Plant-Based Dietary Patterns versus Meat Consumption and Prevalence of Impaired Glucose Intolerance and Diabetes Mellitus: A Cross-Sectional Study in Australian Women
Abstract
1. Introduction
2. Materials and Methods
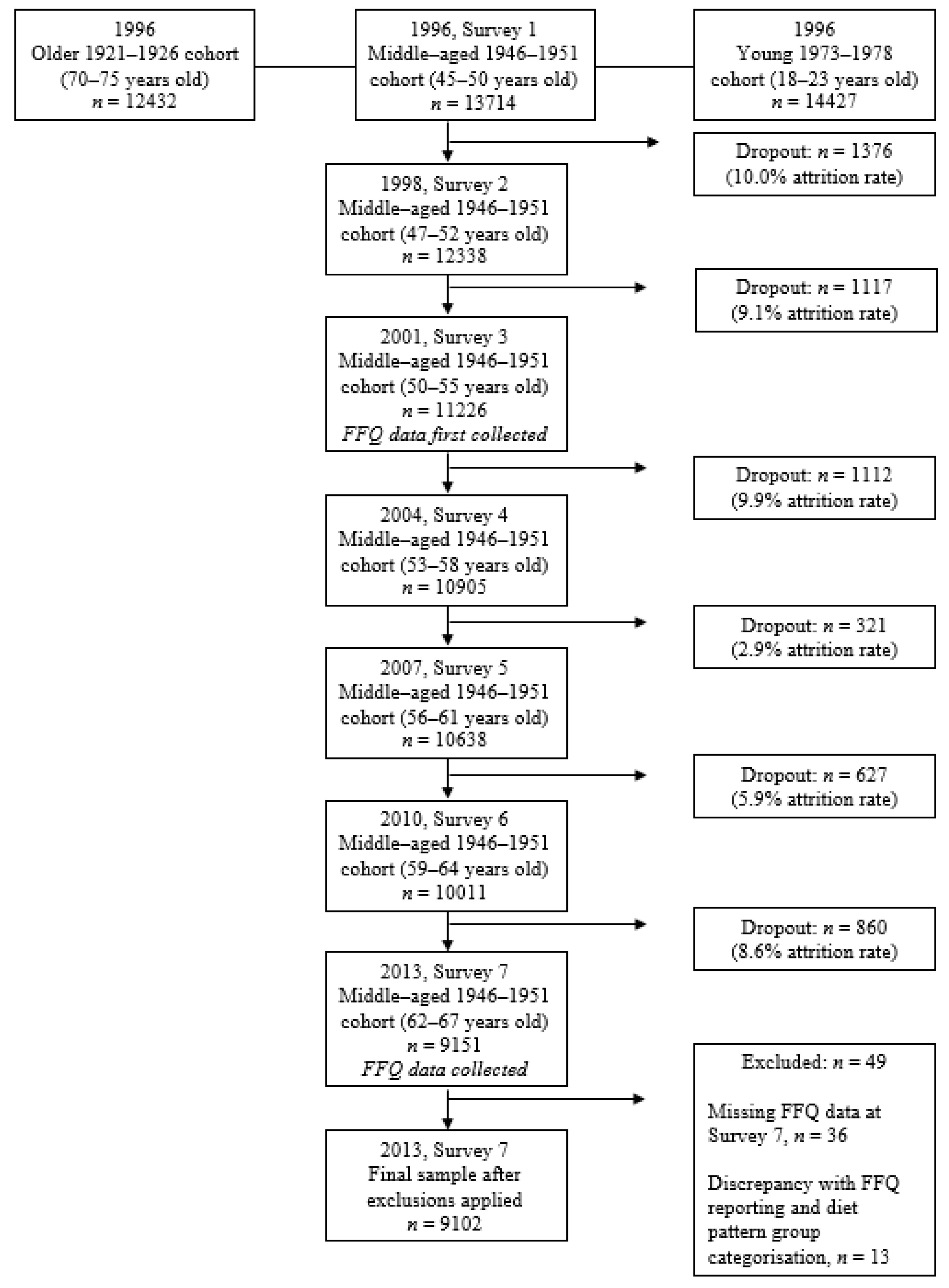
2.1. The Australian Longitudinal Study on Women’s Health (ALSWH)
2.2. Plant-Based Dietary Patterns
2.3. Frequency of Meat Consumption
2.4. Impaired Glucose Tolerance and Diabetes
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Prevalence of IGT and Diabetes across Dietary Patterns
