Abstract
Non-alcoholic fatty liver disease (NAFLD) includes a spectrum of liver alterations that can result in severe disease and even death. Consumption of ultra-processed foods (UPF) has been associated with obesity and related comorbidities. However, the link between UPF and NAFLD has not been sufficiently assessed. We aimed to investigate the prospective association between UPF consumption and liver health biomarkers. Methods: We followed for 1 year 5867 older participants with overweight/obesity and metabolic syndrome (MetS) from the PREDIMED-Plus trial. A validated 143-item semi-quantitative food frequency questionnaire was used to evaluate consumption of UPF at baseline, 6, and 12 months. The degree of processing for foods and beverages (g/day) was established according to the NOVA classification system. The non-invasive fatty liver index (FLI) and hepatic steatosis index (HSI) were used to evaluate liver health at three points in time. The associations between changes in UPF consumption (percentage of total daily dietary intake (g)) and liver biomarkers were assessed using mixed-effects linear models with repeated measurements. Results: In this cohort, UPF consumption at baseline was 8.19% (SD 6.95%) of total daily dietary intake in grams. In multivariable models, each 10% daily increment in UPF consumption in 1 year was associated with significantly greater FLI (β 1.60 points, 95% CI 1.24;1.96 points) and HSI (0.43, 0.29; 0.57) scores (all p-values < 0.001). These associations persisted statistically significant after adjusting for potential dietary confounders and NAFLD risk factors. Conclusions: A higher UPF consumption was associated with higher levels of NAFLD-related biomarkers in older adults with overweight/obesity and MetS.
1. Background
Excessive liver fat accumulation in the absence of alcoholism, known as non-alcoholic fatty liver disease (NAFLD), includes a spectrum of fat-liver alterations affecting approximately 30% of adults worldwide, but its prevalence is increased in persons with type 2 diabetes (70%) and morbid obesity (90%) [1,2]. Although it remains asymptomatic during long time, excess liver fat is an important cause of morbimortality from cardiovascular disease (CVD) and malignancy, in addition to chronic liver disease. Unfortunately, at present, there are gaps in NAFLD diagnosis and limited treatment options [1,2].
Along with physical activity (PA), dietary modifications are considered the cornerstone for NAFLD management [1]. A growing body of evidence suggests that dietary fiber intake and the Mediterranean diet (MedDiet) [3] might be protective, whereas saturated and trans fatty acids (FA), simple sugars, red and processed meat, sugar-sweetened beverages (SSB), and the Western dietary pattern might act as risk factors [4]. However, with the exception of small trials assessing interventions with the MedDiet [3], most of these findings were based on cross-sectional or case-control study designs.
Ultra-processed foods (UPF) are industrial formulations manufactured using a series of processes with no domestic equivalents (i.e., soft drinks, packed snacks, processed meats, and pre-prepared dishes) [5,6]. In addition to their usual poor nutritional composition (i.e., excessive calories, simple sugars, salt, poor quality fat, as well as fiber and vitamin deprivation) [5], UPF typically contains cosmetic additives and substances formed because of extensive processing during manufacturing [7]. On the other hand, UPF are highly palatable, appealing, convenient, microbiologically pure, inexpensive, accessible, and aggressively advertised [5,8]. All these factors explain the steadily increase in UPF consumption despite the health risks associated with their regular intake [9,10].
A recent study conducted in the PREvención con DIeta MEDiterránea Plus (PREDIMED-Plus) cohort found that consumption of UPF, classified according to the NOVA system [11], was associated with greater visceral and total fat accumulation [12]. Other prospective studies with adult cohorts found associations between UPF consumption and higher risks of obesity [13,14,15], CVD [16], type 2 diabetes [17,18], renal dysfunction [19], cancer [20], biological aging [21], and all-cause mortality [22]. However, the link between UPF and NAFLD has not been sufficiently assessed [23]. Only very recently a prospective study by Zhang et al. has been published, reporting a positive link between UPF and NAFLD diagnosed using ultrasonography, within the Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) Cohort Study [24] after a median of 4.2 years of follow-up; but they only measured UPF at baseline, without repeating the dietary assessment during follow-up. Therefore, our aim was to prospectively investigate how concurrent changes in consumption of UPF were associated with liver health in older individuals with overweight/obesity and the metabolic syndrome (MetS) from the Mediterranean area, using three repeated measurements of diet and biomarkers related to NAFLD throughout a 1-year follow-up of PREDIMED-Plus trial.
