Do Overweight People Have Worse Cognitive Flexibility? Cues-Triggered Food Craving May Have a Greater Impact
Abstract
1. Introduction
2. Method
2.1. Participants
2.2. Procedure
2.2.1. Body Mass Index, the z Score of Fat Mass Index, Skeletal Muscle Mass
2.2.2. Cue-Triggered Food Cravings
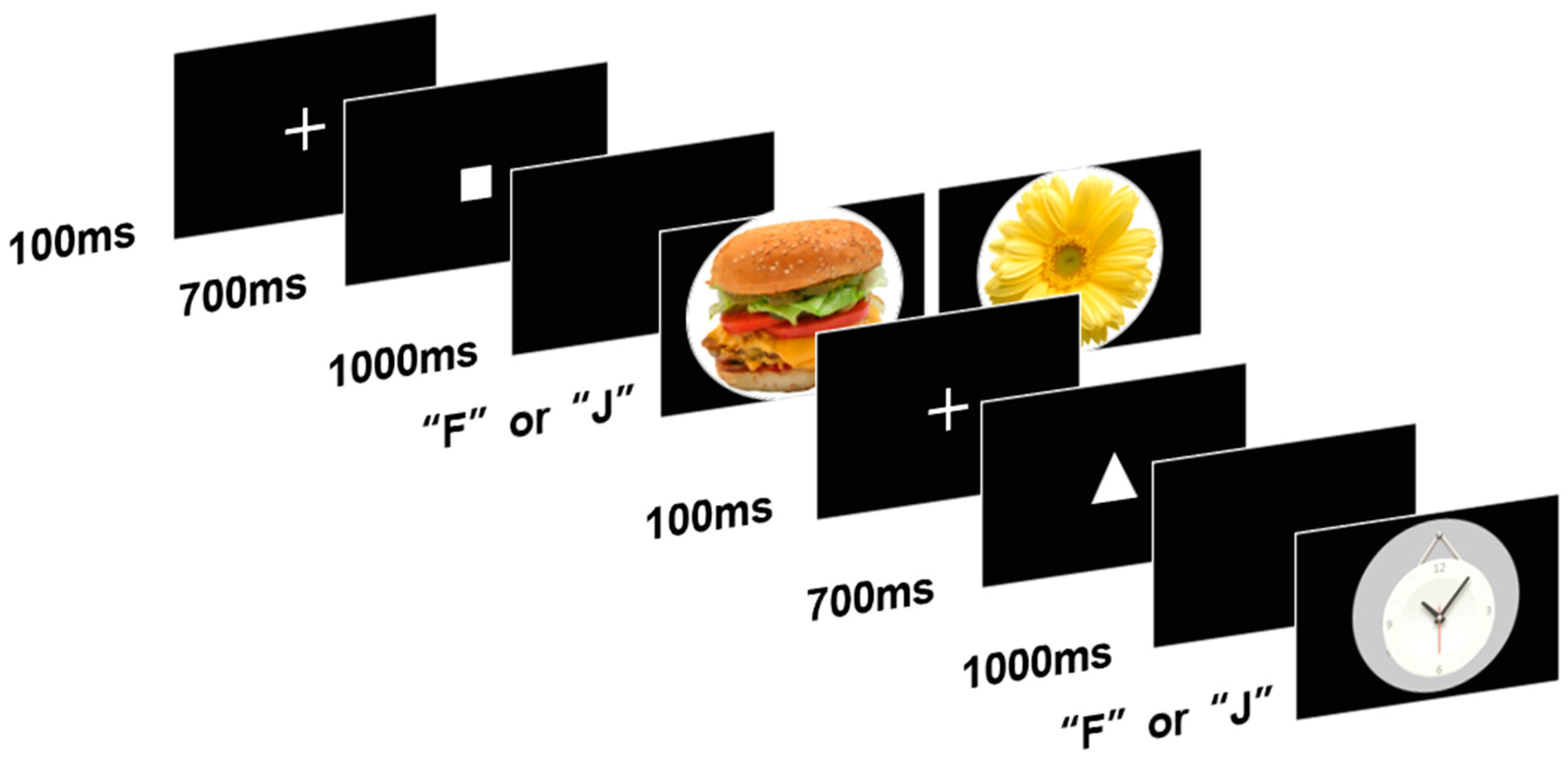
2.2.3. AX-Continuous Performance Test (AX-CPT)
2.3. fMRI Data Acquisition and Processing
2.4. fALFF-Behavior Correlation Analysis
2.5. Region of Interest (ROI)-Wise FC Analysis
2.6. Statistical Analyses
3. Result
3.1. Description and Comparison of Demographics
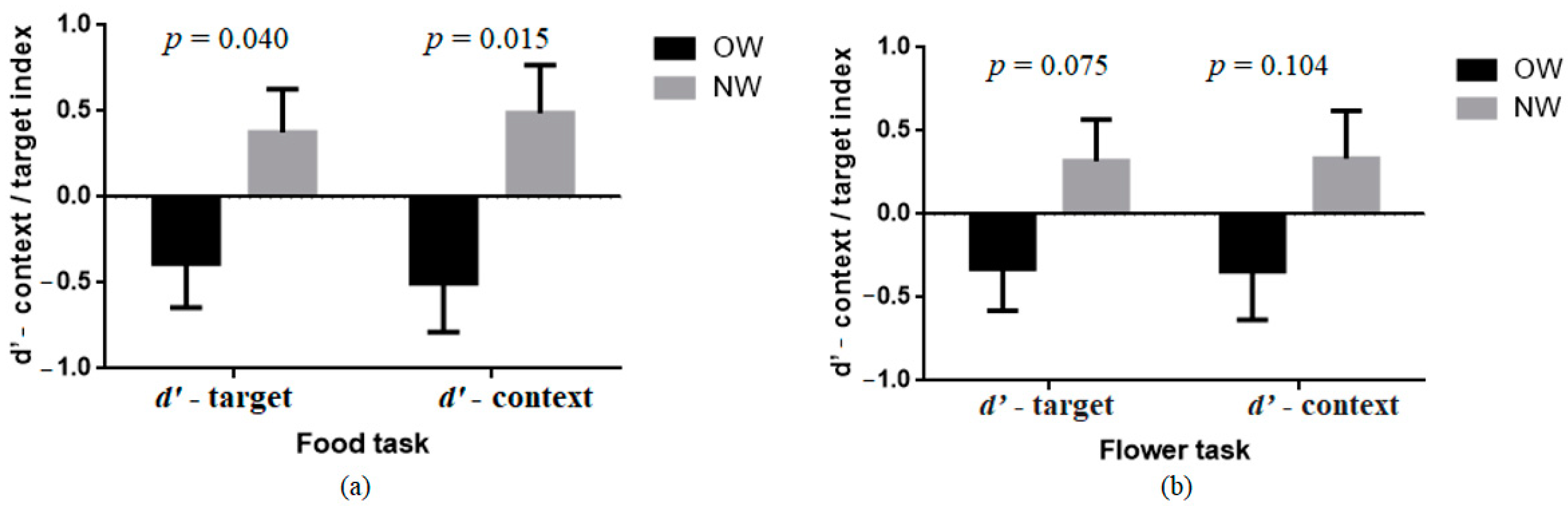
3.2. Group Comparison of d’-Context/Target Index in Food and Flower AX-CPT
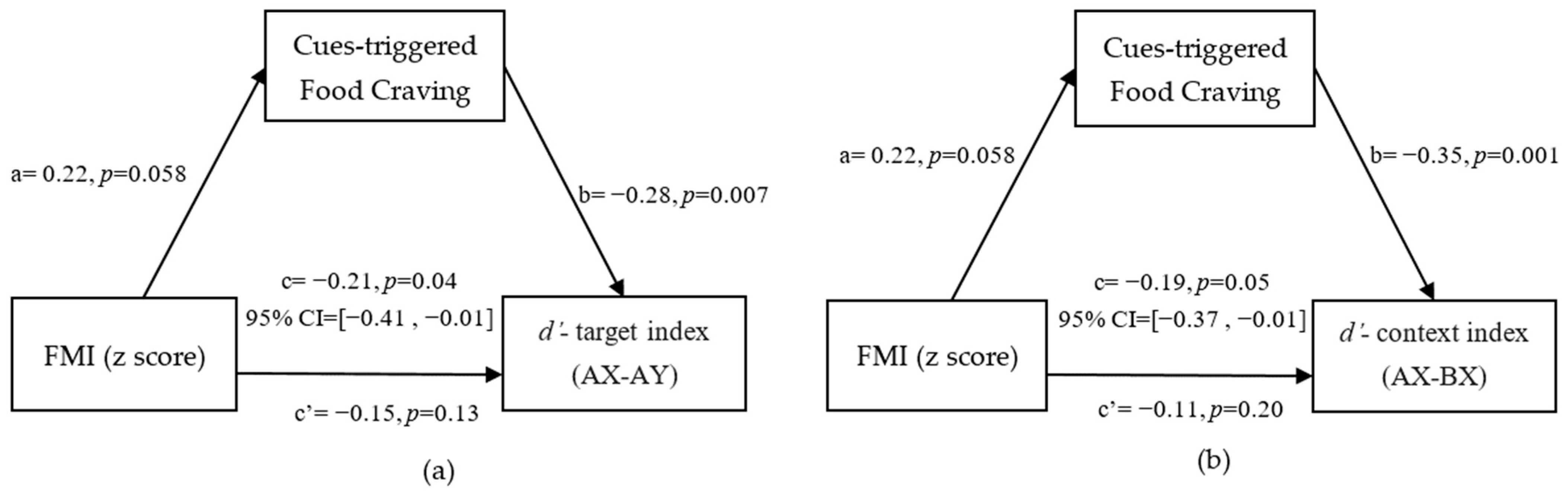
3.3. Mediation from FMI (z-Score) to Reactive and Proactive Control through Cue-Triggered Food Cravings
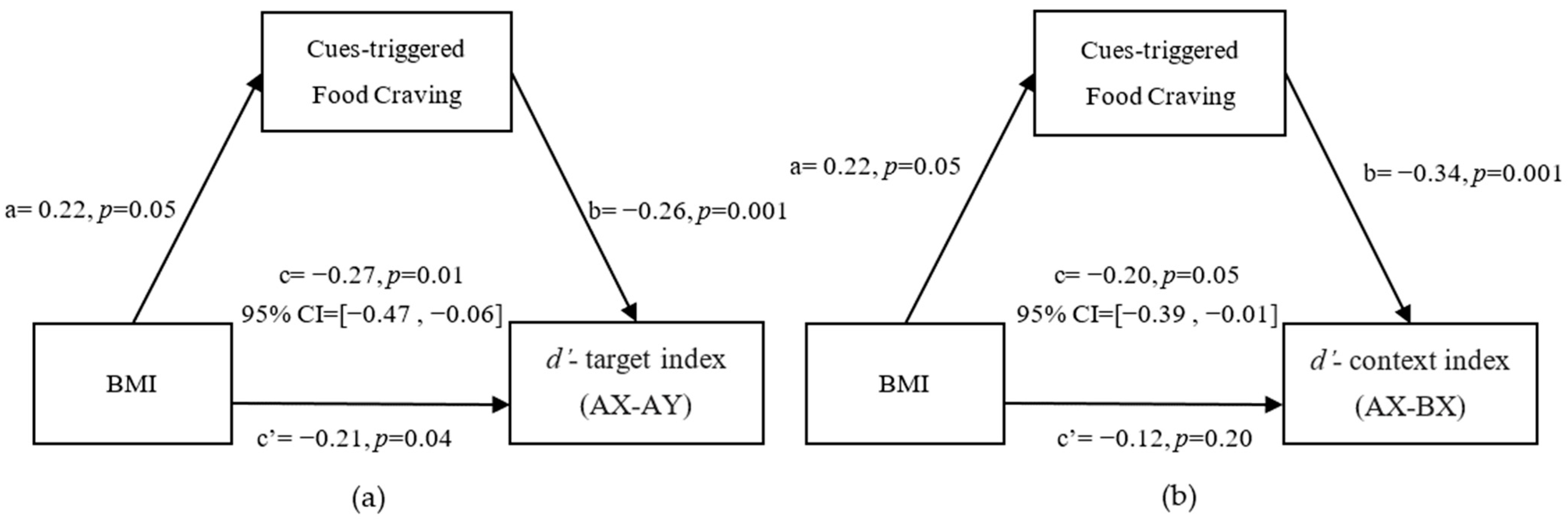
3.4. Mediation from BMI to Reactive and Proactive Control through Cue-Triggered Food Cravings
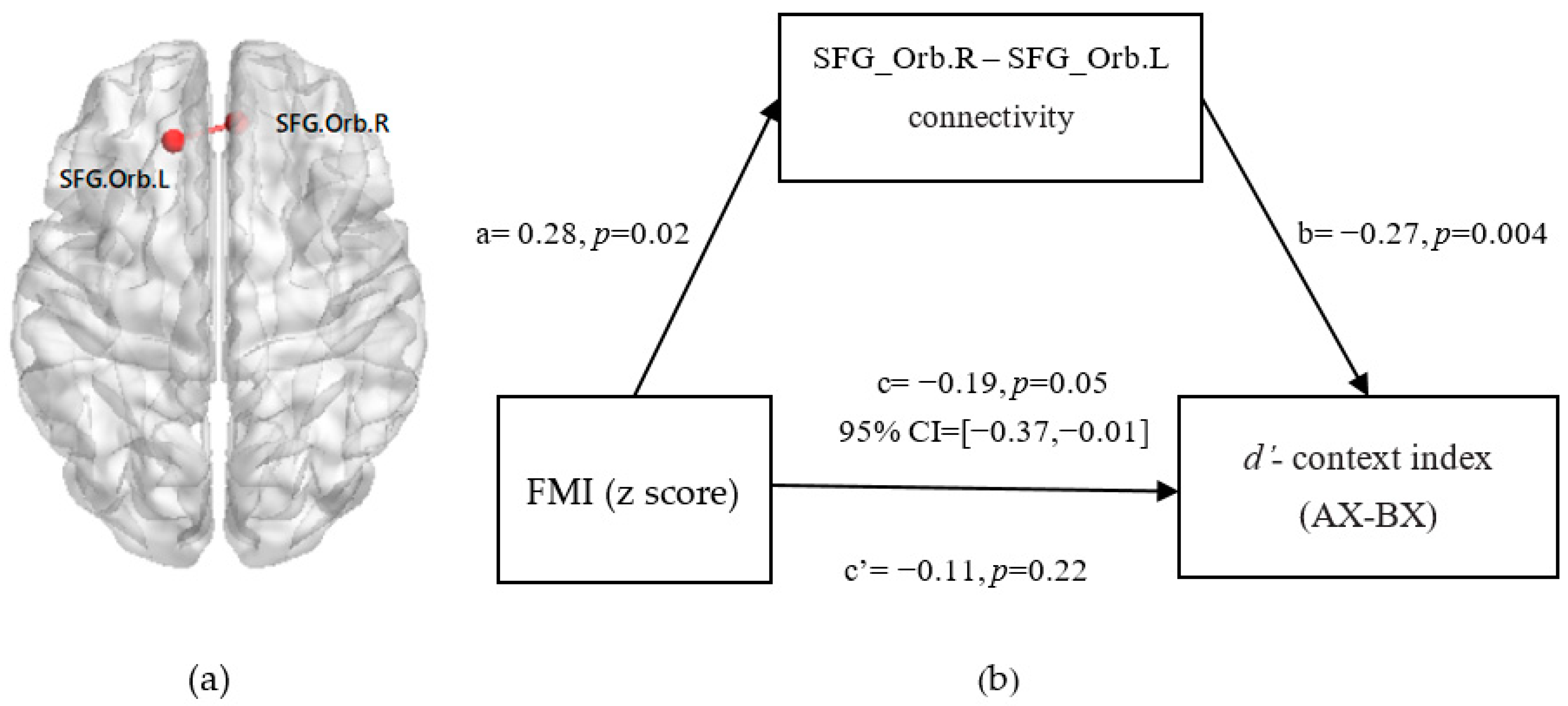
3.5. Mediation from FMI (z-Score) to Reactive and Proactive Control through FC between Bilateral SFG_Orb
3.6. Mediation from BMI to Reactive and Proactive Control through FC between Bilateral SFG_Orb
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. The metabolic syndrome a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014, 383, 970–983. [Google Scholar] [PubMed]
- Wirt, T. Intervention effects of a school-based health promotion programme on obesity related behavioural outcomes. J. Obes. 2014, 2014, 708181. [Google Scholar]
- Raine, L.B. Obesity Visceral Adipose Tissue, and Cognition in Childhood. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2016. [Google Scholar]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Ann. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Groppe, K.; Elsner, B. Executive function and food approach behavior in middle childhood. Front. Psychol. 2014, 5, 447. [Google Scholar] [CrossRef]
