Bioactive Yoghurt Containing Curcumin and Chlorogenic Acid Reduces Inflammation in Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Intervention Yoghurts
2.3. Statistical Analysis
3. Results
3.1. Participant Characterisitics
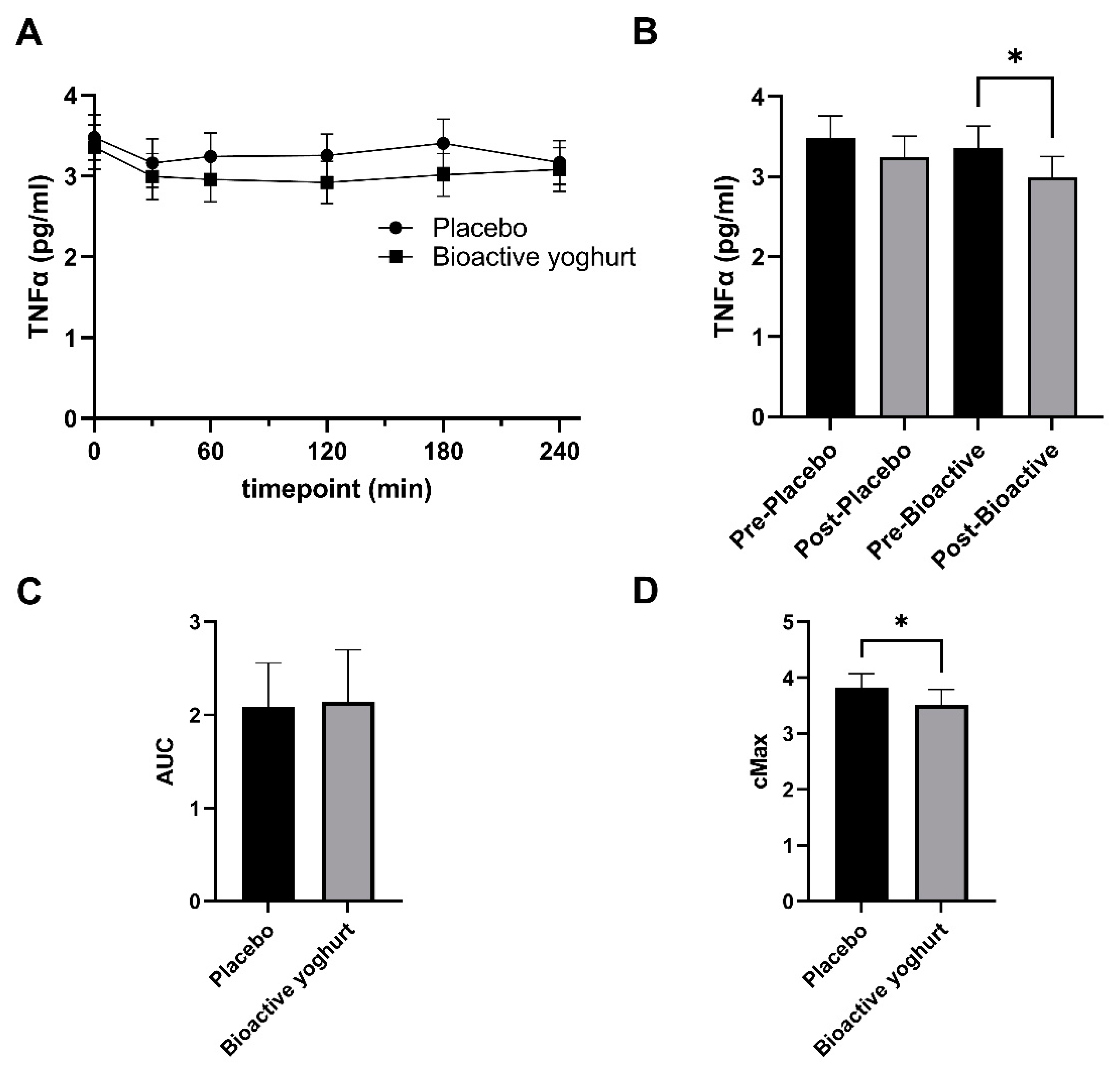
3.2. Influence of Study Yoghurt on Inflammatory Markers
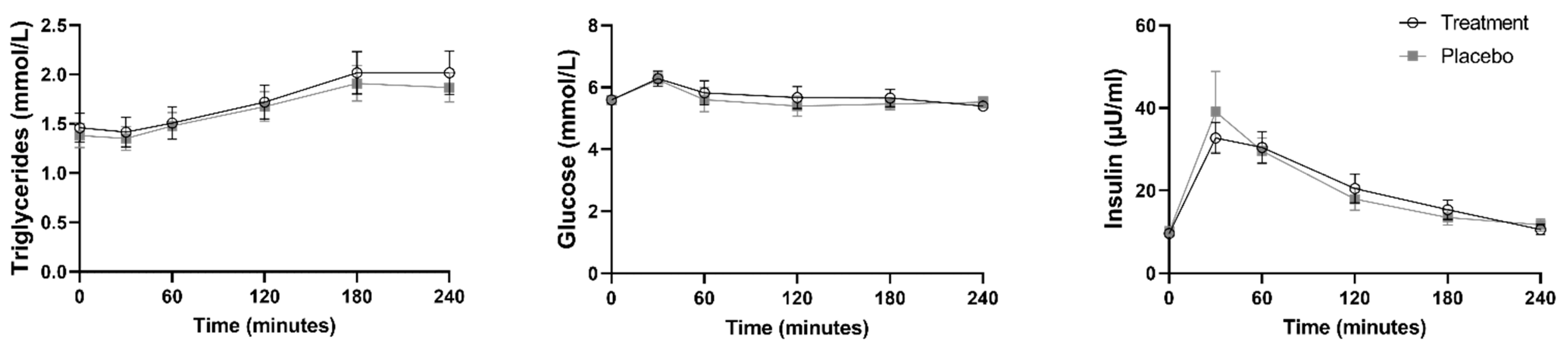
3.3. Study Yoghurts and Metabolic Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, H.I.; Freeman, E.W. Hormone changes associated with the menopausal transition. Minerva Ginecol. 2009, 61, 483. [Google Scholar] [PubMed]
- Auro, K.; Joensuu, A.; Fischer, K.; Kettunen, J.; Salo, P.; Mattsson, H.; Niironen, M.; Kaprio, J.; Eriksson, J.G.; Lehtimäki, T. A metabolic view on menopause and ageing. Nat. Commun. 2014, 5, 4708. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The role of estrogen in insulin resistance: A review of clinical and preclinical data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Bessesen, D.H.; Cox-York, K.A.; Hernandez, T.L.; Erickson, C.B.; Wang, H.; Jackman, M.R.; Van Pelt, R.E. Postprandial triglycerides and adipose tissue storage of dietary fatty acids: Impact of menopause and estradiol. Obesity 2015, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-I.; Chou, P.; Tsai, S.-T. The impact of years since menopause on the development of impaired glucose tolerance. J. Clin. Epidemiol. 2001, 54, 117–120. [Google Scholar] [CrossRef]
- Jackson, K.G.; Abraham, E.C.; Smith, A.M.; Murray, P.; O’Malley, B.; Williams, C.M.; Minihane, A.M. Impact of age and menopausal status on the postprandial triacylglycerol response in healthy women. Atherosclerosis 2010, 208, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch. Intern. Med. 2003, 163, 1306–1316. [Google Scholar] [CrossRef]
- Blaak, E.; Antoine, J.M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef]
- Hyson, D.; Rutledge, J.C.; Berglund, L. Postprandial lipemia and cardiovascular disease. Curr. Atheroscler. Rep. 2003, 5, 437–444. [Google Scholar] [CrossRef]
- Le, N.-A. Postprandial triglycerides, oxidative stress, and inflammation. In Apolipoproteins, Triglycerides and Cholesterol; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Wang, T.; Rohan, T.E.; Gunter, M.J.; Xue, X.; Wactawski-Wende, J.; Rajpathak, S.N.; Cushman, M.; Strickler, H.D.; Kaplan, R.C.; Wassertheil-Smoller, S. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol. Prev. Biomark. 2011, 20, 971–977. [Google Scholar] [CrossRef]