2. Methods
2.1. Study Overview and Population
This study corresponds to longitudinal analyses nested in the ongoing PREDIMED-Plus trial, with data collected at baseline and during the first year of follow-up. Details about the study protocol have been described elsewhere [25,26], and are available at www.predimedplus.com. Briefly, PREDIMED-Plus is a 6-year parallel-group, multicenter randomized clinical trial, aimed to assess the effectiveness of a lifestyle intervention—energy-restricted (er) MedDiet, PA promotion, and behavioral support—on the primary prevention of CVD and weight loss in older individuals with overweight/obesity harboring MetS. The control group received usual health care and recommendations to follow the MedDiet, without advice on energy restriction or PA promotion. The trial was launched in 2013 (the recruitment finished at the end of 2016) in 23 centers across Spain. Community-dwelling older men and women (55–75 years), with body mass index (BMI) ≥ 27 and <40 kg/m2), fulfilling ≥ three criteria for the MetS [27], but free of CVD at baseline, were invited to participate in the trial. Exclusion criteria included self-declared liver disease (chronic hepatitis or cirrhosis), therapy with immunosuppressive drugs, cytotoxic agents or systemic corticosteroids, alcohol abuse or addiction (defined as total daily alcohol intake > 50 g within past 6 months), history of inflammatory bowel disease, and active malignant cancer or history of malignancy within the last 5 years. For a small subset of participants sharing the same household, the randomization was performed as clusters (the couple was used as the unit of randomization). All participants provided written informed consent to a protocol approved by the Research Ethic Committees of all recruiting centers according to the ethical standards of the Declaration of Helsinki. The trial was registered at http://www.isrctn.com/ (ISRCTN89898870).
Out of the 6874 participants randomized for the PREDIMED-Plus trial, participants with history of liver cancer within >5 years before inclusion (n = 2), with missing data on variables of interest at baseline (n = 811), and those who were outside predefined limits for total daily energy intake (<500 or >3500 kcal for women, <800 or >4000 kcal for men) [28] at baseline and during follow-up (n = 194) were excluded from the analysis. After exclusions, a total of 5867 participants were included in the final analyses (see the flowchart of study participants in online Supplementary Figure S1).
2.2. Dietary Habits and Nutrient Intake Assessments
Dietary intake was assessed by trained dietitians during face-to-face interviews at baseline, 6-, and 12-month follow-up visits, using a semi-quantitative food frequency questionnaire (FFQ), repeatedly validated for the Spanish population [29,30]. Intakes of 143 foods and beverages (except water) were calculated by multiplying the common portion size by average consumption frequency (9 possible responses, from never to >6 times/day) over the last year (at baseline visit and over 6-month period at each follow-up visit). Daily intake of beverages was collected in cubic centimeters and then converted into milliliters (1 cc = 1 mL), and further into grams, assuming that 1 mL = 1 g. Food composition tables developed specifically for Spain [31] were used to derive nutrient (sodium(native and added in the form of salt), and cholesterol (both in mg/day), saturated and trans FA, fiber, and alcohol (all in g/day)), as well as total energy intake (kcal/day). The glycemic load was also calculated for each item taking into account the quality (glycemic index) and the amount of carbohydrate as previously described [32]. The glycemic index was determined using average values from the International Tables [33].
2.3. Dietary Data Processing-Based Classification and Ultra-Processed Foods
Items in the FFQ were classified according to the NOVA system [5,11] developed by the Public Health Faculty of the University of São Paulo in Brazil. This system classifies foods and beverages according to the nature, extent, and purpose of their industrial processing into four groups: (1) unprocessed or minimally processed foods (i.e., fresh or frozen fruits and vegetables, eggs, pasteurized milk, meat, seeds, nuts, grains, or plain yogurt); (2) processed culinary ingredients (i.e., oils, fats, sugar, and salt); (3) processed foods (i.e., canned vegetables, canned fish, fruits in syrup, cheeses, fresh bread, beer, and wine); and (4) UPF (i.e., soft drinks, sweet, or savory packed snacks, processed meats, pre-prepared frozen dishes, and ‘instant’ products). Details about the allocation of FFQ items to processing groups with examples are provided in online Supplementary Table S1. Furthermore, items belonging to the UPF group (foods and beverages) were allocated into the following subgroups: dairy products; processed meat; pre-prepared dishes, snacks and fast-foods; sweets; non-alcoholic beverages; and alcoholic beverages (Table 1).

Table 1.
Relative contribution of different food groups to the consumption of UPF in diet of participants at baseline.
Repeated data on the percentage of UPF consumption (and other NOVA groups) was computed as the sum of grams per day consumed from items in the UPF group (determined at baseline, 6-, and 12-month follow-up visits), divided by the total grams of all items consumed per day, and multiplied by 100.
2.4. Socio-Demographic, Lifestyle, Anthropometric, and Clinical Variables Assessment
Information on socio-demographics and health-related issues was collected from participants using a general questionnaire at baseline. Educational level, indicated by the highest educational qualification (professional or academic) achieved, was categorized into three groups (higher education or technician, secondary education, or primary education or less), smoking habits into four groups (current, never, ex-smoker, or insufficient data), whereas history of overweight was categorized into five groups (since childhood, adolescence, adulthood, after childbirth, or since menopause), and prevalence of type 2 diabetes was dichotomized (yes or no).