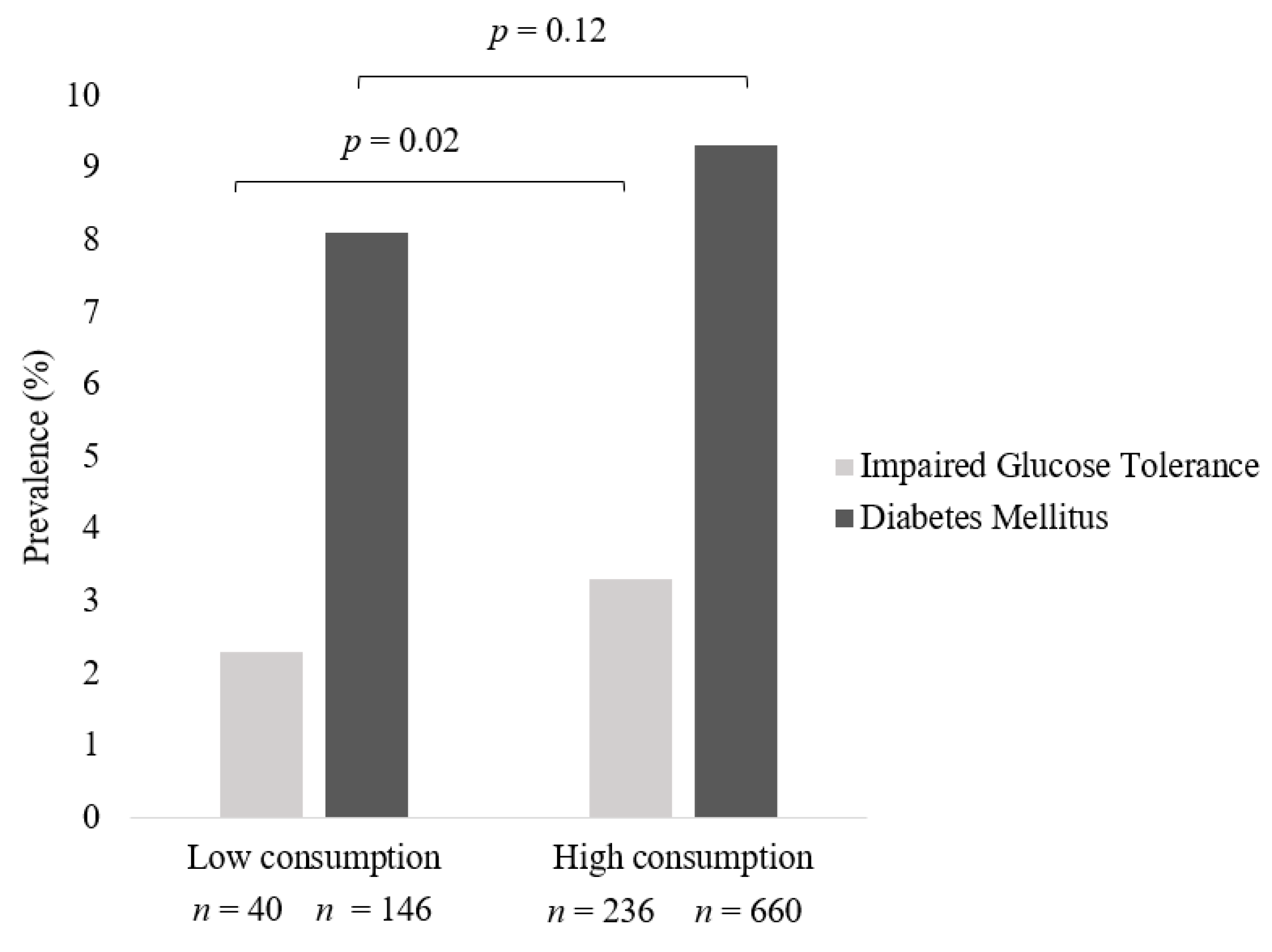
3.3. Prevalence of IGT and Diabetes across Frequency of Meat Intake in All Meat Eaters
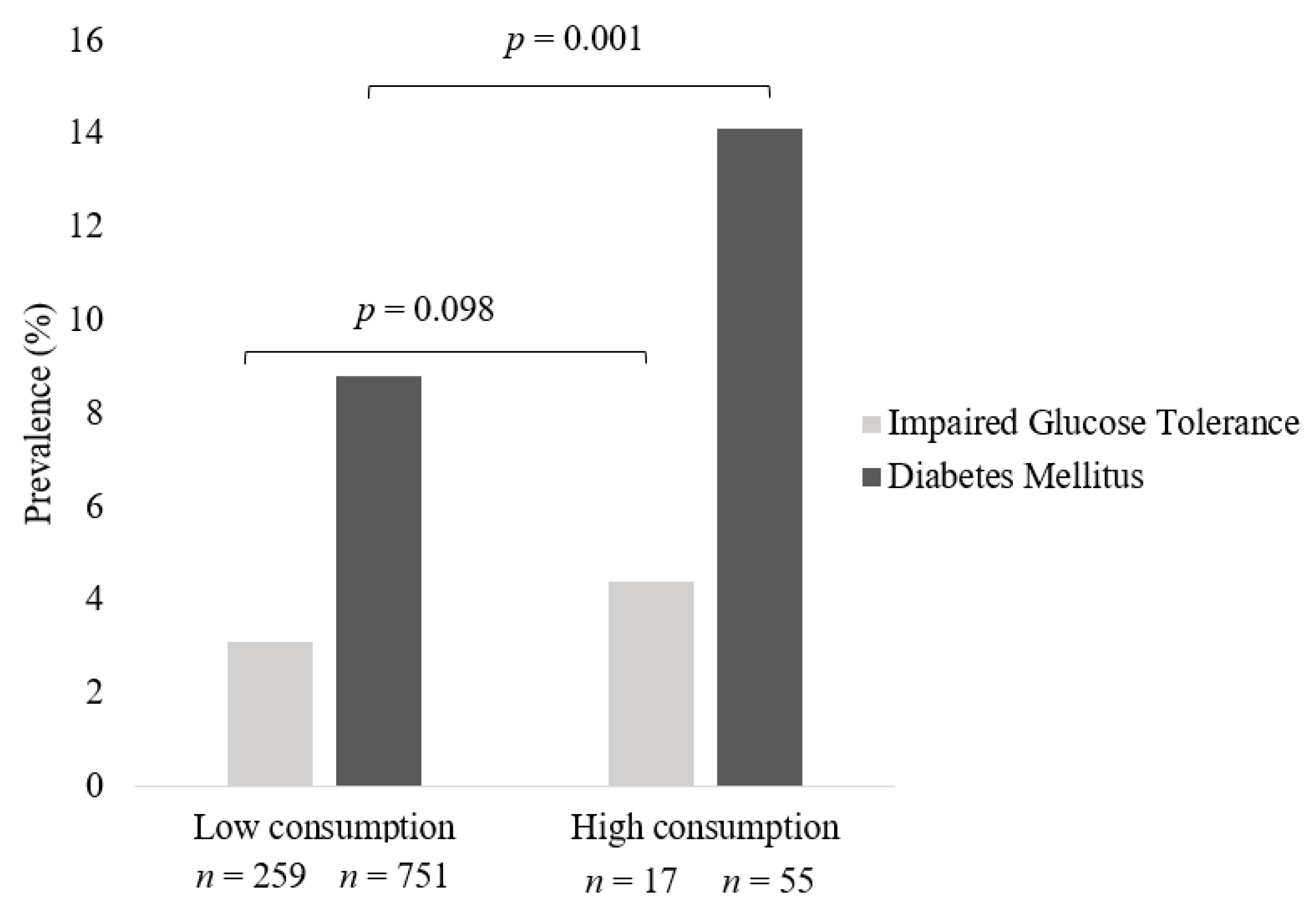
3.4. Prevalence of IGT and Diabetes across Frequency of Processed Meat Intake in All Meat Eaters
3.5. Odds of IGT and Diabetes in All Meat Eaters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A.; Stojanovski, E.; McEvoy, M.; Garg, M.L. Macronutrient Intakes and Development of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. J. Am. Coll. Nutr. 2012, 31, 243–258. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Diabetes [Internet]. 2021 [updated 10 November 2021]. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 10 April 2022).
- Australian Institue of Health and Welfare. Diabetes Canberra: Australian Institue of Health and Welfare. 2020. Available online: https://www.aihw.gov.au/reports/diabetes/diabetes (accessed on 21 March 2021).
