The Role of Bovine and Non-Bovine Milk in Cardiometabolic Health: Should We Raise the “Baa”?
Abstract
:1. Cow Milk Consumption and Cardiometabolic Health
2. Cow Milk Alternatives
3. The Composition and Digestibility of Milks of Different Origin
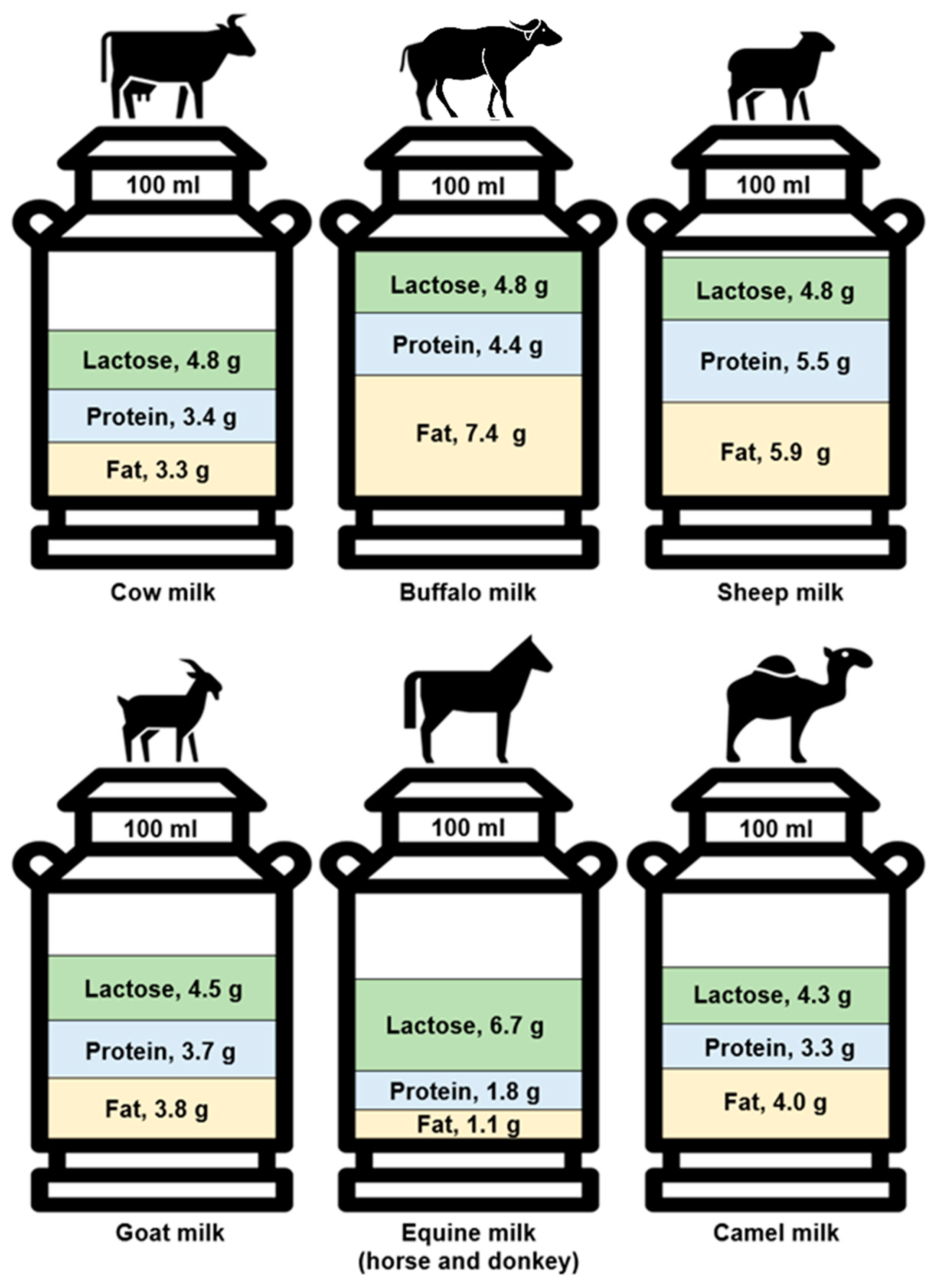
3.1. Composition
3.2. Digestibility
4. The Impact of Milk Origin on Biomarkers of Cardiometabolic Health
4.1. Effects of Milk Origin on Energy Balance & Obesity
4.1.1. Appetite Regulation
4.1.2. Energy Expenditure
4.1.3. Nutrient Processing-Substrate Utilisation and Metabolic Efficiency
4.1.4. Body Weight and Composition
5. Effects of Milk Origin on Insulinaemia, Glycaemia, and Type II Diabetes
5.1. Insulinaemia
5.2. Glycaemia
5.3. Type II Diabetes
6. Effects of Milk Origin on Lipid Metabolism, Aminoacidaemia, and Cardiovascular Health
6.1. Lipidaemia
6.2. Aminoacidaemia
6.3. Cardiovascular Health
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, M.A.; Jacobs, D.R., Jr.; Van Horn, L.; Slattery, M.L.; Kartashov, A.I.; Ludwig, D.S. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The CARDIA Study. JAMA 2002, 287, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.A.; Poppitt, S.D. Milk protein for improved metabolic health: A review of the evidence. Nutr. Metab. 2013, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Chen, C.; Zhu, J.; Tang, W.; Jacobs, D.R.; Shikany, J.M.; Kahe, K. Calcium Intake Is Inversely Related to Risk of Obesity among American Young Adults over a 30-Year Follow-Up. J. Nutr. 2021, 151, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef] [Green Version]
- Poppitt, S.D. Cow’s Milk and Dairy Consumption: Is There Now Consensus for Cardiometabolic Health? Front. Nutr. 2020, 7, 574725. [Google Scholar] [CrossRef]
- Acheson, K.J.; Blondel-Lubrano, A.; Oguey-Araymon, S.; Beaumont, M.; Emady-Azar, S.; Ammon-Zufferey, C.; Monnard, I.; Pinaud, S.; Nielsen-Moennoz, C.; Bovetto, L. Protein choices targeting thermogenesis and metabolism. Am. J. Clin. Nutr. 2011, 93, 525–534. [Google Scholar] [CrossRef]
- Karst, H.; Steiniger, J.; Noack, R.; Steglich, H.D. Diet-induced thermogenesis in man: Thermic effects of single proteins, carbohydrates and fats depending on their energy amount. Ann. Nutr. Metab. 1984, 28, 245–252. [Google Scholar] [CrossRef]
- Lorenzen, J.; Frederiksen, R.; Hoppe, C.; Hvid, R.; Astrup, A. The effect of milk proteins on appetite regulation and diet-induced thermogenesis. Eur. J. Clin. Nutr. 2012, 66, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Harper, A.; James, A.; Flint, A.; Astrup, A. Increased satiety after intake of a chocolate milk drink compared with a carbonated beverage, but no difference in subsequent ad libitum lunch intake. Br. J. Nutr. 2007, 97, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; van Vught, A.J.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.J.; Deutz, N.E.; Westerterp-Plantenga, M.S. Dose-dependent satiating effect of whey relative to casein or soy. Physiol. Behav. 2009, 96, 675–682. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Joanisse, D.R.; Chaput, J.P.; Miegueu, P.; Cianflone, K.; Almeras, N.; Tremblay, A. Milk supplementation facilitates appetite control in obese women during weight loss: A randomised, single-blind, placebo-controlled trial. Br. J. Nutr. 2011, 105, 133–143. [Google Scholar] [CrossRef]
- Ricci, I.; Artacho, R.; Olalla, M. Milk protein peptides with angiotensin I-converting enzyme inhibitory (ACEI) activity. Crit. Rev. Food Sci. Nutr. 2010, 50, 390–402. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Sjogren, P.; Rosell, M.; Skoglund-Andersson, C.; Zdravkovic, S.; Vessby, B.; de Faire, U.; Hamsten, A.; Hellenius, M.L.; Fisher, R.M. Milk-derived fatty acids are associated with a more favorable LDL particle size distribution in healthy men. J. Nutr. 2004, 134, 1729–1735. [Google Scholar] [CrossRef] [Green Version]
- Whigham, L.D.; Watras, A.C.; Schoeller, D.A. Efficacy of conjugated linoleic acid for reducing fat mass: A meta-analysis in humans. Am. J. Clin. Nutr. 2007, 85, 1203–1211. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Leeds, A.A.; Dore, C.J.; Madeiros, S.; Brading, S.; Dornhorst, A. Glycaemic index as a determinant of serum HDL-cholesterol concentration. Lancet 1999, 353, 1045–1048. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Maersk, M.; Belza, A.; Holst, J.J.; Fenger-Gron, M.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: A controlled trial. Eur. J. Clin. Nutr. 2012, 66, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Stampfer, M.J.; Manson, J.E.; Rexrode, K.; Hennekens, C.H.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke 1999, 30, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Folsom, A.R.; Melnick, S.L.; Eckfeldt, J.H.; Sharrett, A.R.; Nabulsi, A.A.; Hutchinson, R.G.; Metcalf, P.A. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: The ARIC study. Atherosclerosis Risk in Communities Study. J. Clin. Epidemiol. 1995, 48, 927–940. [Google Scholar] [CrossRef]
- Massey, L.K. Dairy food consumption, blood pressure and stroke. J. Nutr. 2001, 131, 1875–1878. [Google Scholar] [CrossRef]