At baseline, 6-, and 12-month follow-up visits, total leisure-time PA (METs min/week) was assessed using the validated Minnesota-REGICOR short physical activity questionnaire [34] and sedentary behavior (SB) (h/day) by the validated Spanish version of the Nurses’ Health Study questionnaire [35]. Adherence to the erMedDiet was determined using a 17-item screener, a modified version of a validated 14-item questionnaire [36].
At each visit, trained staff measured in duplicate height and weight according to the study´s protocol using a wall-mounted stadiometer and calibrated scales, respectively. Blood pressure was measured in triplicate using a calibrated oscillometer. Averages of these repeated measurements were taken for analyses. BMI was calculated by dividing weight (kg) by squared height (m), and waist circumference (cm) was determined midway between the lowest rib and the iliac crest using an anthropometric tape.
Fasting blood samples were collected at baseline and thereafter to quantify levels of alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), gamma-glutamyl transferase (GGT, U/L), glucose (mg/dL), high-density lipoprotein cholesterol (HDL-c, mg/dL), triglyceride (mg/dL), and glycated hemoglobin (HbA1c, %) using standard methods. MetS components were ascertained according to guidelines from the International Diabetes Federation/National Heart, Lung and Blood Institute/American Heart Association (2009), namely: waist circumference ≥ 102 cm for men and ≥88 cm for women, triglycerides ≥ 150 mg/dL, HDL-c < 40 mg/dL in men and <50 mg/dL in women, systolic blood pressure ≥ 130 and/or diastolic blood pressure ≥ 85 mmHg (or antihypertensive drug treatment), and fasting glucose ≥ 100 mg/dL (or antidiabetic drug treatment) [27].
2.5. Outcome Assessment: Liver Health
Repeated data of the non-invasive liver health biomarkers were computed using anthropometric and biochemical data collected at baseline, 6-, and 12-month follow-up visits. The fatty liver index (FLI) and hepatic steatosis index (HSI) were used as surrogate measures of NAFLD [37,38]. FLI [37] and HSI [38] are diagnostic algorithms built upon a cluster of liver health biomarkers, including BMI, waist circumference, blood levels of triglycerides, the liver enzyme GGT, the AST/ALT ratio, type 2 diabetes status, and sex (see below). These scores have been previously validated in large populations against imaging techniques, showing high specificity and sensitivity in predicting excess liver fat, with values < 30 ruling out and values ≥ 60 (for FLI) or ≥36 (for HSI) confirming NAFLD [37,38].
FLI = (e(0.953×ln(triglyceride)+0.139×BMI+0.718×ln(GGT)+0.053×waist circumference−15.745))/(1 + e(0.953×ln(triglyceride)+0.139×BMI+0.718×ln(GGT)+0.053×waist circumference−15.745)) × 100
HSI = 8 * ALT/AST + BMI + (2 if type 2 diabetes, 0 otherwise) + (2 if women, 0 otherwise)
2.6. Statistical Analyses
All analyses were performed using the entire analytical sample as an observational cohort (both study arms combined). For descriptive analyses of a participant´s characteristics means and standard deviations (SD), and numbers and percentages (%), were calculated for continuous and categorical variables, respectively. Statistical differences in baseline characteristics by baseline sex-specific quintiles of UPF consumption were assessed using one-way ANOVA or χ2 test, wherever appropriate. The differences in these characteristics over follow-up time were assessed using linear mixed-effects models with random intercepts at recruiting center, cluster family, and patient level.
The same approach with linear mixed-effects modelling with random intercepts (recruiting center, cluster family, and patient) was used to evaluate associations between concurrent 6-month changes in UPF consumption with changes in indices of NAFLD over the first year of follow-up. Our exposure was modelled as repeatedly measured continuous variable (per 10% increment) and as sex-specific quintiles, with the first quintile set as the reference category. The p for linear trend across increasing quintiles was calculated with the use of the median value for each category. In the main analyses, two sets of covariates were used. Model 1 was minimally adjusted for age at inclusion, sex, study arm, and follow-up time (months). Model 2 was additionally adjusted for baseline educational level, smoking habits (all categorical, height, as well as repeatedly measured at baseline and every 6 months thereafter PA, SB, and alcohol intake (all continuous). Selection of covariates was performed using a causal directed acyclic graph approach implemented in the DAGitty free web application [39].
Several sensitivity analyses were performed based on model 2. The potential influence of nutritional factors (characteristics of UPF) was addressed by additional adjustment for repeatedly measured intake of total energy, saturated and trans FA, cholesterol, fiber, glycemic load, sodium (individually and including all factors simultaneously in the model), as well as adherence to erMedDiet (all continuous). Moreover, we controlled for several NAFLD-related risk factors by additional adjustment for repeatedly measured BMI, waist circumference, HbA1c, and number of MetS factors (continuous), as well as for history of overweight self-reported at baseline and type 2 diabetes prevalence at baseline (both categorical). Finally, models were rerun eliminating outliers (1st and 99th percentile) from FLI and HSI, and imputing missing follow-up data (UPF, NAFLD indices and covariables) using the last observation carried forward (LOCF) method.