- Groppe, K.; Elsner, B. The influence of hot and cool executive function on the development of eating styles related to overweight in children. Appetite 2015, 87, 127–136. [Google Scholar] [CrossRef]
- Braver, T.S.; Paxton, J.L.; Locke, H.S.; Barch, D.M. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2009, 106, 7351–7356. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-García, A.; Pérez-Expósito, M.; Schmidt-Río-Valle, J.; Fernández-Serrano, M.J.; Cruz, F.; Pérez-García, M.; López-Belmonte, G.; Martín-Matillas, M.; Martín-Lagos, J.A.; Marcos, A.; et al. Selective alterations within executive functions in adolescents with excess weight. Obesity 2012, 18, 1572–1578. [Google Scholar] [CrossRef]
- Spitoni, G.F.; Ottaviani, C.; Petta, A.M.; Zingaretti, P.; Aragona, M.; Sarnicola, A.; Antonucci, G. Obesity is associated with lack of inhibitory control and impaired heart rate variability reactivity and recovery in response to food stimuli. Int. J. Psychophys. 2017, 116, 77–84. [Google Scholar] [CrossRef]
- Ruge, H.; Jamadar, S.; Zimmermann, U.; Karayanidis, F. The many faces of preparatory control in task switching: Reviewing a decade of fMRI research. Hum. Brain Mapp. 2013, 34, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brockmeyer, T.; Hartmann, M.; Skunde, M.; Herzog, W.; Friederich, H.C. Reward-related decision making in eating and weight disorders: A systematic review and meta-analysis of the evidence from neuropsychological studies. Neurosci. Biobehav. Rev. 2016, 61, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Deng, Z.Y.; Huang, Q.; Zhang, W.X.; Qi, C.Z.; Huang, J.A. Prefrontal cortexmediated executive function as assessed by Stroop task performance associates with weight loss among overweight and obese adolescents and young adults. Behav. Brain Res. 2017, 321, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Korb, F.M.; Jiang, J.; King, J.A.; Egner, T. Hierarchically organized medial frontal cortex-basal ganglia loops selectively control task- and response-selection. J. Neurosci. 2017, 37, 7893–7905. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Huang, Q.; Huang, J.; Zhang, W.; Qi, C.; Xu, X. Association between central obesity and executive function as assessed by stroop task performance: A functional nearinfrared spectroscopy study. J. Innov. Opt. Health Sci. 2018, 11, 1750010. [Google Scholar] [CrossRef]