- Claeys, W.; Verraes, C.; Cardoen, S.; De Block, J.; Huyghebaert, A.; Raes, K.; Dewettinck, K.; Herman, L. Consumption of raw or heated milk from different species: An evaluation of the nutritional and potential health benefits. Food Control. 2014, 42, 188–201. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Schrezenmeir, J. Milk and the metabolic syndrome. Obes. Rev. 2007, 8, 109–118. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food & Agriculture Organization of the United Nations, FAOSTAT Statistics Database. 2018. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 6 January 2021).
- Itan, Y.; Jones, B.L.; Ingram, C.J.; Swallow, D.M.; Thomas, M.G. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol. Biol. 2010, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Bolin, T.D.; Crane, G.G.; Davis, A.E. Lactose intolerance in various ethnic groups in South-East Asia. Australas. Ann. Med. 1968, 17, 300–306. [Google Scholar] [CrossRef]
- Sahi, T. Genetics and epidemiology of adult-type hypolactasia. Scand. J. Gastroenterol. Suppl. 1994, 202, 7–20. [Google Scholar] [CrossRef]
- Park, Y.W. Overview of Bioactive Components in Milk and Dairy Products. In Bioactive Components in Milk and Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 3–12. [Google Scholar]
- Balthazar, C.F.; Pimentel, T.C.; Ferrao, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef]
- El-Agamy, E.I.; Nawar, M.; Shamsia, S.M.; Awad, S.; Haenlein, G.F. Are camel milk proteins convenient to the nutrition of cow milk allergic children? Small Rumin. Res. 2009, 82, 1–6. [Google Scholar] [CrossRef]
- Sheehan, W.J.; Phipatanakul, W. Tolerance to water buffalo milk in a child with cow milk allergy. Ann. Allergy Asthma. Immunol. 2009, 102, 349. [Google Scholar] [CrossRef] [Green Version]
- Carroccio, A.; Cavataio, F.; Montalto, G.; D’Amico, D.; Alabrese, L.; Iacono, G. Intolerance to hydrolysed cow’s milk proteins in infants: Clinical characteristics and dietary treatment. Clin. Exp. Allergy 2000, 30, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Medhammar, E.; Wijesinha-Bettoni, R.; Stadlmayr, B.; Nilsson, E.; Charrondiere, U.R.; Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J. Sci. Food Agric. 2012, 92, 445–474. [Google Scholar] [CrossRef]
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional value and technological suitability of milk from various animal species used for dairy production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Pietrzak-Fiećko, R.; Tomczyński, R.; Smoczyński, S.S. Effect of lactation period on the fatty acid composition in mares’ milk from different breeds. Arch. Anim. Breed. 2013, 56, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Pietrzak-Fiecko, R.; Kamelska-Sadowska, A.M. The Comparison of Nutritional Value of Human Milk with other Mammals’ Milk. Nutrients 2020, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak-Fiecko, R. Relationship between the Content of Chlorinated Hydrocarbons and Fatty Acid Composition of Milk Fat. J. Vet. Res. 2018, 62, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.; Jiménez-Munguía, M.; Mani-López, E.; López-Malo, A. Plant-Based Milk Alternatives: Types, Processes, Benefits, and Characteristics. Food Rev. Int. 2021, 1–32. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. A comprehensive review on the composition and properties of buffalo milk. Dairy Sci. Technol. 2011, 91, 663–699. [Google Scholar] [CrossRef]
- Sawaya, W.; Khalil, J.; Al-Shalhat, A.; Al-Mohammad, H. Chemical composition and nutritional quality of camel milk. J. Food Sci. 1984, 49, 744–747. [Google Scholar] [CrossRef]
- MS Gorban, A.; Izzeldin, O.M. Fatty acids and lipids of camel milk and colostrum. Int. J. Food Sci. Nutr. 2001, 52, 283–287. [Google Scholar] [CrossRef]
- Jahreis, G.; Fritsche, J.; Möckel, P.; Schöne, F.; Möller, U.; Steinhart, H. The potential anticarcinogenic conjugated linoleic acid, cis-9, trans-11 C18: 2, in milk of different species: Cow, goat, ewe, sow, mare, woman. Nutr. Res. 1999, 19, 1541–1549. [Google Scholar] [CrossRef]
- Welsch, U.; Buchheim, W.; Schumacher, U.; Schinko, I.; Patton, S. Structural, histochemical and biochemical observations on horse milk-fat-globule membranes and casein micelles. Histochemistry 1988, 88, 357–365. [Google Scholar] [CrossRef]
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s milk substitutes for children: Nutritional aspects of milk from different mammalian species, special formula and plant-based beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innocente, N.; Parpinel, M.; Rinaldi, A.; Biasutti, M. 4.8.(S4. 36) Composition and Nutritional Value of Donkey Milk. Bull. Int. Dairy Fed. 2012, 1201, 168. [Google Scholar]
- Zervas, G.; Tsiplakou, E. Goat milk. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 498–518. [Google Scholar]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein proteins: Structural and functional aspects. In Milk Proteins–From Structure to Biological Properties and Health Aspects; IntechOpen: Rijeka, Croatia, 2016; pp. 1–17. [Google Scholar]
- Haenlein, G. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Chilliard, Y.; Rouel, J.; Ferlay, A.; Bernard, L.; Gaborit, P.; Raynal-Ljutovac, K.; Lauret, A.; Leroux, C. Optimising goat’s milk and cheese fatty acid composition. In Improving the Fat Content of Foods; Elsevier: Amsterdam, The Netherlands, 2006; pp. 281–312. [Google Scholar]