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Diabetes Australia. Type 2 Diabetes 2020. Available online: https://www.diabetesaustralia.com.au/about-diabetes/type-2-diabetes/ (accessed on 21 March 2021).
- Diabetes Australia. Pre Diabetes Turner, ACT: Diabetes Australia. 2021. Available online: https://www.diabetesaustralia.com.au/about-diabetes/pre-diabetes/ (accessed on 21 March 2021).
- Fardet, A.; Boirie, Y. Associations between food and beverage groups and major diet-related chronic diseases: An exhaustive review of pooled/meta-analyses and systematic reviews. Nutr. Rev. 2014, 72, 741–762. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.P.; Mann, J.; Bennett, P.H.; Temple, N.; Zimmet, P.; Tuomilehto, J.; Lindström, J.; Louheranta, A. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004, 7, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Horikawa, C.; Fujihara, K.; Heianza, Y.; Hirasawa, R.; Yachi, Y.; Sugawara, A.; Tanaka, S.; Shimano, H.; Iida, K.T.; et al. Comparisons of the Strength of Associations With Future Type 2 Diabetes Risk Among Anthropometric Obesity Indicators, Including Waist-to-Height Ratio: A Meta-Analysis. Am. J. Epidemiol. 2012, 176, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Australia. National Position Statement Weight Loss Surgery (Bariatric Surgery) and Its Use in Treating Obesity or Treating and Preventing Diabetes Turner ACT: Diabetes Australia. 2011. Available online: https://www.diabetesaustralia.com.au/wp-content/uploads/Position-statement-Bariatric-Surgery.pdf (accessed on 21 March 2021).
- Lea, E.; Worsley, A. Benefits and barriers to the consumption of a vegetarian diet in Australia. Public Health Nutr. 2003, 6, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Corrin, T.; Papadopoulos, A. Understanding the attitudes and perceptions of vegetarian and plant-based diets to shape future health promotion programs. Appetite 2017, 109, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Olfert, M.D.; Wattick, R.A. Vegetarian Diets and the Risk of Diabetes. Curr. Diabetes Rep. 2018, 18, 101. [Google Scholar] [CrossRef]
- Barnard, N.D.; Katcher, H.I.; Jenkins, D.J.; Cohen, J.; Turner-McGrievy, G. Vegetarian and vegan diets in type 2 diabetes management. Nutr. Rev. 2009, 67, 255–263. [Google Scholar] [CrossRef]
- Ostfeld, R.J. Definition of a plant-based diet and overview of this special issue. J. Geriatr. Cardiol. 2017, 14, 315. [Google Scholar]
- Mihrshahi, S.; Ding, D.; Gale, J.; Allman-Farinelli, M.; Banks, E.; Bauman, A.E. Vegetarian diet and all-cause mortality: Evidence from a large population-based Australian cohort—the 45 and Up Study. Prev. Med. 2017, 97, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.; Ferguson, J.J.A.; Garg, M.L. Effects of Plant-Based Diets on Weight Status in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 4099. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Vang, A.; Singh, P.N.; Lee, J.W.; Haddad, E.H.; Brinegar, C.H. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: Findings from Adventist Health Studies. Ann. Nutr. Metab. 2008, 52, 96–104. [Google Scholar] [CrossRef]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef]
- Micha, R.; Michas, G.; Mozaffarian, D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes--an updated review of the evidence. Curr. Atheroscler. Rep. 2012, 14, 515–524. [Google Scholar] [CrossRef]
- Chen, Z.; Zuurmond, M.G.; van der Schaft, N.; Nano, J.; Wijnhoven, H.A.H.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Eur. J. Epidemiol. 2018, 33, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Pribis, P.; Pencak, R.C.; Grajales, T. Beliefs and attitudes toward vegetarian lifestyle across generations. Nutrients 2010, 2, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; MacDonald-Wicks, L.K.; McEvoy, M.A.; Forder, P.M.; Holder, C.; Oldmeadow, C.; Byles, J.E.; Patterson, A.J. Better diet quality scores are associated with a lower risk of hypertension and non-fatal CVD in middle-aged Australian women over 15 years of follow-up. Public Health Nutr. 2020, 23, 882–893. [Google Scholar] [CrossRef]
- Brown, W.J.; Bryson, L.; Byles, J.E.; Dobson, A.J.; Lee, C.; Mishra, G.; Schofield, M. Women’s Health Australia: Recruitment for a national longitudinal cohort study. Women Health 1998, 28, 23–40. [Google Scholar] [CrossRef]
- Dobson, A.J.; Hockey, R.; Brown, W.J.; Byles, J.E.; Loxton, D.J.; McLaughlin, D.; Tooth, L.; Mishra, G.D. Cohort Profile Update: Australian Longitudinal Study on Women’s Health. Int. J. Epidemiol. 2015, 44, 1547–1547f. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Zeal. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef]
- Ferguson, J.J.; Oldmeadow, C.; Mishra, G.D.; Garg, M.L. Plant-based dietary patterns are associated with lower body weight, BMI and waist circumference in older Australian women. Public Health Nutr. 2022, 25, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Navin Cristina, T.J.; Stewart Williams, J.A.; Parkinson, L.; Sibbritt, D.W.; Byles, J.E. Identification of diabetes, heart disease, hypertension and stroke in mid- and older-aged women: Comparing self-report and administrative hospital data records. Geriatr. Gerontol. Int. 2016, 16, 95–102. [Google Scholar] [CrossRef]
- World Health Organisation. Body Mass Index Copenhagen, Denmark World Health Organisation 2021. [cited 2021 25/5/21]. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 25 May 2021).