Additionally, the proportion mediated by nutritional factors (characteristics of UPF, and adherence to erMedDiet, as an indicator of a healthy dietary pattern) and NAFLD-related biomarkers (known risk factors and components of FLI and HSI scores) in the studied association were quantified. For this, standard steps proposed by Baron and Kenny (1986) with adjustments introduced by Iacobucci et al. [40] were followed using multicovariate-adjusted linear mixed-effects models (Model 2). Details about the method and analyses are presented in online Supplementary Text S1.
Subgroup analyses were also performed by rerunning the model for different strata at baseline: sex (men or women), age (<65 or ≥65 y), type 2 diabetes status (non-diabetics or diabetics), alcohol intake (<20/30 g/day for men/women or ≥20/30 g/day for men/women, respectively), and adherence to erMedDiet (<8 or ≥8 points). Median values were used as a threshold to stratify age and erMedDiet, whereas safe limits accepted by guidelines of scientific associations were used for alcohol intake [41]. The p values for interaction were computed for each scenario rerunning model 2 with a multiplicative interaction term inserted between these variables and exposure in continuous form.
In secondary analyses, model 2 was rerun to explore the associations between concurrent changes in consumption of different food subgroups within UPF (continuous variable) and NAFLD indices.
Analyses were performed using Stata v15. and statistical significances was set at p < 0.05. The last actualized version of the PREDIMED-Plus longitudinal database generated on 22nd December 2020 (202012220958_PREDIMEDplus 2020) was used.
3. Results
Table 2 presents baseline characteristics of study participants according to quintiles of UPF consumption. The analytical sample (n = 5867) comprised 47.8% women with average age at inclusion of 65.0 years (SD 4.9 years). Overall obesity and abdominal obesity (73.1 and 93.0%, respectively), as well as NAFLD screened using FLI and HSI (84.1 and 95.2%, respectively), were highly prevalent. Mean UPF consumption accounted for 8.19% (SD 6.95%) of total daily intake (in grams). Compared to participants with the lowest UPF consumption (Q1, mean consumption 2.12% (SD 0.81%) of total daily intake (in grams)), participants in the highest quintile (Q5, mean UPF consumption 19.0% (SD 7.9%)) were younger and showed less healthy lifestyle habits in terms of physical inactivity; higher sedentariness; intake of energy, saturated and trans FA, cholesterol, sodium, and glycemic load; as well as lower intake of fiber, alcohol, and adherence to MedDiet (p < 0.001 for all comparisons). Moreover, participants in the highest quintile of UPF consumption presented higher values of BMI and WC than their counterparts in the lowest quintile, as well as higher levels of liver health biomarkers, such as blood ALT/AST ratio and triglycerides, as well as both NAFLD indices (p ≤ 0.001). Sweets (28%), non-alcoholic beverages (26%), and processed meats (22%) were the main food subgroups consumed within the UPF category at baseline (Table 1).

Table 2.
Baseline characteristics of study participants according to baseline sex-specific quintiles of UPF.
As shown in online Supplementary Table S2, lifestyle factors and liver health markers improved over time compared to the baseline (p < 0.05 for all comparisons). An increase in the consumption of unprocessed or minimally processed foods and decrease in products with higher degree of processing was observed during the 1-year follow-up period, probably as consequence of MedDiet recommendations, which were given to participants in both study arms (p < 0.001).