- MacGibbon, A.; Taylor, M. Composition and structure of bovine milk lipids. In Advanced Dairy Chemistry Volume 2 Lipids; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–42. [Google Scholar]
- Jahreis, G.; Fritsche, J.; Kraft, J. Species-dependent, seasonal, and dietary variation of conjugated linoleic acid in milk. Adv. Conjug. Linoleic Acid Res. 1999, 1, 215–225. [Google Scholar]
- Martinez-Ferez, A.; Rudloff, S.; Guadix, A.; Henkel, C.A.; Pohlentz, G.; Boza, J.J.; Guadix, E.M.; Kunz, C. Goats’ milk as a natural source of lactose-derived oligosaccharides: Isolation by membrane technology. Int. Dairy J. 2006, 16, 173–181. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Rde, C.A.; Bressan, J.; Paiva, A.C. Effects of protein quality on appetite and energy metabolism in normal weight subjects. Arq. Bras. Endocrinol. Metab. 2010, 54, 45–51. [Google Scholar] [CrossRef] [Green Version]
- FAO. Dietary Protein Quality Evaluation. In Proceedings of the FAO Food and Nutrition, Rome, Italy, 31 March–2 April 2011. [Google Scholar]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Singhal, S.; Baker, R.D.; Baker, S.S. A Comparison of the Nutritional Value of Cow’s Milk and Nondairy Beverages. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein-its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108 (Suppl. 2), S105–S112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef]
- Tsitouras, P.D.; Gucciardo, F.; Salbe, A.D.; Heward, C.; Harman, S.M. High omega-3 fat intake improves insulin sensitivity and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm. Metab. Res. 2008, 40, 199–205. [Google Scholar] [CrossRef] [Green Version]
- St-Onge, M.P.; Jones, P.J. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J. Nutr. 2002, 132, 329–332. [Google Scholar] [CrossRef]
- Ceballos, L.S.; Morales, E.R.; Martinez, L.P.; Extremera, F.G.; Sampelayo, M.R. Utilization of nitrogen and energy from diets containing protein and fat derived from either goat milk or cow milk. J. Dairy Res. 2009, 76, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Michaelidou, A. Factors influencing nutritional and health profile of milk and milk products. Small Rumin. Res. 2008, 79, 42–50. [Google Scholar] [CrossRef]
- El-Zahar, K.; Sitohy, M.; Choiset, Y.; Métro, F.; Haertle, T.; Chobert, J.-M. Peptic hydrolysis of ovine β-lactoglobulin and α-lactalbumin Exceptional susceptibility of native ovine β-lactoglobulin to pepsinolysis. Int. Dairy J. 2005, 15, 17–27. [Google Scholar] [CrossRef]
- Inglingstad, R.A.; Devold, T.G.; Eriksen, E.K.; Holm, H.; Jacobsen, M.; Liland, K.H.; Rukke, E.O.; Vegarud, G.E. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci. Technol. 2010, 90, 549–563. [Google Scholar] [CrossRef] [Green Version]
- El-Zeini, H.M. Microstructure, rheological and geometrical properties of fat globules of milk from different animal species. Pol. J. Food Nutr. Sci. 2006, 56, 147–154. [Google Scholar]
- Argov-Argaman, N. Symposium review: Milk fat globule size: Practical implications and metabolic regulation. J. Dairy Sci. 2019, 102, 2783–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Juárez, M.; Ramos, M.; Haenlein, G. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef] [Green Version]
- Fleming, A.; Schenkel, F.; Chen, J.; Malchiodi, F.; Ali, R.; Mallard, B.; Sargolzaei, M.; Corredig, M.; Miglior, F. Variation in fat globule size in bovine milk and its prediction using mid-infrared spectroscopy. J. Dairy Sci. 2017, 100, 1640–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y. Hypo-allergenic and therapeutic significance of goat milk. Small Rumin. Res. 1994, 14, 151–159. [Google Scholar] [CrossRef]
- Lejeune, M.P.; Kovacs, E.M.; Westerterp-Plantenga, M.S. Additional protein intake limits weight regain after weight loss in humans. Br. J. Nutr. 2005, 93, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendtsen, L.Q.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: A review of the evidence from controlled clinical trials. Adv. Nutr. 2013, 4, 418–438. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.H.; Tecimer, S.N.; Shah, D.; Zafar, T.A. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J. Nutr. 2004, 134, 3011–3015. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.J.; Stote, K.S.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Clevidence, B.A. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J. Nutr. 2011, 141, 1489–1494. [Google Scholar] [CrossRef]
- Rubio-Martin, E.; Garcia-Escobar, E.; Ruiz de Adana, M.S.; Lima-Rubio, F.; Pelaez, L.; Caracuel, A.M.; Bermudez-Silva, F.J.; Soriguer, F.; Rojo-Martinez, G.; Olveira, G. Comparison of the Effects of Goat Dairy and Cow Dairy Based Breakfasts on Satiety, Appetite Hormones, and Metabolic Profile. Nutrients 2017, 9, 877. [Google Scholar] [CrossRef]
- Milan, A.M.; Hodgkinson, A.J.; Mitchell, S.M.; Prodhan, U.K.; Prosser, C.G.; Carpenter, E.A.; Fraser, K.; Cameron-Smith, D. Digestive Responses to Fortified Cow or Goat Dairy Drinks: A Randomised Controlled Trial. Nutrients 2018, 10, 1492. [Google Scholar] [CrossRef] [Green Version]