- Australian Institue of Health and Welfare (AIWH). The Active Australia Survey: A Guide and Manual for Implementation, Analysis and Reporting. Canberra, ACT: AIHW. 2003. Available online: https://www.aihw.gov.au/reports/physical-activity/active-australia-survey/summary (accessed on 25 May 2021).
- Russell, A.; Ball, J.; Brown, W.; Spallek, M. Physical Activity- Survey 2 and later surveys New South Wales: ALSWH; 2001. [updated January 2016; cited 2021 25/5/21]. Available online: https://alswh.org.au/wp-content/uploads/2020/08/DDSSection2.5PA_S2andlater.pdf (accessed on 25 May 2021).
- Russell, A.; Ball, J. Alcohol Intake and Pattern of Alcohol Consumption New South Wales: ALSWH; 2003. [cited 2021 25/5/21]. Available online: https://alswh.org.au/wp-content/uploads/2020/08/DDSSection2.5AlcIntake.pdf (accessed on 25 May 2021).
- Russell, A.; Ball, J. Smoking Status New South Wales: ALSWH; 2003. [updated October 2012; cited 2021 25/5/21]. Available online: https://www.alswh.org.au/wp-content/uploads/2021/01/DDS_section2_5SmokingStatus.pdf (accessed on 25 May 2021).
- Papier, K.; Appleby, P.N.; Fensom, G.K.; Knuppel, A.; Perez-Cornago, A.; Schmidt, J.A.; Tong, T.Y.N.; Key, T.J. Vegetarian diets and risk of hospitalisation or death with diabetes in British adults: Results from the EPIC-Oxford study. Nutr. Diabetes 2019, 9, 7. [Google Scholar] [CrossRef]
- OECD-FAO Agricultural Outlook (Edition 2021) [Internet]. 2021. Available online: https://www.oecd-ilibrary.org/content/data/4bde2d83-en (accessed on 18 October 2021).
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef]
- Lee, Y.; Park, K. Adherence to a Vegetarian Diet and Diabetes Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 603. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: Three cohorts of US men and women. JAMA Intern. Med. 2013, 173, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Richi, E.B.; Baumer, B.; Conrad, B.; Darioli, R.; Schmid, A.; Keller, U. Health Risks Associated with Meat Consumption: A Review of Epidemiological Studies. Int. J. Vitam. Nutr. Res. 2015, 85, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.; Sluik, D.; van Woudenbergh, G.J. Meat consumption, diabetes, and its complications. Curr. Diabetes Rep. 2013, 13, 298–306. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.; Clifton, P. A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism 2015, 64, 768–779. [Google Scholar] [CrossRef]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef]
- Sanders, L.M.; Wilcox, M.L.; Maki, K.C. Red meat consumption and risk factors for type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2022, 1–10. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9, 982. [Google Scholar] [CrossRef]
- Winter, J.E.; MacInnis, R.J.; Wattanapenpaiboon, N.; Nowson, C.A. BMI and all-cause mortality in older adults: A meta-analysis. Am. J. Clin. Nutr. 2014, 99, 875–890. [Google Scholar] [CrossRef]
- 52. Nutrition Education Materials Online (NEMO) Team, Queensland Health. Using Body Mass Index; Queensland Government: Brisbane, Australia, 2017.
