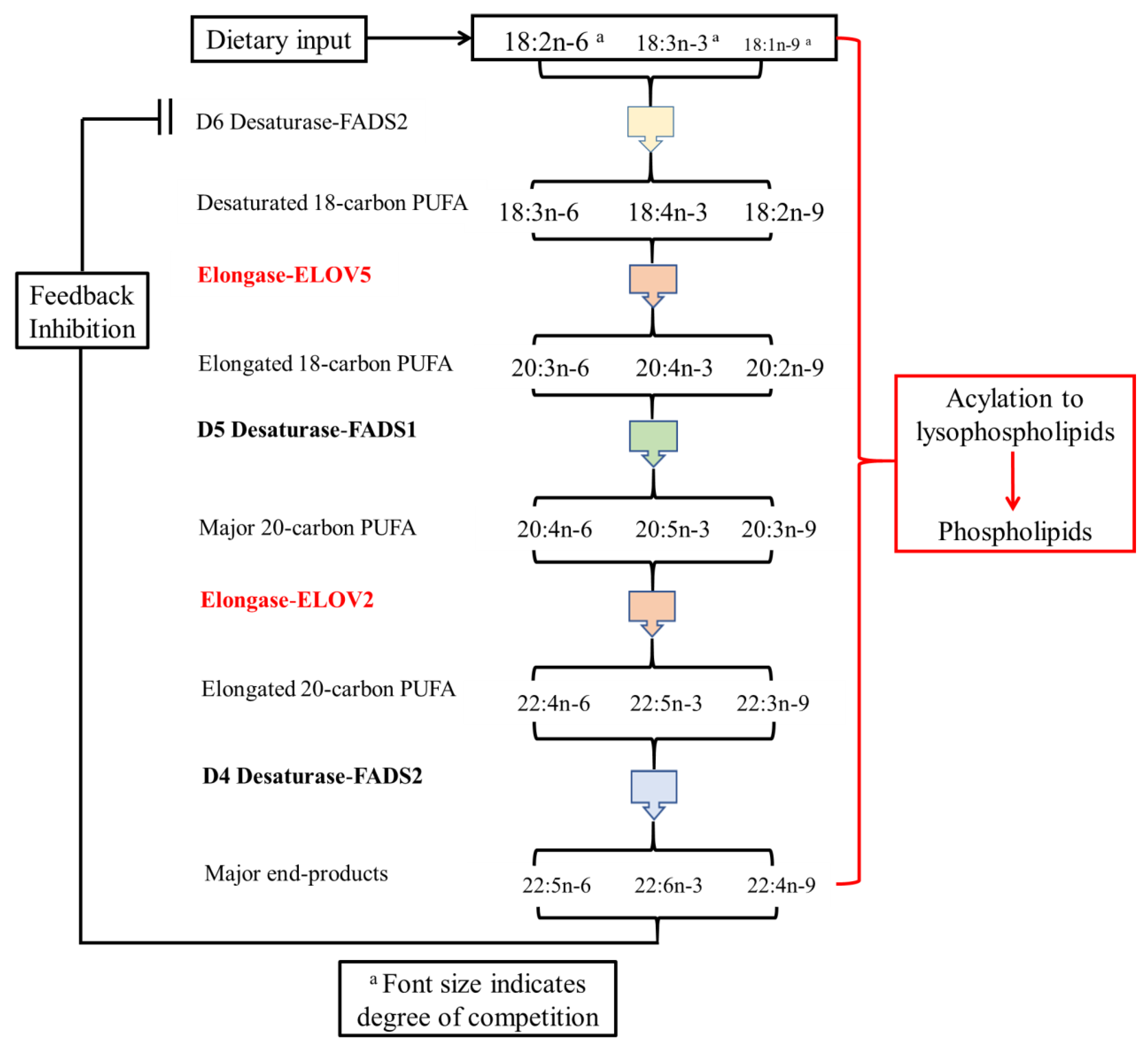
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Analyses
2.3. Statistical Analyses
3. Results
3.1. Diets
3.2. Effect of Increasing Dietary ALA on Tissue Fatty Acids in 3-Week-Old Guinea Pigs
4. Discussion
4.1. General Remarks about the Study
4.2. What Is the Relevance of Retinal 22:5n-6?
4.3. How Is DHA Incorporated into the Brain and Retina?
4.4. The Relevance of This Research Question to Humans
4.5. Strengths and Weaknesses of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Diet Group | 1 a | 2 b | 3 b | 4 b | 5 b |
|---|---|---|---|---|---|
| Fatty Acids | |||||
| 12:0 + 14:0 | nd | 10.9 | 23.7 | 13.8 | 10.9 |
| 16:0 | 8.2 | 28.0 | 18.9 | 24.0 | 13.7 |
| 18:0 | 2.9 | 4.0 | 3.5 | 4.1 | 4.3 |
| 18:1 | 15.3 | 34.5 | 29.8 | 30.0 | 35.3 |
| 18:2n-6 | 72.6 | 18.6 | 17.6 | 17.5 | 18.2 |
| 18:3n-3 | 0.7 | 2.8 | 6.4 | 10.0 | 17.3 |
| 20:1 | nd | nd | nd | nd | nd |
| 20:4n-6 | nd | nd | nd | nd | nd |
| 20:5n-3 | nd | nd | nd | nd | nd |
| 22:5n-3 | nd | nd | nd | nd | nd |
| 22:6n-3 | nd | nd | nd | nd | nd |
| 18:2/18:3 | 103 | 6.6 | 2.75 | 1.75 | 1.05 |
| Diet Group b | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| ALA% in diet lipid | 0.7 | 2.8 | 6.4 | 10.0 | 17.3 |
| Diet LA:ALA | 103 | 6.61 | 2.75 | 1.75 | 1.05 |
| (n) c | (4) | (7) | (4) | (6) | (5) |
| Fatty Acids | |||||
| 16:0 | 19.9 ± 0.6 a | 17.0 ± 0.9 b | 18.0 ± 0.2 b | 19.0 ± 0.8 a | 16.2 ± 0.9 c |
| 18:0 | 19.0 ± 0.8 a | 15.7 ± 0.9 b | 15.8 ± 0.6 b | 18.5 ± 0.9 a | 16.5 ± 0.7 b |
| 16:1n-9 | 0.5 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.1 |
| 18:1n-9 | 12.2 ± 0.8 a | 12.6 ± 0.8 a | 10.7 ± 0.0 b | 12.9 ± 0.7 a | 12.1 ± 0.9 a |
| 18:2n-6 | 2.9 ± 0.2 a | 1.8 ± 0.1 b | 1.9 ± 0.1 b | 2.2 ± 0.3 a | 1.7 ± 0.2 b |
| 20:3n-6 | 1.1 ± 0.0 a | 0.8 ± 0.1 b | 0.8 ± 0.0 b | 0.9 ± 0.1 b | 0.8 ± 0.1 b |
| 20:4n-6 | 10.4 ± 0.6 a | 9.9 ± 0.7 a | 10.5 ± 0.5 a | 9.7 ± 0.5 a b | 9.4 ± 0.5 b |
| 22:4n-6 | 6.0 ± 0.5 a | 5.0 ± 0.4 b | 4.9 ± 0.2 b | 5.0 ± 0.5 b | 4.3 ± 0.4 c |
| 22:5n-6 | 8.3 ± 0.3 a | 7.2 ± 0.7 b | 6.5 ± 0.5 c | 5.0 ± 0.5 d | 4.7 ± 0.5 d |
| 18:3n-3 | nd | nd | nd | nd | nd |