- Alferez, M.J.; Barrionuevo, M.; Lopez Aliaga, I.; Sanz-Sampelayo, M.R.; Lisbona, F.; Robles, J.C.; Campos, M.S. Digestive utilization of goat and cow milk fat in malabsorption syndrome. J. Dairy Res. 2001, 68, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Cano, M.P.G.; Van Nieuwenhove, C.; Chaila, Z.; Bazan, C.; Gonzalez, S. Effects of short-term mild calorie restriction diet and renutrition with ruminant milks on leptin levels and other metabolic parameters in mice. Nutrition 2009, 25, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Roh, C.; Han, J.; Tzatsos, A.; Kandror, K.V. Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E322–E330. [Google Scholar] [CrossRef] [Green Version]
- Mars, M.; de Graaf, C.; de Groot, C.P.; van Rossum, C.T.; Kok, F.J. Fasting leptin and appetite responses induced by a 4-day 65%-energy-restricted diet. Int. J. Obes. 2006, 30, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Moya, T.; Planes-Munoz, D.; Frontela-Saseta, C.; Ros-Berruezo, G.; Lopez-Nicolas, R. Milk whey from different animal species stimulates the in vitro release of CCK and GLP-1 through a whole simulated intestinal digestion. Food Funct. 2020, 11, 7208–7216. [Google Scholar] [CrossRef]
- Uchida, M.; Ohshiba, Y.; Mogami, O. Novel dipeptidyl peptidase-4-inhibiting peptide derived from beta-lactoglobulin. J. Pharmacol. Sci. 2011, 117, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulipano, G.; Cocchi, D.; Caroli, A.M. Comparison of goat and sheep β-lactoglobulin to bovine β-lactoglobulin as potential source of dipeptidyl peptidase IV (DPP-4) inhibitors. Int. Dairy J. 2012, 24, 97–101. [Google Scholar] [CrossRef]
- Vargas-Bello-Perez, E.; Marquez-Hernandez, R.I.; Hernandez-Castellano, L.E. Bioactive peptides from milk: Animal determinants and their implications in human health. J. Dairy Res. 2019, 86, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. [Google Scholar] [CrossRef]
- Seaton, T.B.; Welle, S.L.; Warenko, M.K.; Campbell, R.G. Thermic effect of medium-chain and long-chain triglycerides in man. Am. J. Clin. Nutr. 1986, 44, 630–634. [Google Scholar] [CrossRef]
- Scalfi, L.; Coltorti, A.; Contaldo, F. Postprandial thermogenesis in lean and obese subjects after meals supplemented with medium-chain and long-chain triglycerides. Am. J. Clin. Nutr. 1991, 53, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Matsuo, M.; Taguchi, N.; Takeuchi, H. The thermic effect is greater for structured medium- and long-chain triacylglycerols versus long-chain triacylglycerols in healthy young women. Metabolism 2001, 50, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Fathi, M.; Mensi, N.; Girardier, L. Twenty-four-hour energy expenditure and urinary catecholamines of humans consuming low-to-moderate amounts of medium-chain triglycerides: A dose-response study in a human respiratory chamber. Eur. J. Clin. Nutr. 1996, 50, 152–158. [Google Scholar]
- Hill, J.O.; Peters, J.C.; Yang, D.; Sharp, T.; Kaler, M.; Abumrad, N.N.; Greene, H.L. Thermogenesis in humans during overfeeding with medium-chain triglycerides. Metabolism 1989, 38, 641–648. [Google Scholar] [CrossRef]
- Matsuo, T.; Takeuchi, H. Effects of structured medium- and long-chain triacylglycerols in diets with various levels of fat on body fat accumulation in rats. Br. J. Nutr. 2004, 91, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Lasekan, J.B.; Rivera, J.; Hirvonen, M.D.; Keesey, R.E.; Ney, D.M. Energy expenditure in rats maintained with intravenous or intragastric infusion of total parenteral nutrition solutions containing medium- or long-chain triglyceride emulsions. J. Nutr. 1992, 122, 1483–1492. [Google Scholar] [CrossRef]
- Posati, L.P.; Orr, M.L. Composition of Foods—Dairy and Egg Products: Raw, Processed, Prepared; Agricultural Research Service, US Department of Agriculture: Beltsville, MD, USA, 1976. [Google Scholar]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [Green Version]
- Ravussin, E.; Lillioja, S.; Anderson, T.E.; Christin, L.; Bogardus, C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J. Clin. Investig. 1986, 78, 1568–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layman, D.K. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261S–267S. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, J.T.; Dillard, C.J.; German, J.B. Milk beyond essential nutrients: The metabolic food. Aust. J. Dairy Technol. 2005, 60, 77. [Google Scholar]
- Dulloo, A.G. The search for compounds that stimulate thermogenesis in obesity management: From pharmaceuticals to functional food ingredients. Obes. Rev. 2011, 12, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Aliaga, I.; Alferez, M.J.; Barrionuevo, M.; Nestares, T.; Sanz Sampelayo, M.R.; Campos, M.S. Study of nutritive utilization of protein and magnesium in rats with resection of the distal small intestine. Beneficial effect of goat milk. J. Dairy Sci. 2003, 86, 2958–2966. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Ranvir, S.; Gandhi, K.; Mann, B. Profiling and distribution of minerals content in cow, buffalo and goat milk. Indian J. Dairy Sci. 2019, 72, 480–488. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.M.; Heaney, R.P.; Recker, R.R.; Lappe, J.M.; Barger-Lux, M.J.; Rafferty, K.; Hinders, S. Calcium intake and body weight. J. Clin. Endocrinol. Metab. 2000, 85, 4635–4638. [Google Scholar] [CrossRef]