- Chevalier, N.; Sheffield, T.D.; Nelson, J.M.; Clark, C.A.C.; Wiebe, S.A.; Espy, K.A. Underpinnings of the costs of flexibility in preschool children: The roles of inhibition and working memory. Dev. Neuropsychol. 2012, 37, 99–118. [Google Scholar] [CrossRef]
- Braver, T.S.; Gray, J.R.; Burgess, G.C. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. Var. Work. Mem. 2007, 75, 106. [Google Scholar]
- Jimura, K.; Locke, H.S.; Braver, T.S. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc. Natl. Acad. Sci. USA 2010, 107, 8871–8876. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.W.; Cohen, J.D.; Stenger, V.A.; Carter, C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef]
- Redick, T.S. Cognitive control in context: Working memory capacity and proactive control. Acta Psychol. 2014, 145, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Quan, H.; Song, S.; Zhang, X.; Yang, C.; Chen, H. Decreased conflict control in overweight Chinese females: Behavioral and event-related potentials evidence. Nutrients 2019, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, Y.; Song, S.; Wang, Y.; Zhang, X.; Chen, H. Effects of food stimuli on event-related potentials of restrained eating subgroups during task switching. Neurosci. Lett. 2021, 754, 135853. [Google Scholar] [CrossRef]
- Ewing, S.W.F.; Claus, E.D.; Hudson, K.A.; Filbey, F.M.; Jimenez, E.Y.; Lisdahl, K.M.; Kong, A.S. Overweight adolescents’ brain response to sweetened beverages mirrors addiction pathways. Brain Imaging Behav. 2017, 11, 925–935. [Google Scholar] [CrossRef]