- Pacifici, R.; Brown, C.; Puscheck, E.; Friedrich, E.; Slatopolsky, E.; Maggio, D.; McCracken, R.; Avioli, L.V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc. Natl. Acad. Sci. USA 1991, 88, 5134–5138. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-San Nicolás, A.; SÁnchez-RodrÍguez, M.A.; Zacarías-Flores, M.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Relationship between central obesity and oxidative stress in premenopausal versus postmenopausal women. Nutr. Hosp. 2020, 37, 267–274. [Google Scholar] [PubMed]
- Zamboni, M.; Armellini, F.; Harris, T.; Turcato, E.; Micciolo, R.; Bergamo-Andreis, I.A.; Bosello, O. Effects of age on body fat distribution and cardiovascular risk factors in women. Am. J. Clin. Nutr. 1997, 66, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Austin, H.; Austin, J.M.; Partridge, E.E.; Hatch, K.D.; Shingleton, H.M. Endometrial cancer, obesity, and body fat distribution. Cancer Res. 1991, 51, 568–572. [Google Scholar] [PubMed]
- Malutan, A.M.; Dan, M.; Nicolae, C.; Carmen, M. Proinflammatory and anti-inflammatory cytokine changes related to menopause. Prz. Menopauzalny Menopause Rev. 2014, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Carr, M.C.; Murdoch, S.J.; Mitchell, E.; Woods, N.F.; Wener, M.H.; Chandler, W.L.; Boyko, E.J.; Brunzell, J.D. Adipokines, inflammation, and visceral adiposity across the menopausal transition: A prospective study. J. Clin. Endocrinol. Metab. 2009, 94, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Fenkci, S.; Rota, S.; Sabir, N.; Sermez, Y.; Guclu, A.; Akdag, B. Relationship of serum interleukin-6 and tumor necrosis factor α levels with abdominal fat distribution evaluated by ultrasonography in overweight or obese postmenopausal women. J. Investig. Med. 2006, 54, 455–460. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C. Obesity, inflammation, and postmenopausal breast cancer: Therapeutic implications. Sci. World J. 2011, 11, 2020–2036. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Volpato, S.; Guralnik, J.M.; Ferrucci, L.; Balfour, J.; Chaves, P.; Fried, L.P.; Harris, T.B. Cardiovascular disease, interleukin-6, and risk of mortality in older women: The women’s health and aging study. Circulation 2001, 103, 947–953. [Google Scholar] [CrossRef]
- Liu, S.; Tinker, L.; Song, Y.; Rifai, N.; Bonds, D.E.; Cook, N.R.; Heiss, G.; Howard, B.V.; Hotamisligil, G.S.; Hu, F.B. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch. Intern. Med. 2007, 167, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Grigor, J.; Winger, R.; Win, A. Functional food product development—Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases. Eur. Cardiol. Rev. 2019, 14, 117. [Google Scholar] [CrossRef]