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.; Zemel, P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000, 14, 1132–1138. [Google Scholar] [CrossRef]
- Zemel, M.B.; Richards, J.; Milstead, A.; Campbell, P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes. Res. 2005, 13, 1218–1225. [Google Scholar] [CrossRef]
- Booth, A.O.; Huggins, C.E.; Wattanapenpaiboon, N.; Nowson, C.A. Effect of increasing dietary calcium through supplements and dairy food on body weight and body composition: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1013–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, M.A.; Sunehag, A.L.; Rodriguez, L.A.; Haymond, M.W. Galactose promotes fat mobilization in obese lactating and nonlactating women. Am. J. Clin. Nutr. 2011, 93, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Charrière, N.; Montani, J.-P.; Dulloo, A.G. Postprandial thermogenesis and respiratory quotient in response to galactose: Comparison with glucose and fructose in healthy young adults. J. Nutr. Sci. 2016, 5, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.B.; Weiss, J.; Boufath, N. Effects of dietary monosaccharides on sympathetic nervous system activity in adipose tissues of male rats. Diabetes 2004, 53, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Goseki-Sone, M.; Maruyama, R.; Sogabe, N.; Hosoi, T. Effects of dietary lactose on long-term high-fat-diet-induced obesity in rats. Obesity 2007, 15, 2605–2613. [Google Scholar] [CrossRef]
- Cataldi, T.R.; Angelotti, M.; Bianco, G. Determination of mono-and disaccharides in milk and milk products by high-performance anion-exchange chromatography with pulsed amperometric detection. Anal. Chim. Acta 2003, 485, 43–49. [Google Scholar] [CrossRef]
- Mack, P. A preliminary nutrition study of the value of goat’s milk in the diet of children. In Yearbook of the American Goat Society; American Goat Society, Inc.: Mena, AR, USA, 1952. [Google Scholar]
- Razafindrakoto, O.; Ravelomanana, N.; Rasolofo, A.; Rakotoarimanana, R.D.; Gourgue, P.; Coquin, P.; Briend, A.; Desjeux, J.F. Goat’s milk as a substitute for cow’s milk in undernourished children: A randomized double-blind clinical trial. Pediatrics 1994, 94, 65–69. [Google Scholar] [CrossRef]
- Guo, H.Y.; Pang, K.; Zhang, X.Y.; Zhao, L.; Chen, S.W.; Dong, M.L.; Ren, F.Z. Composition, physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. J. Dairy Sci. 2007, 90, 1635–1643. [Google Scholar] [CrossRef]
- Park, Y. Rheological characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 73–87. [Google Scholar] [CrossRef]
- Shamsia, S. Nutritional and therapeutic properties of camel and human milks. Int. J. Genet. Mol. Biol. 2009, 1, 052–058. [Google Scholar]
- Kanwal, R.; Ahmed, T.; Mirza, B. Comparative analysis of quality of milk collected from buffalo, cow, goat and sheep of Rawalpindi/Islamabad region in Pakistan. Asian J. Plant Sci. 2004, 3, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Barłowska, J. Nutritional Value and Technological Usability of Milk From Cows of 7 Breeds Maintained in Poland. Postdoctoral Thesis, Agriculture Academy, University of Life Sciences, Lublin, Poland, 2007. [Google Scholar]
- Mohapatra, A.; Shinde, A.K.; Singh, R. Sheep milk: A pertinent functional food. Small Rumin. Res. 2019, 181, 6–11. [Google Scholar] [CrossRef]
- Park, Y.W.; Mahoney, A.W.; Hendricks, D.G. Bioavailability of iron in goat milk compared with cow milk fed to anemic rats. J. Dairy Sci. 1986, 69, 2608–2615. [Google Scholar] [CrossRef]
- Korish, A.A. The antidiabetic action of camel milk in experimental type 2 diabetes mellitus: An overview on the changes in incretin hormones, insulin resistance, and inflammatory cytokines. Horm. Metab. Res. 2014, 46, 404–411. [Google Scholar] [CrossRef]
- Ha, E.; Zemel, M.B. Functional properties of whey, whey components, and essential amino acids: Mechanisms underlying health benefits for active people (review). J. Nutr. Biochem 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Master, P.B.Z.; Macedo, R.C.O. Effects of dietary supplementation in sport and exercise: A review of evidence on milk proteins and amino acids. Crit. Rev. Food Sci. Nutr. 2021, 61, 1225–1239. [Google Scholar] [CrossRef]
- Astrup, A. The satiating power of protein—A key to obesity prevention? Am. J. Clin. Nutr. 2005, 82, 1–2. [Google Scholar] [CrossRef]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S–1329S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.T.; Astrup, A.; Sjodin, A. Are Dietary Proteins the Key to Successful Body Weight Management? A Systematic Review and Meta-Analysis of Studies Assessing Body Weight Outcomes after Interventions with Increased Dietary Protein. Nutrients 2021, 13, 3193. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [Green Version]
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and health attributes of milk and milk imitations. Eur. J. Nutr. 2020, 59, 19–34. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poppitt, S.D. Milk proteins and human health. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2020; pp. 651–669. [Google Scholar]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Recio, I.; de la Fuente, M.A.; Juárez, M.; Ramos, M. Bioactive components in sheep milk. In Bioactive Components in Milk and Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 83–104. [Google Scholar]
