Oral Administration of Nicotinamide Mononucleotide Increases Nicotinamide Adenine Dinucleotide Level in an Animal Brain
Abstract
:1. Introduction
2. Materials and Methods
Animals
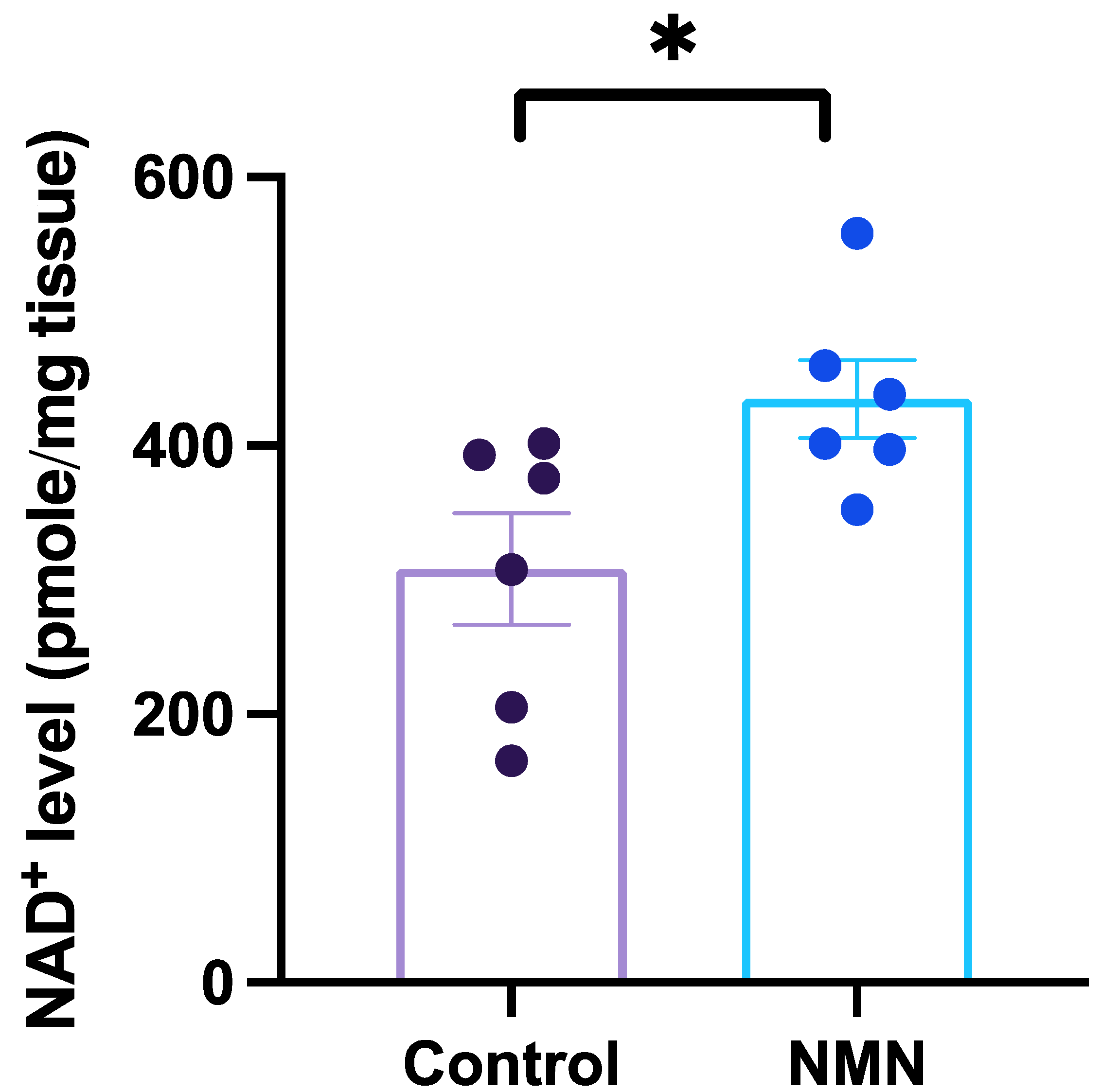
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Goodman, R.P.; Markhard, A.L.; Shah, H.; Sharma, R.; Skinner, O.S.; Clish, C.B.; Deik, A.; Patgiri, A.; Hsu, Y.-H.H.; Masia, R. Hepatic NADH reductive stress underlies common variation in metabolic traits. Nature 2020, 583, 122–126. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in aging: Molecular mechanisms and translational implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Caudal, A.; Abell, L.; Gowda, G.N.; Tian, R. Targeting NAD+ metabolism as interventions for mitochondrial disease. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Baur, J.A.; Imai, S.-I. NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shade, C. The Science Behind NMN—A Stable, Reliable NAD+ Activator and Anti-Aging Molecule. Integr. Med. A Clin. J. 2020, 19, 12. [Google Scholar]
- Wang, G.; Han, T.; Nijhawan, D.; Theodoropoulos, P.; Naidoo, J.; Yadavalli, S.; Mirzaei, H.; Pieper, A.A.; Ready, J.M.; McKnight, S.L. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 2014, 158, 1324–1334. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S.-I. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet-and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poddar, S.K.; Sifat, A.E.; Haque, S.; Nahid, N.A.; Chowdhury, S.; Mehedi, I. Nicotinamide mononucleotide: Exploration of diverse therapeutic applications of a potential molecule. Biomolecules 2019, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, M.J.; Yoshida, M.; Johnson, S.; Takikawa, A.; Usui, I.; Tobe, K.; Nakagawa, T.; Yoshino, J.; Imai, S.-I. SIRT1-mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab. 2015, 21, 706–717. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Yang, W.; Gao, Z.; Jia, P. Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci. Lett. 2017, 647, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peek, C.B.; Affinati, A.H.; Ramsey, K.M.; Kuo, H.-Y.; Yu, W.; Sena, L.A.; Ilkayeva, O.; Marcheva, B.; Kobayashi, Y.; Omura, C. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 2013, 342, 1243417. [Google Scholar] [CrossRef] [Green Version]
- Stromsdorfer, K.L.; Yamaguchi, S.; Yoon, M.J.; Moseley, A.C.; Franczyk, M.P.; Kelly, S.C.; Qi, N.; Imai, S.-I.; Yoshino, J. NAMPT-mediated NAD+ biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 2016, 16, 1851–1860. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.S.; Abraham, D.M.; Hershberger, K.A.; Bhatt, D.P.; Mao, L.; Cui, H.; Liu, J.; Liu, X.; Muehlbauer, M.J.; Grimsrud, P.A. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight 2017, 2, e93885. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Wang, S.-R.; Huang, X.-Z.; Xie, Q.-H.; Xu, Y.-Y.; Shang, D.; Hao, C.-M. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1–dependent manner. J. Am. Soc. Nephrol. 2017, 28, 2337–2352. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.B.; Kubota, S.; Ban, N.; Yoshida, M.; Santeford, A.; Sene, A.; Nakamura, R.; Zapata, N.; Kubota, M.; Tsubota, K. NAMPT-mediated NAD+ biosynthesis is essential for vision in mice. Cell Rep. 2016, 17, 69–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Picciotto, N.E.; Gano, L.B.; Johnson, L.C.; Martens, C.R.; Sindler, A.L.; Mills, K.F.; Imai, S.I.; Seals, D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 2016, 15, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.R.; Imai, S.i. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, J.; Imai, S.-I. Accurate measurement of nicotinamide adenine dinucleotide (NAD+) with high-performance liquid chromatography. Sirtuins 2013, 1077, 203–215. [Google Scholar]