- Goldman, M.; Szucs-Reed, R.P.; Jagannathan, K.; Ehrman, R.N.; Wang, Z.; Li, Y.; Suh, J.J.; Kampman, K.; O’Brien, C.P.; Childress, A.R.; et al. Reward-related brain response and craving correlates of marijuana cue exposure: A preliminary study in treatment-seeking marijuana-dependent subjects. J. Addict. Med. 2013, 7, 8–16. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Lv, H.; Li, M.Y.; Yu, F.X.; Wang, Z.; Ding, H.Y.; Wang, L.X.; Zhao, K.X.; Zhang, Z.Y.; et al. Integration of neural reward processing and appetite-related signaling in obese females: Evidence from resting-state fMRI. J. Magn. Reason. Imaging 2019, 50, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends cognit. sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed]
- García-García, I.; Jurado, M.Á.; Garolera, M.; Segura, B.; Sala-Llonch, R.; Marqués-Iturria, I.; Junqué, C. Alterations of the salience network in obesity: A resting-state fMRI study. Hum. Brain Mapp. 2013, 34, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Doornweerd, S.; van Duinkerken, E.; de Geus, E.J.; Arbab-Zadeh, P.; Veltman, D.J.; IJzerman, R.G. Overweight is associated with lower resting state functional connectivity in females after eliminating genetic effects: A twin study. Hum. Brain Mapp. 2017, 38, 5069–5081. [Google Scholar] [CrossRef]
- Khan, N.; Raine, L.; Drollette, E.; Scudder, M.; Pontifex, M.; Hillman, C. Differences in cognitive flexibility between healthy weight and obese children: An erp study. Faseb J. 2014, 28, 629-6. [Google Scholar] [CrossRef]
- Schag, K.; Schonleber, J.; Teufel, M.; Zipfel, S.; Giel, K.E. Food-related impulsivity in obesity and binge eating Disorder—A systematic review. Obes. Rev. 2013, 14, 477–495. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee. World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Consultation, W.H.O. Obesity: Preventing and managing the global epidemic. WHO Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Baker, J.F.; Giles, J.T.; Weber, D.; Leonard, M.B.; Zemel, B.S.; Long, J.; Katz, P.P. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology 2017, 56, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.F.; Long, J.; Leonard, M.B.; Harris, T.; Delmonico, M.J.; Santanasto, A.; Weber, D.R. Estimation of skeletal muscle mass relative to adiposity improves prediction of physical performance and incident disability. J. Gerontol. Ser. A 2018, 73, 946–952. [Google Scholar] [CrossRef]