- Thota, R.N.; Dias, C.B.; Abbott, K.A.; Acharya, S.H.; Garg, M.L. Curcumin alleviates postprandial glycaemic response in healthy subjects: A cross-over, randomized controlled study. Sci. Rep. 2018, 8, 13679. [Google Scholar] [CrossRef]
- Sahebkar, A.; Cicero, A.F.; Simental-Mendía, L.E.; Aggarwal, B.B.; Gupta, S.C. Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacol. Res. 2016, 107, 234–242. [Google Scholar] [CrossRef]
- White, C.M.; Pasupuleti, V.; Roman, Y.M.; Li, Y.; Hernandez, A.V. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 146, 104280. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Ahmed Nasef, N.; Loveday, S.M.; Golding, M.; Martins, R.N.; Shah, T.M.; Clarke, M.; Coad, J.; Moughan, P.J.; Garg, M.L.; Singh, H. Food matrix and co-presence of turmeric compounds influence bioavailability of curcumin in healthy humans. Food Funct. 2019, 10, 4584–4592. [Google Scholar] [CrossRef]
- Bisht, A.; Dickens, M.; Rutherfurd-Markwick, K.; Thota, R.; Mutukumira, A.N.; Singh, H. Chlorogenic acid potentiates the anti-inflammatory activity of curcumin in LPS-stimulated THP-1 cells. Nutrients 2020, 12, 2706. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Dupont, W.D.; Plummer, W.D., Jr. Power and Sample Size Calculations: A Review and Computer Program. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef]
- National Heart, Lung, Blood Institute, National Institute of Diabetes, Kidney Diseases (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report; National Institutes of Health, National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1998. [Google Scholar]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: A systematic review. Adv. Nutr. 2017, 8, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Herieka, M.; Erridge, C. High-fat meal induced postprandial inflammation. Mol. Nutr. Food Res. 2014, 58, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Smith, K.; Rivera, A.; Orosz, Z.; Ungvari, Z. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am. J. Pathol. 2007, 170, 388–398. [Google Scholar] [CrossRef]
- Moreau, K.L.; Deane, K.D.; Meditz, A.L.; Kohrt, W.M. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 2013, 230, 390–396. [Google Scholar] [CrossRef]
- Nanes, M.S. Tumor necrosis factor-α: Molecular and cellular mechanisms in skeletal pathology. Gene 2003, 321, 1–15. [Google Scholar] [CrossRef]
- Hotamisligil, G. Molecular mechanisms of insulin resistance and the role of the adipocyte. Int. J. Obes. 2000, 24, S23–S27. [Google Scholar] [CrossRef]
- Ilesanmi-Oyelere, B.L.; Schollum, L.; Kuhn-Sherlock, B.; McConnell, M.; Mros, S.; Coad, J.; Roy, N.C.; Kruger, M.C. Inflammatory markers and bone health in postmenopausal women: A cross-sectional overview. Immun. Ageing 2019, 16, 15. [Google Scholar] [CrossRef]