- Glaser, J.A.; Foerst, D.L.; McKee, G.D.; Quave, S.A.; Budde, W.L. Trace analyses for wastewaters. Environ. Sci. Technol. 1981, 15, 1426–1435. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis; Macmillan: New York, NY, USA, 2010. [Google Scholar]
- Hong, W.; Mo, F.; Zhang, Z.; Huang, M.; Wei, X. Nicotinamide mononucleotide: A promising molecule for therapy of diverse diseases by targeting NAD+ metabolism. Front. Cell Dev. Biol. 2020, 8, 246. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.-I.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.-K.; Chong, J.L.; Buhr, E.D.; Lee, C. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.-I. “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 1584–1590. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-Y.; Wang, F.; Zhang, X.-Y.; Huang, P.; Lu, Y.-B.; Wei, E.-Q.; Zhang, W.-P. Nicotinamide phosphoribosyltransferase may be involved in age-related brain diseases. PLoS ONE 2012, 7, e44933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöndorf, D.C.; Ivanyuk, D.; Baden, P.; Sanchez-Martinez, A.; De Cicco, S.; Yu, C.; Giunta, I.; Schwarz, L.K.; Di Napoli, G.; Panagiotakopoulou, V. The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep. 2018, 23, 2976–2988. [Google Scholar] [CrossRef]
- Stein, L.R.; Wozniak, D.F.; Dearborn, J.T.; Kubota, S.; Apte, R.S.; Izumi, Y.; Zorumski, C.F.; Imai, S.-I. Expression of Nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. J. Neurosci. 2014, 34, 5800–5815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; De Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef]
- Liu, D.; Pitta, M.; Jiang, H.; Lee, J.-H.; Zhang, G.; Chen, X.; Kawamoto, E.M.; Mattson, M.P. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: Evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol. Aging 2013, 34, 1564–1580. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Tang, L.; Wei, W.; Hong, Y.; Chen, H.; Ying, W.; Chen, S. Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease. Exp. Ther. Med. 2014, 8, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Long, A.N.; Owens, K.; Schlappal, A.E.; Kristian, T.; Fishman, P.S.; Schuh, R.A. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Long, A.; Owens, K.; Kristian, T. Nicotinamide mononucleotide inhibits post-ischemic NAD+ degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol. Dis. 2016, 95, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Hu, X.; Yang, Y.; Takata, T.; Sakurai, T. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016, 1643, 1–9. [Google Scholar] [CrossRef]