- Lajous, M.; Bijon, A.; Fagherazzi, G.; Rossignol, E.; Boutron-Ruault, M.C.; Clavel-Chapelon, F. Processed and unprocessed red meat consumption and hypertension in women. Am. J. Clin. Nutr. 2014, 100, 948–952. [Google Scholar] [CrossRef]
- Zhang, R.; Fu, J.; Moore, J.B.; Stoner, L.; Li, R. Processed and Unprocessed Red Meat Consumption and Risk for Type 2 Diabetes Mellitus: An Updated Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2021, 18, 10788. [Google Scholar] [CrossRef] [PubMed]
- Pounis, G.D.; Tyrovolas, S.; Antonopoulou, M.; Zeimbekis, A.; Anastasiou, F.; Bountztiouka, V.; Metallinos, G.; Gotsis, E.; Lioliou, E.; Polychronopoulos, E.; et al. Long-term animal-protein consumption is associated with an increased prevalence of diabetes among the elderly: The Mediterranean islands (MEDIS) study. Diabetes Metab. 2010, 36 Pt 1, 484–490. [Google Scholar] [CrossRef] [PubMed]
| Vegan (n = 8) | Lacto-Ovo Vegetarian (n = 48) | Semi-Vegetarian (n = 45) | Pesco-Vegetarian (n = 74) | Regular Meat Eater (n = 8927) | |
|---|---|---|---|---|---|
| Times per week consumed: | |||||
| Beef, veal, chicken, lamb, pork, bacon, ham, corned beef, luncheon meats or salami, sausages or frankfurters | 0 | 0 | ≤1 | 0 | 0 or ≥1 |
| Fish, steamed, grilled, or baked; fish, fried (include take-away), fish, tinned (salmon, tuna, sardines etc.) | 0 | 0 | 0 or ≤1 | ≥1 | 0 or ≥1 |
| Total of above categories | 0 | 0 | ≤ 1 | ≥1 | ˃1 |
| Usual eating habits 2 | |||||
| Milk, cheese, ice-cream, yoghurt | Nil | Y | N/A | N/A | N/A |
| Butter, butter, and margarine blends | Nil | Y | N/A | N/A | N/A |
| Eggs | Nil | Y | N/A | N/A | N/A |
| Vegan (n = 8) | Lacto-Ovo Vegetarian (n = 48) | Pesco-Vegetarian (n = 74) | Semi-Vegetarian (n = 45) | Regular Meat Eater (n = 8927) | Total (n = 9102) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n 2 | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | p 1 | |
| Age (years) | 64.4 | 1.8 | 64.1 | 1.5 | 64.3 | 1.5 | 64.6 | 1.5 | 64.3 | 1.5 | 64.3 | 1.5 | 0.78 | ||||||
| Height (cm) | 162.4 | 6.7 | 162.7 | 5.4 | 162.8 | 7.1 | 162.9 | 6.7 | 162.9 | 6.6 | 8916 | 162.9 | 6.6 | 9091 | 1.00 | ||||
| Weight (kg) | 63.7 | 9.7 | 66.4 | 15.3 | 47 | 63.7 | 13.4 | 72 | 71.0 | 13.4 | 43 | 73.8 | 15.6 | 8629 | 73.7 | 15.6 | 8799 | <0.001 | |
| WC (cm) | 79.9 | 12.6 | 7 | 87.3 | 13.7 | 45 | 83.0 | 11.8 | 68 | 89.0 | 13.2 | 38 | 91.4 | 13.7 | 7980 | 91.3 | 13.7 | 8138 | <0.001 |
| BMI (kg/m2) | 24.1 | 3.1 | 25.0 | 5.0 | 47 | 24.0 | 4.5 | 72 | 26.8 | 5.3 | 43 | 27.8 | 5.7 | 8620 | 27.8 | 5.7 | 8790 | <0.001 | |
| Overweight or obese 3 | 2 (25.0) | 21 (44.7) | 25 (34.7) | 27 (62.8) | 5536 (64.2) | 5611 (63.8) | <0.001 | ||||||||||||
| IGT 4 | 0 (0.0) | 1 (2.17) | 0 (0.0) | 1 (2.2) | 275 (3.1) | 277 (3.0) | |||||||||||||
| Diabetes | 0 (0.0) | 5 (10.9) | 0 (0.0) | 6 (13.3) | 800 (9.1) | 811 (9.0) | |||||||||||||