- Piché, M.-È.; Lemieux, S.; Weisnagel, S.J.; Corneau, L.; Nadeau, A.; Bergeron, J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am. J. Cardiol. 2005, 96, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas-Melo, F.; Sereno, J.; Teixeira-Lemos, E.; Ribeiro, S.; Rocha-Pereira, P.; Cotterill, E.; Teixeira, F.; Reis, F. Markers of increased cardiovascular risk in postmenopausal women: Focus on oxidized-LDL and HDL subpopulations. Dis. Markers 2013, 35, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Llaneza, P.; González, C.; Fernandez-Iñarrea, J.; Alonso, A.; Diaz, F.; Arnott, I.; Ferrer-Barriendos, J. Soy isoflavones, diet and physical exercise modify serum cytokines in healthy obese postmenopausal women. Phytomedicine 2011, 18, 245–250. [Google Scholar] [CrossRef]
- Manning, P.J.; Sutherland, W.H.; Hendry, G.; De Jong, S.A.; McGrath, M.; Williams, S.M. Changes in circulating postprandial proinflammatory cytokine concentrations in diet-controlled type 2 diabetes and the effect of ingested fat. Diabetes Care 2004, 27, 2509–2511. [Google Scholar] [CrossRef]
- Poppitt, S.D.; Keogh, G.F.; Lithander, F.E.; Wang, Y.; Mulvey, T.B.; Chan, Y.-K.; McArdle, B.H.; Cooper, G.J. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-α, and C-reactive protein to a high-fat dietary load. Nutrition 2008, 24, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Nappo, F.; Esposito, K.; Cioffi, M.; Giugliano, G.; Molinari, A.M.; Paolisso, G.; Marfella, R.; Giugliano, D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: Role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 2002, 39, 1145–1150. [Google Scholar] [CrossRef]
- Payette, C.; Blackburn, P.; Lamarche, B.; Tremblay, A.; Bergeron, J.; Lemieux, I.; Després, J.-P.; Couillard, C. Sex differences in postprandial plasma tumor necrosis factor–α, interleukin-6, and C-reactive protein concentrations. Metabolism 2009, 58, 1593–1601. [Google Scholar] [CrossRef]
- Blackburn, P.; Després, J.P.; Lamarche, B.; Tremblay, A.; Bergeron, J.; Lemieux, I.; Couillard, C. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity 2006, 14, 1747–1754. [Google Scholar] [CrossRef]
- Cho, G.J.; Lee, J.H.; Park, H.T.; Shin, J.H.; Hong, S.C.; Kim, T.; Hur, J.Y.; Lee, K.W.; Park, Y.K.; Kim, S.H. Postmenopausal status according to years since menopause as an independent risk factor for the metabolic syndrome. Menopause 2008, 15, 524–529. [Google Scholar]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef]