- Johnson, S.; Wozniak, D.F.; Imai, S. CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. npj Aging Mech. Dis. 2018, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Klimova, N.; Long, A.; Kristian, T. Nicotinamide mononucleotide alters mitochondrial dynamics by SIRT3-dependent mechanism in male mice. J. Neurosci. Res. 2019, 97, 975–990. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, L.; Farokhi-Sisakht, F.; Badalzadeh, R.; Khabbaz, A.; Mahmoudi, J.; Sadigh-Eteghad, S. Nicotinamide mononucleotide and melatonin alleviate aging-induced cognitive impairment via modulation of mitochondrial function and apoptosis in the prefrontal cortex and hippocampus. Neuroscience 2019, 423, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Spinnler, R.; Gorski, T.; Stolz, K.; Schuster, S.; Garten, A.; Beck-Sickinger, A.G.; Engelse, M.A.; de Koning, E.J.; Körner, A.; Kiess, W. The adipocytokine Nampt and its product NMN have no effect on beta-cell survival but potentiate glucose stimulated insulin secretion. PLoS ONE 2013, 8, e54106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, G.M.; Youngson, N.A.; Doyle, B.M.; Sinclair, D.A.; Morris, M.J. Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: Comparison with exercise. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, L.; Vafaee, M.S.; Badalzadeh, R. Melatonin and nicotinamide mononucleotide attenuate myocardial ischemia/reperfusion injury via modulation of mitochondrial function and hemodynamic parameters in aged rats. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 240–250. [Google Scholar] [CrossRef]
- Jafari-Azad, A.; Hosseini, L.; Rajabi, M.; Høilund-Carlsen, P.F.; Vafaee, M.S.; Feyzizadeh, S.; Badalzadeh, R. Nicotinamide mononucleotide and melatonin counteract myocardial ischemia-reperfusion injury by activating SIRT3/FOXO1 and reducing apoptosis in aged male rats. Mol. Biol. Rep. 2021, 48, 3089–3096. [Google Scholar] [CrossRef]
- Tsubota, K. The first human clinical study for NMN has started in Japan. npj Aging Mech. Dis. 2016, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Irie, J.; Inagaki, E.; Fujita, M.; Nakaya, H.; Mitsuishi, M.; Yamaguchi, S.; Yamashita, K.; Shigaki, S.; Ono, T.; Yukioka, H. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr. J. 2020, 67, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, M.; Yoshino, J.; Kayser, B.D.; Patti, G.J.; Franczyk, M.P.; Mills, K.F.; Sindelar, M.; Pietka, T.; Patterson, B.W.; Imai, S.-I. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 2021, 372, 1224–1229. [Google Scholar] [CrossRef]
| Mice Model | Intervention | Percentage of NAD+ Increased in Brain Tissues | Effects | Reference |
|---|---|---|---|---|
| Triple transgenic Alzheimer’s disease model mice | NMM (40 μg/g/day) for eight months | Unspecified | Reduced beta amyloid (Aβ), improved brain bioenergetics and preserved mitochondrial functionality. | Liu, et al. [36] |
| C57BL/6N | NMN (i.p. 500 mg/kg/day) single dose. | Hippocampal tissue; 34–39% within 15 min. | Unspecified | Stein and Imai [23] |
| C57BL/6N | NMN (drinking water; 100 or 300 mg/kg/day) for 12 months | Unspecified | Maintain neural stem/progenitor cells proliferation and self-renewal with age. | Stein and Imai [23] |
| PC12 cells (Parkinson’s disease cellular model) | NMN (0.1 mM to 1 mM). The treated cells were incubated for 24 h. | - | Reduced the rotenone-induced apoptosis and restored intracellular NAD+ level and ATP. | Lu, et al. [37] |
| C57BL/6N Adipose tissue-specific Nampt KO (ANKO) | NMN (i.p. 500 mg/kg /day) single dose | Individual hypothalamic nuclei (Arc, VMH, DMH, and LH); 1.5 to 3.5-fold increase 1 h after NMN administration. | Improved physical activity of the mice compared with control in the first half of the 12 h dark time. | Yoon, et al. [13] |