- Poppitt, S.D.; Strik, C.M.; MacGibbon, A.K.; McArdle, B.H.; Budgett, S.C.; McGill, A.T. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol. Behav. 2010, 101, 161–167. [Google Scholar] [CrossRef]
- Kaviani, S.; Cooper, J.A. Appetite responses to high-fat meals or diets of varying fatty acid composition: A comprehensive review. Eur. J. Clin. Nutr. 2017, 71, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Storkson, J.M.; Albright, K.J.; Liu, W.; Pariza, M.W. Evidence that the trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 1999, 34, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Belury, M.A. Dietary conjugated linoleic acid in health: Physiological effects and mechanisms of action. Annu. Rev. Nutr. 2002, 22, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Belury, M.A.; Mahon, A.; Banni, S. The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J. Nutr. 2003, 133, 257S–260S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, S.; Liu, W.; Storkson, J.; Ha, Y.; Pariza, M. Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J. Food Compos. Anal. 1992, 5, 185–197. [Google Scholar] [CrossRef]
- Larsen, T.M.; Toubro, S.; Astrup, A. Efficacy and safety of dietary supplements containing CLA for the treatment of obesity: Evidence from animal and human studies. J. Lipid Res. 2003, 44, 2234–2241. [Google Scholar] [CrossRef] [Green Version]
- Gaullier, J.M.; Halse, J.; Hoivik, H.O.; Hoye, K.; Syvertsen, C.; Nurminiemi, M.; Hassfeld, C.; Einerhand, A.; O’Shea, M.; Gudmundsen, O. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br. J. Nutr. 2007, 97, 550–560. [Google Scholar] [CrossRef] [Green Version]
- den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef] [Green Version]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef]
- Zemel, M.B.; Teegarden, D.; Van Loan, M.; Schoeller, D.; Matkovic, V.; Lyle, R.; Craig, B. Role of dairy products in modulating weight and fat loss: A multi-center trial. FASEB J. 2004, 18, 4–5. [Google Scholar]
- Zemel, M.B. Role of calcium and dairy products in energy partitioning and weight management. Am. J. Clin. Nutr. 2004, 79, 907S–912S. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, F.Q.; Gannon, M.C. Quantitative importance of dietary constituents other than glucose as insulin secretagogues in type II diabetes. Diabetes Care 1988, 11, 72–76. [Google Scholar] [CrossRef]
- Coe, S.; Ryan, L. Impact of polyphenol-rich sources on acute postprandial glycaemia: A systematic review. J. Nutr. Sci. 2016, 5, e24. [Google Scholar] [CrossRef]
- Gunnerud, U.; Holst, J.J.; Ostman, E.; Bjorck, I. The glycemic, insulinemic and plasma amino acid responses to equi-carbohydrate milk meals, a pilot- study of bovine and human milk. Nutr. J. 2012, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Floyd, J.C., Jr.; Fajans, S.S.; Conn, J.W.; Knopf, R.F.; Rull, J. Stimulation of insulin secretion by amino acids. J. Clin. Investig. 1966, 45, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.; Saris, W.H.; Verhagen, H.; Wagenmakers, A.J. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowicz, D.; Froy, O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J. Nutr. Biochem 2013, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Bjorck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O.; Ahren, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetologia 2014, 57, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel milk protein hydrolysates generated with trypsin. J. Funct. Foods 2017, 34, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Song, J.J.; Wang, Q.; Du, M.; Ji, X.M.; Mao, X.Y. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J. Dairy Sci. 2017, 100, 6885–6894. [Google Scholar] [CrossRef] [Green Version]
- Comerford, K.B.; Pasin, G. Emerging Evidence for the Importance of Dietary Protein Source on Glucoregulatory Markers and Type 2 Diabetes: Different Effects of Dairy, Meat, Fish, Egg, and Plant Protein Foods. Nutrients 2016, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Tan, K.W.J.; Han, C.M.S.; Leow, M.K.; Henry, C.J. Impact of preloading either dairy or soy milk on postprandial glycemia, insulinemia and gastric emptying in healthy adults. Eur. J. Nutr. 2015, 56, 77–87. [Google Scholar] [CrossRef]
- Sun, L.; Tan, K.W.; Siow, P.C.; Henry, C.J. Soya milk exerts different effects on plasma amino acid responses and incretin hormone secretion compared with cows’ milk in healthy, young men. Br. J. Nutr. 2016, 116, 1216–1221. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.; Singh, G.; Nayak, K.; Kochar, D.; Sharma, R.; Beniwal, R.; Rastogi, P.; Gupta, R. Prevalence of diabetes in camel-milk consuming Raica rural community of north-west Rajasthan. Int. J. Diab. Dev. Ctries. 2004, 24, 109–114. [Google Scholar]
- Yagil, R.; Zagorski, O.; Van Creveld, C.; Saran, A. Science and Camel’s Milk Production. In Proceedings of the Chameux et Dromedaries, Animaux Laitiers (Dromedaries and Camels, Milking Animals); Saint Martin, G., Ed.; Expansion Scientifique Francais: Paris, France; pp. 75–89. Available online: https://bengreenfieldfitness.com/wp-content/uploads/2017/02/Science-and-camel%E2%80%99s-milk-production.pdf (accessed on 6 January 2021).