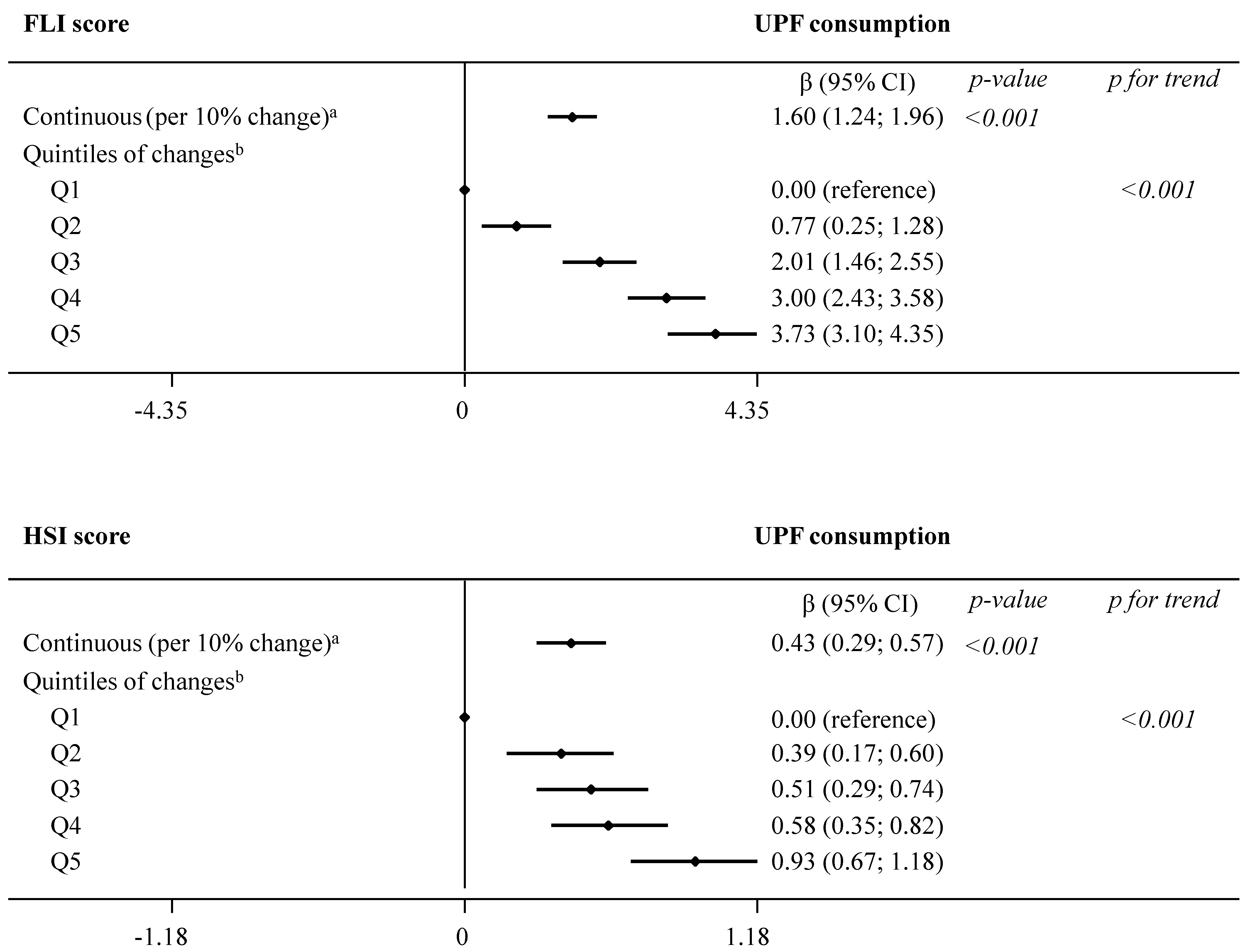
Results from the main analysis evaluating the association between concurrent changes in UPF consumption and changes in indices of NAFLD are summarized in Figure 1 and presented in detail in online Supplementary Table S3. In model 2, we observed significant (p < 0.001) associations between each daily 10% increment in UPF consumption and greater FLI (β 1.60, 95% CI 1.24; 1.96) and HSI (0.43, 0.29; 0.57) over the 1-year follow-up. Comparison across increasing quintiles of UPF consumption revealed a significant dose–response relationship with both NAFLD indices (p for trend < 0.001): FLI (β estimates for Q5 3.73, 95% CI 3.10; 4.35) and HSI (0.93, 0.67; 1.18).
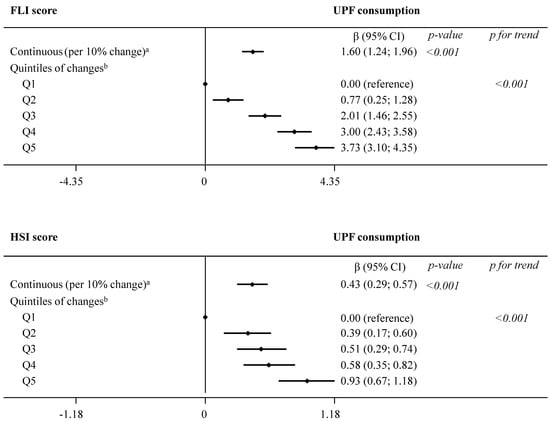
Figure 1.
Dose–response relationship in the association between concurrent changes in UPF consumption (% of g/day) and changes in NAFLD indices during 1 year of follow-up (fully adjusted model 2). The consumption of UPF was expressed as a percentage of total food and beverage intake in g/day. Daily intake of beverages was collected in cubic centimeters and then converted into milliliters (1 cc = 1 mL), and further into grams, assuming that 1 mL = 1 g. Mixed-effects linear modelling for repeated measures with random intercepts at recruiting center, cluster family, and patient level were used after controlling in fully adjusted model 2 for baseline variables, such as age, sex, study arm, educational level, smoking habits, and height, as well as repeatedly measured physical activity, sedentary behavior, alcohol intake, and follow-up time. a Estimates β are interpreted as changes in NAFLD associated with increments of 10% in UPF consumption. b Estimates β are interpreted as changes in NAFLD indices in each sex-specific quintile of UPF consumption, compared to quintile 1, the reference category. Abbreviations: FLI—fatty liver index; HSI—hepatic steatosis index; NAFLD—non-alcoholic fatty liver disease; UPF—ultra-processed foods.