| APPswe/PS1dE9 (AD-Tg) mice | NMN (s.c. 100 mg/kg/day) for every other day for 28 days. | Forebrain tissue was examined after 24 h NMN injection; the % of increased NAD+ level was unspecified. | Increased mitochondrial respiratory function and decreased amyloid precursor protein (APP). | Long, et al. [38] |
| C57BL/6N | MNM (oral gavage; 300 mg/kg) single dose. | Cortex; ~10% increased within 60 min | Unspecified | Mills, et al. [15] |
| C57BL/6N | NMN (drinking water; 100 and 300 mg/kg/day) for 12 months. | Unspecified | Improved the rod cells functions in aged mice. | Mills, et al. [15] |
| C57BL/6 | NMN (i.p. 62.5 mg/kg/day) Single dose. | Hippocampal tissue was examined; the % of increased NAD+ level was unspecified. | Ameliorated hippocampal CA1 injury. | Park, et al. [39] |
| Wister rat (Alzheimer’s diease model) | NMN (i.p. 500 mg/kg/day) for 10 days. | Hippocampal tissue was examined after the treatment; the % of increased NAD+ level was unspecified. | Restored the level of NAD+ and ATP; eliminated ROS accumulation in hippocampal tissue. | Wang, et al. [40] |
| APPswe/PS1dE9 double transgenic (AD-Tg) mice | NMN (s.c. 100 mg/kg/day) every other day for 28 days | Unspecified | Decreased β-amyloid production and increased cognitive function. | Yao, et al. [14] |
| C57BL/6 (CA1-specific Nampt knockdown mice) | NMN (oral gavage. 300 mg/kg/day) for three weeks. | Hippocampal tissue was examined; the % of increased NAD+ level was unspecified. | Increased level of NAD+ and improved cognitive function in old 20-month-old mice. | Johnson, et al. [41] |
| C57BL/6 | NMN (i.p. 62.5 mg/kg/day) single dose. | Hippocampal tissue was examined after 24 h; the % of increased NAD+ level was unspecified. | Reduced mitochondrial fission and ROS in the hippocampus. | Klimova, et al. [42] |
| Wister rats | NMN (i.p. 100 mg/kg/day) every other day for 28 days. | Hippocampal and Prefrontal cortex tissue were examined; the % of increased NAD+ level was unspecified. | Alleviate aging-induced memory impairment; improved mitochondrial function and reduced apoptosis in brain tissues. | Hosseini, et al. [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramanathan, C.; Lackie, T.; Williams, D.H.; Simone, P.S.; Zhang, Y.; Bloomer, R.J. Oral Administration of Nicotinamide Mononucleotide Increases Nicotinamide Adenine Dinucleotide Level in an Animal Brain. Nutrients 2022, 14, 300. https://doi.org/10.3390/nu14020300
Ramanathan C, Lackie T, Williams DH, Simone PS, Zhang Y, Bloomer RJ. Oral Administration of Nicotinamide Mononucleotide Increases Nicotinamide Adenine Dinucleotide Level in an Animal Brain. Nutrients. 2022; 14(2):300. https://doi.org/10.3390/nu14020300
Chicago/Turabian StyleRamanathan, Chidambaram, Thomas Lackie, Drake H. Williams, Paul S. Simone, Yufeng Zhang, and Richard J. Bloomer. 2022. "Oral Administration of Nicotinamide Mononucleotide Increases Nicotinamide Adenine Dinucleotide Level in an Animal Brain" Nutrients 14, no. 2: 300. https://doi.org/10.3390/nu14020300
APA StyleRamanathan, C., Lackie, T., Williams, D. H., Simone, P. S., Zhang, Y., & Bloomer, R. J. (2022). Oral Administration of Nicotinamide Mononucleotide Increases Nicotinamide Adenine Dinucleotide Level in an Animal Brain. Nutrients, 14(2), 300. https://doi.org/10.3390/nu14020300