| 20:5n-3 | 0.2 ± 0.0 a | 0.6 ± 0.1 b | 0.5 ± 0.0 b | 0.4 ± 0.1 b | 0.5 ± 0.1 b |
| 22:5n-3 | 0.4 ± 0.0 a | 0.5 ± 0.1 b | 0.8 ± 0.0 c | 0.9 ± 0.1 d | 0.8 ± 0.1 c |
| 22:6n-3 | 1.7 ± 0.1 a | 2.6 ± 0.2 b | 3.6 ± 0.1 c | 5.2 ± 0.2 d | 6.9 ± 0.2 e |
| Diet Group b | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| ALA% in diet lipid | 0.7 | 2.8 | 6.4 | 10.0 | 17.3 |
| Diet LA:ALA | 103 | 6.61 | 2.75 | 1.75 | 1.05 |
| (n) c | (4) | (7) | (4) | (6) | (5) |
| Fatty Acids | |||||
| 16:0 | 9.2 ± 0.5 a | 12.5 ± 0.1 b | 12.7 ± 0.1 b | 12.7 ± 0.4 b | 11.0 ± 0.5 c |
| 18:0 | 30.4 ± 0.5 a | 29.0 ± 0.2 b | 27.9 ± 0.5 c | 28.9 ± 0.4 b | 29.4 ± 0.3 b |
| 16:1n-9 | nd | 0.4 ± 0.0 a | 0.3 ± 0.0 b | 0.3 ± 0.0 b | 0.2 ± 0.0 c |
| 18:1n-9 | 3.4 ± 0.1 a | 10.9 ± 0.5 b | 9.7 ± 0.0 c | 10.3 ± 0.0 c d | 10.8 ± 0.9 b d |
| 18:2n-6 | 44.2 ± 0.5 a | 34.2 ± 0.2 b | 35.4 ± 0.1 c | 33.6 ± 0.2 b d | 32.7 ± 0.7 e |
| 20:3n-6 | 0.4 ± 0.1 a | 0.7 ± 0.0 b | 0.8 ± 0.0 b | 0.7 ± 0.0 b | 1.0 ± 0.0 c |
| 20:4n-6 | 5.8 ± 0.5 a | 5.9 ± 0.3 a | 5.6 ± 0.3 a | 5.7 ± 0.1 a | 4.9 ± 0.2 b |
| 22:4n-6 | 0.8 ± 0.1 a | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.5 ± 0.0 b c | 0.4 ± 0.1 c |
| 22:5n-6 | 0.7 ± 0.1 a | 0.5 ± 0.0 b | 0.5 ± 0.0 b | 0.5 ± 0.0 b | 0.3 ± 0.0 c |
| 18:3n-3 | 0.1 ± 0.0 a | 0.4 ± 0.1 b | 0.5 ± 0.0 b | 1.1 ± 0.1 c | 1.6 ± 0.2 d |
| 22:5n-3 | nd | 0.3 ± 0.0 a | 0.5 ± 0.0 b | 0.6 ± 0.0 c | 1.0 ± 0.2 d |
| 22:6n-3 | nd | 0.3 ± 0.0 c | 0.4 ± 0.0 b | 0.6 ± 0.0 c | 0.9 ± 0.1 d |
References
- Fliesler, S.J.; Anderson, R.E. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef]
- Jastrzebska, B.; Debinski, A.; Filipek, S.; Palczewski, K. Role of membrane integrity on G protein-coupled receptors: Rhodopsin stability and function. Prog. Lipid Res. 2011, 50, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, T.G.; Benolken, R.M.; Anderson, R.E. Visual Membranes: Specificity of Fatty Acid Precursors for the Electrical Response to Illumination. Science 1975, 188, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.; Casperd, N.; Sinclair, A. The long chain metabolites of linoleic and linolenic acids in liver and brain in herbivores and carnivores. Comp. Biochem. Physiol. B 1976, 54, 395–401. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Chen, C.T.; Trepanier, M.O.; Stavro, P.M.; Bazinet, R.P. Whole body synthesis rates of DHA from al-pha-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. J. Lipid Res. 2014, 55, 62–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffrey, B.G.; Weisinger, H.S.; Neuringer, M.; Mitchell, D.C. The role of docosahexaenoic acid in retinal function. Lipids 2001, 36, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J. Docosahexaenoic acid and the brain—What is its role? Asia Pac. J. Clin. Nutr. 2019, 28, 675–688. [Google Scholar] [PubMed]
- Weisinger, H.S.; Vingrys, A.J.; Sinclair, A.J. Effect of dietary n-3 deficiency on the electroretinogram in the guinea pig. Ann. Nutr. Metab. 1996, 40, 91–98. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Niu, S.-L.; Litman, B.J. Quantifying the differential effects of DHA and DPA on the early events in visual signal transduction. Chem. Phys. Lipids 2012, 165, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Weisinger, H.S.; Vingrys, A.J.; Sinclair, A.J. The effect of docosahexaenoic acid on the electroretinogram of the guinea pig. Lipids 1996, 31, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Abedin, L.; Lien, E.L.; Vingrys, A.J.; Sinclair, A.J. The effects of dietary α-linolenic acid compared with docosahexaenoic acid on brain, retina, liver, and heart in the guinea pig. Lipids 1999, 34, 475–482. [Google Scholar] [CrossRef]
- Morrison, J.L.; Botting, K.J.; Darby, J.R.T.; David, A.L.; Dyson, R.M.; Gatford, K.L.; Gray, C.; Herrera, E.A.; Hirst, J.J.; Kim, B.; et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J. Physiol. 2018, 596, 5535–5569. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.H.; Baghurst, K.I.; Potter, B.J.; Hetzel, B.S. Foetal brain development in the sheep. Neuropathol. Appl. Neurobiol. 1979, 5, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Tolcos, M.; Bateman, E.; O’Dowd, R.; Markwick, R.; Vrijsen, K.; Rehn, A.; Rees, S. Intrauterine growth restriction affects the maturation of myelin. Exp. Neurol. 