- Stawarczyk, D.; Majerus, S.; Catale, C.; D’Argembeau, A. Relationships between mind-wandering and attentional control abilities in young adults and adolescents. Acta Psychol. 2014, 148, 25–36. [Google Scholar] [CrossRef]
- Stanislaw, H.; Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999, 31, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Pauli-Pott, U.; Albayrak, O.; Hebebrand, J.; Pott, W. Does inhibitory control capacity in overweight and obese children and adolescents predict success in a weight-reduction program? Eur. Child Adolesc. Psychiatry 2010, 19, 135–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lawrence, M.; Claire, H.; Victoria, S.; Antonio, C. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity 2011, 19, 1382–1387. [Google Scholar]
- Reyes, S.; Peirano, P.; Peigneux, P.; Lozoff, B.; Algarin, C. Inhibitory control in otherwise healthy overweight 10-year-old children. Int. J. Obes. 2015, 39, 1230–1235. [Google Scholar] [CrossRef]
- Aviram-Friedman, R.; Kafri, L.; Baz, G.; Alyagon, U.; Zangen, A. Prisoners of addictive cues: Biobehavioral markers of overweight and obese adults with food addiction. Nutrients 2020, 12, 3563. [Google Scholar] [CrossRef] [PubMed]
- Werthmann, J.; Roefs, A.; Nederkoorn, C.; Mogg, K.; Bradley, B.P.; Jansen, A. Can (not) take my eyes off it: Attention bias for food in overweight participants. Health Psychol. 2012, 30, 561. [Google Scholar] [CrossRef]
- Hardman, C.A.; Jones, A.; Burton, S.; Duckworth, J.J.; McGale, L.S.; Mead, B.R.; Roberts, C.; Field, M.; Werthmann, J. Food-related attentional bias and its associations with appetitive motivation and body weight: A systematic review and meta-analysis. Appetite 2020, 157, 104986. [Google Scholar] [CrossRef]
- Pessoa, L. How do emotion and motivation direct executive control? Trends Cognitive Sci. 2009, 13, 160–166. [Google Scholar] [CrossRef]