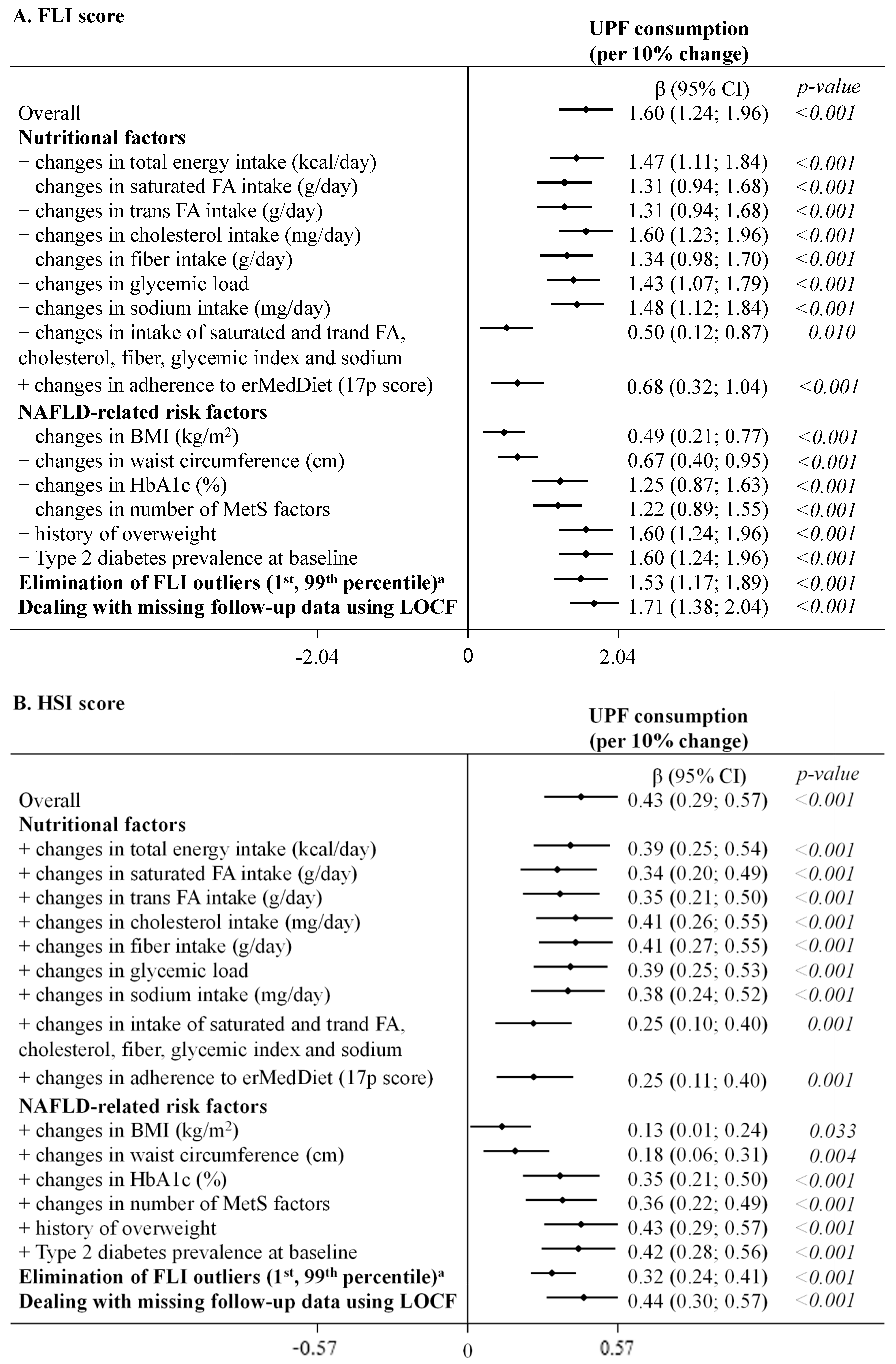
As highlighted in Figure 2 (UPF coded as continuous variable) and shown in detail in online Supplementary Table S4 (UPF coded as continuous and sex-specific quintiles), results remained statistically significant after further adjustments in sensitivity analysis. Only simultaneous adjustment for several factors related to nutritional quality of the diet (saturated and trans FA, cholesterol, fiber, glycemic load and sodium) and an adherence to erMedDiet, as well as BMI and waist circumference, decreased point estimates, yet the associations of UPF consumption with both NAFLD indices remained statistically significant. A similar pattern was observed when the exposure was coded in quintiles (online Supplementary Table S4).

Figure 2.