- Zagorski, O.; Maman, A.; Yaffe, A.; Meisler, A.; Van Creveld, C.; Yagil, R. Insulin in milk-a comparative study. Int. J. Anim. Sci. 1998, 13, 241–244. [Google Scholar]
- Agrawal, R.P.; Jain, S.; Shah, S.; Chopra, A.; Agarwal, V. Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 1048–1052. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.; Al-Senaidy, A.; Skrzypczak-Jankun, E.; Jankun, J. A study of the anti-diabetic agents of camel milk. Int. J. Mol. Med. 2012, 30, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Trinchese, G.; Cavaliere, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Chun, J.T.; Penna, E.; Negri, R.; Muredda, L.; Demurtas, A.; et al. Human Milk and Donkey Milk, Compared to Cow Milk, Reduce Inflammatory Mediators and Modulate Glucose and Lipid Metabolism, Acting on Mitochondrial Function and Oleylethanolamide Levels in Rat Skeletal Muscle. Front. Physiol. 2018, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Belury, M.; Vanden Heuvel, J. Modulation of diabetes by conjugated linoleic acid. Adv. Conjug. Linoleic Acid Res. 1999, 1, 404–411. [Google Scholar]
- Li, K.; Sinclair, A.J.; Zhao, F.; Li, D. Uncommon Fatty Acids and Cardiometabolic Health. Nutrients 2018, 10, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riserus, U.; Arner, P.; Brismar, K.; Vessby, B. Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care 2002, 25, 1516–1521. [Google Scholar] [CrossRef] [Green Version]
- Ryder, J.W.; Portocarrero, C.P.; Song, X.M.; Cui, L.; Yu, M.; Combatsiaris, T.; Galuska, D.; Bauman, D.E.; Barbano, D.M.; Charron, M.J.; et al. Isomer-specific antidiabetic properties of conjugated linoleic acid. Improved glucose tolerance, skeletal muscle insulin action, and UCP-2 gene expression. Diabetes 2001, 50, 1149–1157. [Google Scholar] [CrossRef] [Green Version]
- Foster-Powell, K.; Miller, J.B. International tables of glycemic index. Am. J. Clin. Nutr. 1995, 62, 871S–890S. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of Physicochemical and Glycaemic Properties of Commercial Plant-Based Milk Substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Ercan, N.; Nuttall, F.Q.; Gannon, M.C.; Redmon, J.B.; Sheridan, K.J. Effects of glucose, galactose, and lactose ingestion on the plasma glucose and insulin response in persons with non-insulin-dependent diabetes mellitus. Metabolism 1993, 42, 1560–1567. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. [Google Scholar] [CrossRef] [Green Version]
- Huth, P.J.; Park, K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012, 3, 266–285. [Google Scholar] [CrossRef] [Green Version]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drouin-Chartier, J.P.; Cote, J.A.; Labonte, M.E.; Brassard, D.; Tessier-Grenier, M.; Desroches, S.; Couture, P.; Lamarche, B. Comprehensive Review of the Impact of Dairy Foods and Dairy Fat on Cardiometabolic Risk. Adv. Nutr. 2016, 7, 1041–1051. [Google Scholar] [CrossRef]
- Greenberger, N.J.; Skillman, T.G. Medium-chain triglycerides. N. Engl. J. Med. 1969, 280, 1045–1058. [Google Scholar] [CrossRef] [Green Version]
- Paszczyk, B.; Tońska, E.; Łuczyńska, J. Health-promoting value of cow, sheep and goat milk and yogurts. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2019, 69, 182–192. [Google Scholar] [CrossRef]
- Penn, D.; Dolderer, M.; Schmidt-Sommerfeld, E. Carnitine concentrations in the milk of different species and infant formulas. Biol. Neonate. 1987, 52, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Uniacke-Lowe, T.; Huppertz, T.; Fox, P.F. Equine milk proteins: Chemistry, structure and nutritional significance. Int. Dairy J. 2010, 20, 609–629. [Google Scholar] [CrossRef]
- Chan, A.H.; D’Souza, R.F.; Beals, J.W.; Zeng, N.; Prodhan, U.; Fanning, A.C.; Poppitt, S.D.; Li, Z.; Burd, N.A.; Cameron-Smith, D. The degree of aminoacidemia after dairy protein ingestion does not modulate the postexercise anabolic response in young men: A randomized controlled trial. J. Nutr. 2019, 149, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; McGregor, R.A.; D’Souza, R.F.; Thorstensen, E.B.; Markworth, J.F.; Fanning, A.C.; Poppitt, S.D.; Cameron-Smith, D. Consumption of Milk Protein or Whey Protein Results in a Similar Increase in Muscle Protein Synthesis in Middle Aged Men. Nutrients 2015, 7, 8685–8699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weijzen, M.E.G.; van Gassel, R.J.J.; Kouw, I.W.K.; Trommelen, J.; Gorissen, S.H.M.; van Kranenburg, J.; Goessens, J.P.B.; van de Poll, M.C.G.; Verdijk, L.B.; van Loon, L.J.C. Ingestion of Free Amino Acids Compared with an Equivalent Amount of Intact Protein Results in More Rapid Amino Acid Absorption and Greater Postprandial Plasma Amino Acid Availability without Affecting Muscle Protein Synthesis Rates in Young Adults in a Double-Blind Randomized Trial. J. Nutr. 2021. [Google Scholar] [CrossRef]
- Sarwar, G.; Botting, H.G.; Davis, T.A.; Darling, P.; Pencharz, P.B. Free amino acids in milks of human subjects, other primates and non-primates. Br. J. Nutr. 1998, 79, 129–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenness, R. Composition and characteristics of goat milk: Review 1968−1979. J. Dairy Sci. 1980, 63, 1605–1630. [Google Scholar] [CrossRef]
- Milan, A.M.; Samuelsson, L.M.; Shrestha, A.; Sharma, P.; Day, L.; Cameron-Smith, D. Circulating Branched Chain Amino Acid Concentrations Are Higher in Dairy-Avoiding Females following an Equal Volume of Sheep Milk Relative to Cow Milk: A Randomized Controlled Trial. Front. Nutr. 2020, 7, 553674. [Google Scholar] [CrossRef] [PubMed]