- Braem, S.; King, J.A.; Korb, F.M.; Krebs, R.M.; Notebaert, W.; Egner, T. Affective modulation of cognitive control is determined by performance-contingency and mediated by ventromedial prefrontal and cingulate cortex. J. Neurosci. 2013, 33, 16961–16970. [Google Scholar] [CrossRef]
- Grabenhorst, F.; Rolls, E.T.; Bilderbeck, A. How cognition modulates affective responses to taste and flavor: Top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb. Cortex 2008, 18, 1549–1559. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; O’Doherty, J.; Rolls, E.T.; Andrews, C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb. Cortex 2003, 13, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Lacadie, C.; Skudlarski, P.; Fulbright, R.K.; Rounsaville, B.J.; Kosten, T.R.; Wexler, B.E. Neural activity associated with stress-induced cocaine craving: A functional magnetic resonance imaging study. Psychopharmacology 2005, 183, 171–180. [Google Scholar] [CrossRef]
- Grüsser, S.M.; Wrase, J.; Klein, S.; Hermann, D.; Smolka, M.N.; Ruf, M.; Weber-Fahr, W.; Flor, H.; Mann, K.; Braus, D.F.; et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology 2004, 175, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Filbey, F.M.; Schacht, J.P.; Myers, U.S.; Chavez, R.S.; Hutchison, K.E. Marijuana craving in the brain. Proc. Natl. Acad. Sci. USA 2009, 106, 13016–13021. [Google Scholar] [CrossRef] [PubMed]
- Demos, K.E.; Heatherton, T.F.; Kelley, W.M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012, 32, 5549–5552. [Google Scholar] [CrossRef]
- Stoeckel, L.E.; Weller, R.E.; Cook, E.W.; Twieg, D.B.; Knowlton, R.C.; Cox, J.E. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008, 41, 636–647. [Google Scholar] [CrossRef]
- Yokum, S.; Ng, J.; Stice, E. Attentional bias to food images associated with elevated weight and future weight gain: An fMRI study. Obesity 2011, 19, 1775–1783. [Google Scholar] [CrossRef]