Summary of the sensitivity analysis for the association between concurrent changes in UPF consumption (% of g/day, continuous variable) and changes in NAFLD indices during 1 year of follow-up (fully adjusted model 2). The consumption of UPF was expressed as a percentage of total food and beverage intake in g/day. Daily intake of beverages was collected in cubic centimeters and then converted into milliliters (1 cc = 1 mL), and further into grams, assuming that 1 mL = 1 g. Mixed-effects linear modelling for repeated measures with random intercepts at recruiting center, cluster family and patient level were used after controlling in fully adjusted model 2 for baseline variables, such as age, sex, study arm, educational level, smoking habits, and height, as well as repeatedly measured physical activity, sedentary behavior, alcohol intake, follow-up time, and use of antidiabetic medications (for models with HbA1c). Estimates β are interpreted as changes in NAFLD indices associated with increments of 10% in UPF consumption. a Outliers (1st, 99th percentile) in the outcome variables were eliminated at baseline and follow-up (for FLI total n = 318, for HSI total n = 316). Abbreviations: BMI—body mass index; erMedDiet—energy-restricted Mediterranean Diet; FA—fatty acids; FLI—Fatty liver index; HbA1c—glycated hemoglobin; HSI—Hepatic steatosis index; LOCF—last observation carried forward; MetS—metabolic syndrome; NAFLD—non-alcoholic fatty liver disease; UPF—ultra-processed foods.
In the mediation analysis, we found that changes in nutritional factors partly mediated the association with concurrent changes in NAFLD indices (Table 3 and online Supplementary Table S5). Among them, changes in nutritional characteristics of UPF, such as saturated and trans FA, mediated 17–21% of the associations for both indices, and fiber and glycemic load explained 15% and 11% of the association for FLI, respectively; whereas changes in intake of total energy, sodium, and cholesterol did not mediate any of the associations. Moreover, changes in adherence to erMedDiet acted as mediator in 58% and 43% for FLI and HSI, respectively. As far as NAFLD-related biomarkers (known risk factors and components of both scores) are concerned, changes in BMI were responsible in 69% for the association between concurrent changes in UPF consumption and both FLI and HSI; whereas, waist circumference was responsible in 56% of the association for FLI and in 82% for HSI. Furthermore, in the case of FLI, the association with UPF was driven by changes in triglycerides and MetS factors (both in 26%), followed by changes in Hba1c (14%). In turn, in case of HSI, the association was driven by the ALT/AST ratio (39%), ALT and MetS factors (both in 16%), and changes in HbA1c (15%). Changes in GGT and AST did not mediate the respective associations for FLI and HSI.

Table 3.
Proportion of the association between concurrent changes in UPF consumption (% of g/day, continuous variable) and changes in NAFLD indices during 1 year of follow-up mediated through nutritional factors and NAFLD-related biomarkers (fully adjusted model 2).
In subgroup analyses (online Supplementary Table S6), we found that the direct association between UPF consumption and FLI was slightly more pronounced in non-diabetics (β 1.73, 95% CI 1.29; 2.18, p < 0.001) than in diabetics (1.29, 0.68; 1.90, <0.001) (p for interaction = 0.027).
In additional analyses (online Supplementary Table S7), we found that all UPF subgroups contributed to observed associations with NAFLD indices. In particular, pre-prepared dishes, snacks, and fast-foods, as well as processed meats and sweets, showed the strongest statistically significant associations (all p-values < 0.001) with both liver scores. In turn, the subgroup of alcoholic beverages was only strongly associated with FLI.
4. Discussion
In this large prospective cohort study of older adults with overweight/obesity and MetS from Spain, a Mediterranean country, we found that UPF consumption was associated with worse liver health, assessed using biomarkers related to NAFLD. This direct association was ascertained using sophisticated analyses and validated tools, and was robust after accounting for a wide range of indicators related to nutritional quantity and quality of the diet and NAFLD risk.
Given the mounting body of evidence showing associations of UPF consumption with well-known risk factors for NAFLD, such as obesity [12,13,14,15], type 2 diabetes [17], or hypertension [42,43], our findings were not unexpected. Moreover, they are in line with recent evidence on the role of diet in NAFLD [4], pointing to several UPF, such as processed meat [44] and SSB [45], as culprits in objectively-determined NAFLD development. However, studies on diet-NAFLD risk have rarely been prospective, and few of them aggregated foods according to the nature, extent, and purpose of processing [23,24]. In this sense, our findings corroborate recent results from TCLSIH prospective cohort in China, showing the link between UPF consumption and risk of developing NAFLD, diagnosed using ultrasonography [24]. Here, we support this link using repeatedly measured dietary habits in a different population from a Mediterranean country. It needs to be underlined that processing not always has to be negative, as it also can increase the safety and shelf-life of foods and beverages. However, ultra-processing combines several ingredients with little, if any, intact whole foods, which results in the creation of new products with nutritionally imbalanced properties [5,6].