2011, 232, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Luo, N.L.; Borenstein, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, L.D.; Innis, S.M. Docosahexaenoic acid in developing brain and retina of piglets fed high or low α-linolenate formula with and without fish oil. Lipids 1992, 27, 89–93. [Google Scholar] [CrossRef]
- Su, H.-M.; Huang, M.-C.; Saad, N.M.; Nathanielsz, P.W.; Brenna, J.T. Fetal baboons convert 18:3n-3 to 22:6n-3 in vivo: A stable isotope tracer study. J. Lipid Res. 2001, 42, 581–586. [Google Scholar] [CrossRef]
- Bourre, J.-M.; Francois, M.; Youyou, A.; Dumont, O.; Piciotti, M.; Pascal, G.; Durand, G. The Effects of Dietary α-Linolenic Acid on the Composition of Nerve Membranes, Enzymatic Activity, Amplitude of Electrophysiological Parameters, Resistance to Poisons and Performance of Learning Tasks in Rats. J. Nutr. 1989, 119, 1880–1892. [Google Scholar] [CrossRef]
- Galli, C.T.; Paoletti, R. Effects of dietary fatty acids on the fatty acid composition of brain ethanolamine phosphoglyceride: Reciprocal replacemernt of n-6 and n-3 polyunsaturated fatty acids. Biochim. Biophys. Acta 1971, 248, 449–454. [Google Scholar] [CrossRef]
- Brenner, R.R.; Peluffo, R.O. Regulation of unsaturated fatty acids biosynthesis 1. Effect of unsaturated fatty acid of 18 carbons on the microsomal desaturation of linoleic acid into γ-linolenic acid. Biochim. Biophys. Acta 1969, 176, 471–479. [Google Scholar] [CrossRef]
- Holman, R.T. The Slow Discovery of the Importance of ω3 Essential Fatty Acids in Human Health. J. Nutr. 1998, 128, 427S–433S. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.S.; Calandria, J.M.; Gordon, W.C.; Jun, B.; Zhou, Y.; Gelfman, C.M.; Li, S.; Jin, M.; Knott, E.J.; Chang, B.; et al. Adiponectin receptor 1 conserves docosahex-aenoic acid and promotes photoreceptor cell survival. Nat. Commun. 