- Donofry, S.D.; Jakicic, J.M.; Rogers, R.J.; Watt, J.C.; Roecklein, K.A.; Erickson, K.I. Comparison of Food Cue–Evoked and Resting-State Functional Connectivity in Obesity. Psychosom. Med. 2020, 82, 261. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Padilla, M.; Verdejo-Román, J.; Fernández-Serrano, M.J.; Del Paso, G.A.R.; Verdejo-García, A. Increased food choice-evoked brain activation in adolescents with excess weight: Relationship with subjective craving and behavior. Appetite 2018, 131, 7–13. [Google Scholar] [CrossRef]
- Manippa, V.; van der Laan, L.N.; Brancucci, A.; Smeets, P.A. Health body priming and food choice: An eye tracking study. Food Qual. Prefer. 2019, 72, 116–125. [Google Scholar] [CrossRef]
- Cano-Ibáñez, N.; Gea, A.; Ruiz-Canela, M.; Corella, D.; Salas-Salvadó, J.; Schröder, H.; Navarrete-Muñoz, E.M.; Romaguera, D.; Martínez, J.A.; Barón-López, F.J.; et al. Diet quality and nutrient density in subjects with metabolic syndrome: Influence of socioeconomic status and lifestyle factors. A cross-sectional assessment in the PREDIMED-Plus study. Clin. Nutr. 2020, 39, 1161–1173. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The epidemiology of obesity: A big picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef]
- Egnell, M.; Seconda, L.; Neal, B.; Mhurchu, C.N.; Rayner, M.; Jones, A.; Touvier, M.; Kesse-Guyot, E.; Hercberg, S.; Julia, C. Prospective associations of the original Food Standards Agency nutrient profiling system and three variants with weight gain, overweight and obesity risk: Results from the French NutriNet-Santé cohort. Br. J. Nutr. 2021, 125, 902–914. [Google Scholar] [CrossRef]
- Kakoschke, N.; Hawker, C.; Castine, B.; de Courten, B.; Verdejo-Garcia, A. Smartphone-Based cognitive bias modification training improves healthy food choice in obesity: A pilot study European Eating Disorders Review. Eur. Eat. Disord. Rev. 2018, 26, 526–532. [Google Scholar] [CrossRef]

| Overweight Group (n = 34) | Normal-Weight Group (n = 35) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Age (years) | 19.71 | 1.40 | 19.63 | 0.69 | 0.29 | 0.772 |
| Sex (male%) | 50% | - | 37% | - | 1.07 | 0.288 |
| Hunger | 66.82 | 23.62 | 54.51 | 23.78 | 2.16 | 0.035 |