- Riserus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Salamon, R.; Salamon, S.; Csapó-Kiss, Z.; Csapó, J. Composition of mare’s colostrum and milk I. Fat content, fatty acid composition and vitamin contents. Acta Univ. Sapientiae Aliment. 2009, 2, 119–131. [Google Scholar]
- Barreto, Í.M.L.G.; Rangel, A.H.d.N.; Urbano, S.A.; Bezerra, J.d.S.; Oliveira, C.A.d.A. Equine milk and its potential use in the human diet. Food Sci. Technol. 2019, 39, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension 2001, 37, 1053–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomilehto, J.; Lindstrom, J.; Hyyrynen, J.; Korpela, R.; Karhunen, M.L.; Mikkola, L.; Jauhiainen, T.; Seppo, L.; Nissinen, A. Effect of ingesting sour milk fermented using Lactobacillus helveticus bacteria producing tripeptides on blood pressure in subjects with mild hypertension. J. Hum. Hypertens. 2004, 18, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Turpeinen, A.M.; Ikonen, M.; Kivimaki, A.S.; Kautiainen, H.; Vapaatalo, H.; Korpela, R. A spread containing bioactive milk peptides Ile-Pro-Pro and Val-Pro-Pro, and plant sterols has antihypertensive and cholesterol-lowering effects. Food Funct. 2012, 3, 621–627. [Google Scholar] [CrossRef]
- Turpeinen, A.M.; Jarvenpaa, S.; Kautiainen, H.; Korpela, R.; Vapaatalo, H. Antihypertensive effects of bioactive tripeptides-a random effects meta-analysis. Ann. Med. 2013, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef] [Green Version]
- Politis, I.; Theodorou, G. Angiotensin I-converting (ACE)-inhibitory and anti-inflammatory properties of commercially available Greek yoghurt made from bovine or ovine milk: A comparative study. Int. Dairy J. 2016, 58, 46–49. [Google Scholar] [CrossRef]
- Murakami, M.; Tonouchi, H.; Takahashi, R.; Kitazawa, H.; Kawai, Y.; Negishi, H.; Saito, T. Structural analysis of a new anti-hypertensive peptide (beta-lactosin B) isolated from a commercial whey product. J. Dairy Sci. 2004, 87, 1967–1974. [Google Scholar] [CrossRef] [Green Version]
- Geerlings, A.; Villar, I.; Zarco, F.H.; Sánchez, M.; Vera, R.; Gomez, A.Z.; Boza, J.; Duarte, J. Identification and characterization of novel angiotensin-converting enzyme inhibitors obtained from goat milk. J. Dairy Sci. 2006, 89, 3326–3335. [Google Scholar] [CrossRef]
- Silva, S.V.; Pihlanto, A.; Malcata, F.X. Bioactive peptides in ovine and caprine cheeselike systems prepared with proteases from Cynara cardunculus. J. Dairy Sci. 2006, 89, 3336–3344. [Google Scholar] [CrossRef] [Green Version]
- Morgan, T.; Nowson, C.; Snowden, R.; Teow, B.H.; Hadji, E.; Hodgson, M.; Anderson, A.; Wilson, D.; Adam, W. The effect of sodium potassium, calcium and magnesium on blood pressure. Recent Adv. Clin. Nutr. 1986, 2, 94. [Google Scholar]
- Houston, M.C.; Harper, K.J. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hypertension. J. Clin. Hypertens. 2008, 10, 3–11. [Google Scholar] [CrossRef]
| Milk Origin | Global Milk Production (%) | Global Milk Production (kg) |
|---|---|---|
| Cow | 81.3 | 714,400,000,000 |
| Buffalo | 14.8 | 130,300,000,000 |
| Goat | 2.2 | 18,900,000,000 |
| Sheep | 1.3 | 11,800,000,000 |
| Camel | 0.4 | 3,200,000,000 |
| Milk Origin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cow | Buffalo | Sheep | Goat | Equine | Camel | Soy | Oat | Rice | Almond | |
| Total fat (%) | 3.3 | 7.4 | 5.9 | 3.8 | 1.1 | 4.0 | 2.0 | 2.2 | 1.0 | 1.1 |
| MCT (% of total fat) | 10.5 | 7.1 | 21.8 | 23.0 | 15.2 | 1.5 | n.d. | n.d. | n.d. | 0.2 |
| CLA (% of total fat) | 0.7 | 0.5 | 1.2 | 0.6 | 0.1 | 0.9 | n/a | n/a | n/a | n/a |
| SFA (% of total fat) | 68.4 | 70.8 | 65.0–75.0 | 65.0–73.8 | 38.0–61.0 | 66.1 | 14.3 | 18.9 | 12.0 | 22.6 |
| MFG diameter (µm) | 3.8 | 8.7 | 3.8 | 3.2 | 2.8 | 3.0 | n/a | n/a | n/a | n/a |
| Total protein (%) | 3.4 | 4.4 | 5.5 | 3.7 | 1.8 | 3.3 | 2.6 | 1.0 | 0.5 | 0.6 |
| Casein:whey | 82:18 | 82:18 | 76:24 | 78:22 | 52:48 | 73:27–76:24 | n/a | n/a | n/a | n/a |
| Lactose (%) | 4.8 | 4.8 | 4.8 | 4.5 | 6.9 | 4.3 | n/a | n/a | n/a | n/a |
| Galactose (%) | 4.0 | 3.3 | 0.3 | 0.6 | <0.1 | <0.1 | n/a | n/a | n/a | n/a |
| GI (0–100) | 27–37 | - | - | - | 89.3 (donkey) | - | 31–37 | 69 | 79–92 | 49–64 |
| Energy (kJ) | 316.9–373.0 | 345.0 | 593.2 | 301.8 | 184.2–205.1 | 328.3 | 179.9 | 195.8 | 225.9 | 126.8 |
| Calcium (mg) | 119.8 | 183.9 | 181.7 | 130.4 | 92.9 | 106.0 | 113.0 | 120.0 | 118.0 | 160.0 |
| Potassium (mg) | 145.0 | 101.6 | 120.0 | 181.0 | 50.5 | 156.0 | 122.0 | 162.0 | 27.0 | 67.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penhaligan, J.; Poppitt, S.D.; Miles-Chan, J.L. The Role of Bovine and Non-Bovine Milk in Cardiometabolic Health: Should We Raise the “Baa”? Nutrients 2022, 14, 290. https://doi.org/10.3390/nu14020290
Penhaligan J, Poppitt SD, Miles-Chan JL. The Role of Bovine and Non-Bovine Milk in Cardiometabolic Health: Should We Raise the “Baa”? Nutrients. 2022; 14(2):290. https://doi.org/10.3390/nu14020290
Chicago/Turabian StylePenhaligan, Jack, Sally D. Poppitt, and Jennifer L. Miles-Chan. 2022. "The Role of Bovine and Non-Bovine Milk in Cardiometabolic Health: Should We Raise the “Baa”?" Nutrients 14, no. 2: 290. https://doi.org/10.3390/nu14020290
APA StylePenhaligan, J., Poppitt, S. D., & Miles-Chan, J. L. (2022). The Role of Bovine and Non-Bovine Milk in Cardiometabolic Health: Should We Raise the “Baa”? Nutrients, 14(2), 290. https://doi.org/10.3390/nu14020290