2015, 6, 6228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazinet, R.P.; Bernoud-Hubac, N.; Lagarde, M. How the plasma lysophospholipid and unesterified fatty acid pools supply the brain with docosahexaenoic acid. Prostaglandins Leukot. Essent. Fat. Acids 2018, 142, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Gordon, W.C.; Rodriguez de Turco, E.B. Docosahexaenoic Acid Uptake and Metabolism in Photorecep-tors: Retinal Conservation by an Efficient Retinal Pigment Epithelial Cell-Mediated Recycling Process. Adv. Exp. Med. Biol. 1992, 318, 295–306. [Google Scholar]
- Blasbalg, T.L.; Hibbeln, J.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; Pablo, G.S. Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients 2019, 11, 2365. [Google Scholar] [CrossRef] [Green Version]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R.C. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Fritsche, K. Linoleic Acid. Adv. Nutr. Int. Rev. J. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Shakya-Shrestha, S.; Lentjes, M.; Wareham, N.J.; Khaw, K.-T. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of α-linolenic acid to long-chain n-3 polyunsaturated fatty acids: Results from the EPIC-Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1040–1051, Erratum in Am. J. Clin. Nutr. 2011, 93, 676. [Google Scholar] [CrossRef]
- Allès, B.; Baudry, J.; Méjean, C.; Touvier, M.; Péneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of Sociodemographic and Nutritional Characteristics between Self-Reported Vegetarians, Vegans, and Meat-Eaters from the NutriNet-Santé Study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef]
- Dato, S.M.A.; Brenner, R.R. Comparative effects of docosa-4,7,10,13,16-pentaenoic acid and docosa-4,7,10,13,16,19-hexaenoic acid on the desaturation of linoleic acid and α-linolenic acid. Lipids 1970, 5, 1013–1015. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.; Brenna, J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef]
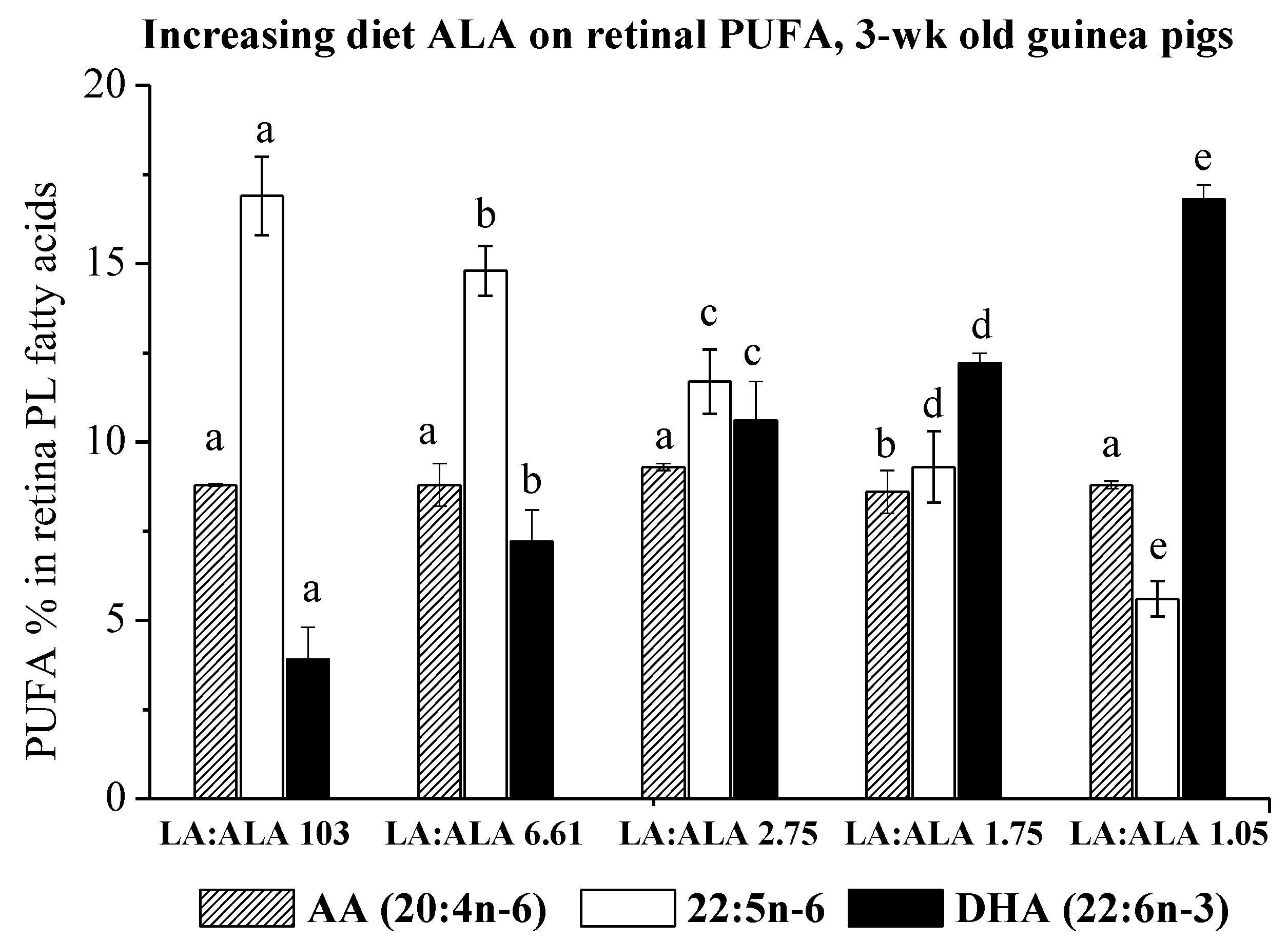
| Diet Group b | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| ALA% in diet lipid | 0.7 | 2.8 | 6.4 | 10.0 | 17.3 |
| Diet LA:ALA | 103 | 6.61 | 2.75 | 1.75 | 1.05 |
| (n) c | (4) | (7) | (4) | (6) | (5) |
| Fatty Acids | |||||
| 14:0 | 0.6 ± 0.0 | 0.6 ± 0.1 | 0.6 ± 0.0 | 0.6 ± 0.1 | 0.6 ± 0.0 |