| BMI (kg/m2) | 27.65 | 2.49 | 21.16 | 1.66 | 12.79 | 0.001 |
| FMI (z score) | 0.42 | 0.75 | −0.88 | 0.76 | 7.10 | 0.001 |
| SMM (kg) | 22.98 | 4.38 | 20.66 | 4.98 | 2.67 | 0.107 |
| OW (n = 34) | NW (n = 35) | |||||
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | F | p | η2 | ||
| Food task | d’-target index | −0.39 (1.94) | 0.38 (0.99) | 4.39 * | 0.040 | 0.06 |
| d’-context index | −0.50 (2.09) | 0.49 (1.00) | 6.26 * | 0.015 | 0.09 | |
| Flower task | d’-target index | −0.33 (1.82) | 0.32 (1.10) | 3.27 | 0.075 | 0.05 |
| d’-context index | −0.34 (2.20) | 0.33 (1.05) | 2.72 | 0.104 | 0.04 |
| Regions | Side | BA | Peak MNI Coordinates | Peak Intensity | Cluster Size (Voxels) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Correlation with fALFF | |||||||
| Superior frontal Gyrus_Orb | R | 11 | 6 | 42 | −27 | 4.105 | 24 |
| SFG_Orb as the seed | |||||||
| Superior frontal Gyrus_Orb | L | 11 | −15 | 36 | −24 | 5.359 | 55 |
| Superior frontal Gyrus_Orb | R | 11 | 12 | 47 | −18 | 6.374 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Li, Q.; Jiang, Y.; Liu, Y.; Xu, A.; Liu, X.; Chen, H. Do Overweight People Have Worse Cognitive Flexibility? Cues-Triggered Food Craving May Have a Greater Impact. Nutrients 2022, 14, 240. https://doi.org/10.3390/nu14020240
Song S, Li Q, Jiang Y, Liu Y, Xu A, Liu X, Chen H. Do Overweight People Have Worse Cognitive Flexibility? Cues-Triggered Food Craving May Have a Greater Impact. Nutrients. 2022; 14(2):240. https://doi.org/10.3390/nu14020240
Chicago/Turabian StyleSong, Shiqing, Qingqing Li, Yan Jiang, Yong Liu, Aidi Xu, Xinyuan Liu, and Hong Chen. 2022. "Do Overweight People Have Worse Cognitive Flexibility? Cues-Triggered Food Craving May Have a Greater Impact" Nutrients 14, no. 2: 240. https://doi.org/10.3390/nu14020240
APA StyleSong, S., Li, Q., Jiang, Y., Liu, Y., Xu, A., Liu, X., & Chen, H. (2022). Do Overweight People Have Worse Cognitive Flexibility? Cues-Triggered Food Craving May Have a Greater Impact. Nutrients, 14(2), 240. https://doi.org/10.3390/nu14020240