- Ghandadi, M.; Sahebkar, A. Curcumin: An effective inhibitor of interleukin-6. Curr. Pharm. Des. 2017, 23, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M. Investigation of the effects of curcumin on serum cytokines in obese individuals: A randomized controlled trial. Sci. World J. 2014, 2014, 898361. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Potentiality of probiotic yoghurt as a functional food—A review. Nutr. Food Sci. 2018, 49, 182–202. [Google Scholar] [CrossRef]

| Yoghurt | ||||
|---|---|---|---|---|
| Macronutrient | Coconut Cream | Coconut Sugar | Breakfast Bar | Total |
| Fat (g) | 30.5 | 0.012 | 9.6 | 40.11 |
| -Saturated fat (g) | 28.9 | 0.012 | 1.1 | 30.01 |
| Carbohydrate (g) | 2.6 | 1.007 | 33.9 | 37.51 |
| -Sugar (g) | 2.6 | 0.9116 | 7.5 | 11.01 |
| Fibre (g) | 0 | 0.0106 | 7.3 | 7.31 |
| Bioactive | Placebo | |||
|---|---|---|---|---|
| Ingredient | g | % | g | % |
| Pasteurized Kara™ coconut and coconut sugar | 1184.58 | 99.818 | 1270.9 | 100 |
| Coffee extract * | 1.17 | 0.099 | 0 | 0 |
| Curcumin C3 complex | 0.99 | 0.083 | 0 | 0 |
| Fermentation culture | 0.027 | 0.0023 | 0.027 | 0.0021 |
| Characteristic | Median (IQR) |
|---|---|
| n | 16 |
| Age (years) | 58 (55 to 61) |
| Last menstrual cycle (years) * | 7 (3 to 19) |
| Height (m) | 1.7 (1.6 to 1.7) |
| Weight (Kg) | 76 (72 to 94) |
| BMI | 27 (25 to 35) |
| Systolic | 126 (109 to 140) |
| Diastolic | 84 (74 to 93) |
| Physical activity (MET-equivalent/min) | 2541 (1239 to 3728) |
| Physical activity category | 2 (2 to 3) |
| HOMA-IR | 2.1 (1.8 to 2.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed Nasef, N.; Thota, R.N.; Mutukumira, A.N.; Rutherfurd-Markwick, K.; Dickens, M.; Gopal, P.; Singh, H.; Garg, M.L. Bioactive Yoghurt Containing Curcumin and Chlorogenic Acid Reduces Inflammation in Postmenopausal Women. Nutrients 2022, 14, 4619. https://doi.org/10.3390/nu14214619
Ahmed Nasef N, Thota RN, Mutukumira AN, Rutherfurd-Markwick K, Dickens M, Gopal P, Singh H, Garg ML. Bioactive Yoghurt Containing Curcumin and Chlorogenic Acid Reduces Inflammation in Postmenopausal Women. Nutrients. 2022; 14(21):4619. https://doi.org/10.3390/nu14214619
Chicago/Turabian StyleAhmed Nasef, Noha, Rohith N. Thota, Anthony N. Mutukumira, Kay Rutherfurd-Markwick, Martin Dickens, Pramod Gopal, Harjinder Singh, and Manohar L. Garg. 2022. "Bioactive Yoghurt Containing Curcumin and Chlorogenic Acid Reduces Inflammation in Postmenopausal Women" Nutrients 14, no. 21: 4619. https://doi.org/10.3390/nu14214619
APA StyleAhmed Nasef, N., Thota, R. N., Mutukumira, A. N., Rutherfurd-Markwick, K., Dickens, M., Gopal, P., Singh, H., & Garg, M. L. (2022). Bioactive Yoghurt Containing Curcumin and Chlorogenic Acid Reduces Inflammation in Postmenopausal Women. Nutrients, 14(21), 4619. https://doi.org/10.3390/nu14214619