| 16:0 | 20.3 ± 0.4 a | 19.1 ± 0.9 b | 19.5 ± 1.0 ab | 19.9 ± 0.8 ab | 19.1 ± 0.8 b |
| 17:0 | 0.5 ± 0.2 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 |
| 18:0 | 23.5 ± 0.0 | 22.6 ± 0.7 | 23.1 ± 0.3 | 23.7 ± 1.0 | 22.6 ± 0.3 |
| 16:1n-9 | 0.6 ± 0.0 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 |
| 18:1n-9 | 7.8 ± 1.2 a | 9.4 ± 0.7 b | 8.9 ± 0.8 b | 8.7 ± 0.8 b | 9.4 ± 0.3 b |
| 18:2n-6 | 1.9 ± 0.4 a | 1.7 ± 0.2 b | 1.6 ± 0.1 b | 1.6 ± 0.2 b | 1.7 ± 0.1 b |
| 20:3n-6 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.8 ± 0.1 | 0.8 ± 0.0 |
| 20:4n-6 | 8.8 ± 0.0 a | 8.8 ± 0.6 a | 9.3 ± 0.1 a | 8.6 ± 0.6 b | 8.8 ± 0.1 a |
| 22:4n-6 | 4.2 ± 0.2 a | 3.7 ± 0.1 b | 3.6 ± 0.2 b | 3.1 ± 0.1 c | 3.0 ± 0.1 c |
| 22:5n-6 | 16.9 ± 1.1 a | 14.8 ± 0.7 b | 11.7 ± 0.9 c | 9.3 ± 1.0 d | 5.6 ± 0.5 e |
| 24:4n-6 | 2.5 ± 0.2 a | 2.3 ± 0.2 a | 2.1 ± 0.2 b | 1.9 ± 0.2 b | 1.6 ± 0.1 c |
| 24:5n-6 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 18:3n-3 | nd | nd | nd | nd | nd |
| 20:5n-3 | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.1 ± 0.0 a | 0.2 ± 0.1 a | 0.1 ± 0.0 b |
| 22:5n-3 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.9 ± 0.2 | 1.3 ± 0.1 | 1.5 ± 0.2 |
| 22:6n-3 | 3.9 ± 1.0 a | 7.2 ± 0.9 b | 10.6 ± 1.1 c | 12.2 ± 0.3 d | 16.8 ± 0.3 e |
| 24:5n-3 | nd | 0.2 ± 0.0 a | 0.4 ± 0.0 a | 0.6 ± 0.1 b | 0.8 ± 0.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinclair, A.J.; Guo, X.-F.; Abedin, L. Dietary Alpha-Linolenic Acid Supports High Retinal DHA Levels. Nutrients 2022, 14, 301. https://doi.org/10.3390/nu14020301
Sinclair AJ, Guo X-F, Abedin L. Dietary Alpha-Linolenic Acid Supports High Retinal DHA Levels. Nutrients. 2022; 14(2):301. https://doi.org/10.3390/nu14020301
Chicago/Turabian StyleSinclair, Andrew J., Xiao-Fei Guo, and Lavinia Abedin. 2022. "Dietary Alpha-Linolenic Acid Supports High Retinal DHA Levels" Nutrients 14, no. 2: 301. https://doi.org/10.3390/nu14020301
APA StyleSinclair, A. J., Guo, X.-F., & Abedin, L. (2022). Dietary Alpha-Linolenic Acid Supports High Retinal DHA Levels. Nutrients, 14(2), 301. https://doi.org/10.3390/nu14020301