| Residence | <0.008 | ||||||||||||||||||
| Major cities | 5 (62.5) | 23 (47.9) | 35 (47.3) | 19 (43.2) | 3408 (38.3) | 3490 (38.5) | |||||||||||||
| Inner regional | 3 (37.5) | 23 (47.9) | 30 (40.5) | 12 (27.3) | 3541 (39.8) | 3609 (39.8) | |||||||||||||
| Outer regional | 0 (0.0) | 1 (2.1) | 8 (10.8) | 11 (25.0) | 1697 (19.1) | 1717 (18.9) | |||||||||||||
| Remote | 0 (0.0) | 1 (2.1) | 1 (1.4) | 2(4.6) | 254 (2.9) | 258 (2.8) | |||||||||||||
| Employment | 0.096 | ||||||||||||||||||
| Retired/never worked | 4 (57.1) | 21 (43.8) | 37 (50.0) | 29 (65.9) | 5158 (58.8) | 5249 (58.7) | |||||||||||||
| Not retired | 3 (42.9) | 27 (56.3) | 37 (50.0) | 15 (34.1) | 3608 (41.2) | 3690 (41.3) | |||||||||||||
| Smoking Status | 0.22 | ||||||||||||||||||
| Not at all | 8 (100.0) | 46 (97.9) | 69 (94.5) | 40 (88.9) | 8271 (93.2) | 8434 (93.2) | |||||||||||||
| < weekly | 0 (0.0) | 1 (2.1) | 1 (1.4) | 1 (2.2) | 43 (0.5) | 46 (0.5) | |||||||||||||
| Weekly | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 37 (0.4) | 37 (0.4) | |||||||||||||
| Daily | 0 (0.0) | 0 (0.0) | 3 (4.1) | 4 (8.9) | 522 (5.9) | 529 (5.8) | |||||||||||||
| Alcohol Intake | <0.001 | ||||||||||||||||||
| Never | 5 (62.5) | 14 (29.8) | 22 (29.7) | 22 (53.7) | 1411 (16.3) | 1474 (16.7) | |||||||||||||
| 1–4/day | 3 (37.5) | 33 (70.2) | 52 (70.3) | 19 (46.3) | 7156 (82.7) | 7263 (82.3) | |||||||||||||
| ˃5/day | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 86 (1.0) | 86 (1.0) | |||||||||||||
| Supplement Intake 5 | |||||||||||||||||||
| Fish Oils | 1 (14.3) | 11 (23.9) | 42 (57.5) | 13 (29.5) | 4162 (47.2) | 4229 (47.1) | <0.001 | ||||||||||||
| Vitamin D | 1 (12.5) | 18 (39.1) | 33 (45.2) | 13 (30.2) | 3074 (34.7) | 3139 (35.0) | 0.22 | ||||||||||||
| CoQ10 | 1 (14.3) | 6 (13.3) | 9 (12.3) | 5 (11.4) | 443 (5.1) | 464 (5.2) | 0.001 | ||||||||||||
| HRT | 0 (0.0) | 2 (4.2) | 10 (13.5) | 2 (4.4) | 832 (9.3) | 846 (9.3) | 0.36 | ||||||||||||
| Physical Activity (mins per week 6 | |||||||||||||||||||
| M | IQR | n | M | IQR | n | M | IQR | n | M | IQR | n | M | IQR | n | M | IQR | n | ||
| Sedentary | 100 | (30,240) | 165 | (45,390) | 180 | (90,300) | 73 | 75 | (0,240) | 42 | 120 | (30,300) | 8675 | 120 | (30,300) | 8846 | 0.062 | ||
| Low | 15 | (0,150) | 30 | (0,130) | 0 | (0,90) | 73 | 0 | (0,30) | 42 | 0 | (0,120) | 8735 | 0 | (0,120) | 8906 | 0.19 | ||
| Moderate | 0 | (0,15) | 0 | (0,45) | 0 | (0,60) | 73 | 0 | (0,0) | 41 | 0 | (0,0) | 8763 | 0 | (0,0) | 8933 | 0.072 | ||
| High | 150 | (0,240) | 125 | (30,300) | 120 | (0,300) | 73 | 60 | (0,125) | 41 | 150 | (0,300) | 8659 | 150 | (0,300) | 8829 | 0.042 | ||
| Vegetarians 1 (n = 128) | All Meat Eaters 2 (n = 8875) | Total (n = 9003) | |
|---|---|---|---|
| IGT | 1(0.8) | 276 (3.1) | 277 |
| Diabetes | 5 (3.9) | 806 (9.1) | 811 |
| Total Sample 1 | All Meat Eaters 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | SE | 95% CI | p | OR | SE | 95% CI | p | |
| Frequency of all meat intake | ||||||||
| Diabetes Mellitus | ||||||||
| ⋅Low intake of meat | 1.0 | - | reference | - | 1.0 | - | reference | - |
| ⋅High intake of meat—Crude model | 1.2 | 0.1 | 1.0, 1.4 | 0.06 | 1.2 | 0.1 | 1.0, 1.4 | 0.12 |
| ⋅High intake of meat—Adjusted model | 1.1 | 0.1 | 0.9, 1.4 | 0.28 | 1.1 | 0.1 | 0.9, 1.4 | 0.37 |
| Impaired Glucose Tolerance | ||||||||
| ⋅Low intake of meat | 1.0 | - | reference | - | 1.0 | - | reference | - |
| ⋅High intake of meat—Crude model | 1.6 | 0.3 | 1.1, 2.2 | 0.01 | 1.5 | 0.3 | 1.1, 2.1 | 0.02 |
| ⋅High intake of meat—Adjusted model | 1.4 | 0.3 | 1.0, 2.0 | 0.07 | 1.4 | 0.3 | 0.9, 2.0 | 0.10 |
| Frequency of processed meat intake | ||||||||
| Diabetes Mellitus | ||||||||
| ⋅Low intake of processed meat | 1.0 | - | reference | - | 1.0 | - | reference | - |
| ⋅High intake of processed meat—Crude model | 1.7 | 0.3 | 1.3, 2.3 | <0.0001 | 1.7 | 0.3 | 1.3, 2.3 | <0.0001 |
| ⋅High intake of processed meat—Adjusted model | 1.2 | 0.2 | 0.8, 1.7 | 0.44 | 1.1 | 0.2 | 0.8, 1.7 | 0.46 |
| Impaired Glucose Tolerance | ||||||||
| ⋅Low intake of processed meat | 1.0 | - | reference | - | 1.0 | - | reference | - |
| ⋅High intake of processed meat—Crude model | 1.5 | 0.4 | 0.9, 2.4 | 0.13 | 1.5 | 0.4 | 0.9, 2.4 | 0.15 |
| ⋅High intake of processed meat—Adjusted model | 1.1 | 0.3 | 0.6, 2.0 | 0.80 | 1.1 | 0.3 | 0.6, 2.0 | 0.281 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baleato, C.L.; Ferguson, J.J.A.; Oldmeadow, C.; Mishra, G.D.; Garg, M.L. Plant-Based Dietary Patterns versus Meat Consumption and Prevalence of Impaired Glucose Intolerance and Diabetes Mellitus: A Cross-Sectional Study in Australian Women. Nutrients 2022, 14, 4152. https://doi.org/10.3390/nu14194152
Baleato CL, Ferguson JJA, Oldmeadow C, Mishra GD, Garg ML. Plant-Based Dietary Patterns versus Meat Consumption and Prevalence of Impaired Glucose Intolerance and Diabetes Mellitus: A Cross-Sectional Study in Australian Women. Nutrients. 2022; 14(19):4152. https://doi.org/10.3390/nu14194152
Chicago/Turabian StyleBaleato, Courtney L., Jessica J. A. Ferguson, Christopher Oldmeadow, Gita D. Mishra, and Manohar L. Garg. 2022. "Plant-Based Dietary Patterns versus Meat Consumption and Prevalence of Impaired Glucose Intolerance and Diabetes Mellitus: A Cross-Sectional Study in Australian Women" Nutrients 14, no. 19: 4152. https://doi.org/10.3390/nu14194152
APA StyleBaleato, C. L., Ferguson, J. J. A., Oldmeadow, C., Mishra, G. D., & Garg, M. L. (2022). Plant-Based Dietary Patterns versus Meat Consumption and Prevalence of Impaired Glucose Intolerance and Diabetes Mellitus: A Cross-Sectional Study in Australian Women. Nutrients, 14(19), 4152. https://doi.org/10.3390/nu14194152