Several putative mechanisms of action could be responsible for the link between UPF and liver fat accumulation. The first is the nutritional characteristics of UPF, which is poor due to the industrial manipulations that they undergo. For instance, the incorporation of sizable amounts of saturated and trans FA may increase product stability and palatability [46], and these nutrients have been associated with increased liver fat in humans and rodents [4,47]. It should be mentioned that there has been significant progress towards 2023, the target for elimination of industrially produced trans FA around the world [48]. In the European Union countries, the regulation limiting the use of artificial trans FA came into force just recently (April 2021) [49]. However, given that dietary data used in this study were collected before that (2013–2017), we could still estimate the intake of trans FA in this population. Furthermore, low fiber content is a common attribute of UPF, and the breakdown of natural food matrix during ultra-transformation might also reduce its quantity. Recent findings from a large cross-sectional study showed an inverse association of dietary fiber with NAFLD [50], and this could be explained through the effects of fiber on microbiota as well as on satiation and satiety. UPF, including beverages, usually lead to postprandial hyperglycemia due to a high content of refined carbohydrates, such as white flour and sugar, and simultaneous fiber, water, and protein deprivation [51]. Food glycemic responses have been implicated in liver fat mass accretion through alterations in glucose, insulin, and lipid metabolism [52,53]. In our multivariate analyses, we found that the associations between UPF and liver scores were attenuated but remained statistically significant after further adjustment for these nutritional factors (i.e., saturated and trans FA, fiber, and glycemic load). Mediation analyses revealed that the quality of fat (saturated and trans FA) and carbohydrate (fiber and glycemic load) contributed (11 to 21%) to this pooled effect. This could indicate that these nutritional attributes of UPF might explain part of the observed associations, but clearly not the overall effect. This suggests that mechanisms beyond the nutritional dimension of UPF might also be responsible for the observed associations.
Another potential mechanism responsible for the association between UPF and liver health could be related to additives used during the ultra-processing of these products. In this regard, although the health properties of non-nutritional additives are relatively underexplored in humans, current research performed in rodents and cell lines suggests that they can be harmful for the liver, and the effect could be partly mediated through imbalances of gut microbiota [54]. Some artificial sweeteners (i.e., saccharin, aspartame), emulsifiers (i.e., polysorbate 80), preservatives (i.e., benzoic acid), and flavor enhancers (i.e., monosodium glutamate) could lead to transaminitis, steatosis, degradation, and toxicity in the liver of rodents [54,55,56,57]. We could not explore the potential mediating role of additive content because this information is not yet available in most food composition tables.
In addition, UPF composition may indirectly lead to excessive hepatic lipid accumulation, given their ability to displace healthy foods affecting overall diet quality. In mediation analyses we confirmed that a low adherence to MedDiet explained approximately half of the studied association. Overall diet quality and consumption of UPF are two different but complementary nutritional dimensions to consider in relation to health, given that they could offset one another. A high-quality dietary pattern such as the MedDiet is presumed to be beneficial for NAFLD management [3,4]. An interesting result obtained in our sensitivity analyses suggests that the associations between consumption of UPF and NAFLD indices were attenuated after adjusting for MedDiet adherence, albeit it remained statistically significant. All in all, the potential mechanisms by which UPF consumption may be related to NAFLD is speculative and warrants future studies.
Although not the sole determinant, obesity is recognized as a major risk factor for NAFLD [1,2], whereas type 2 diabetes and MetS are considered as clinical factors that coexist with NAFLD in a bi-directional relationship [58,59]. In this sense, we found that the direct associations between UPF consumption and liver health scores remained significant after adjustment for BMI or waist circumference, albeit their strength markedly diminished. Mediation analyses confirmed that a substantial proportion of the association was driven by obesity, either overall (69%) or abdominal (56% for FLI and 82% for his). In turn, the proportion mediated by type 2 diabetes, MetS, or triglycerides was lower. We have previously reported in the PREDIMED-Plus cohort by using imaging technique that consumption of UPF affected to a similar extent visceral and total fat [12], potentially leading to NAFLD and other diseases. Other large cross-sectional and prospective studies in adults have also shown strong and direct association between UPF and type 2 diabetes [17], MetS [60], and hypertension [42,43]. Regarding markers of liver function, only in the case of the HSI score was the association driven in part by its enzymatic component ALT, and particularly the ALT/AST ratio. The latter has been considered as more accurate than each of these enzymes alone [61]. There is evidence from cross-sectional and cohort studies with healthy adults on a relationship of SSB and fast foods with greater levels of ALT and the ALT/AST ratio [45,62,63]. It needs to be underlined that all PREDIMED-Plus participants were overweight/obese with MetS, and some also had type 2 diabetes (≈27%); hence, future longitudinal studies with healthier individuals are warranted to ascertain what mechanisms underly the association between UPF and liver health.
Beyond the prospective design, control for a wide set of confounders, and performance of a series of sensitivity and stratified analyses, a marked strength of the present study was the use of a large and homogenous sample of men and women within a narrow range of age, BMI, and health conditions. Of note, a unique feature of the present study is that both exposure and outcome were repeatedly measured at the same points in time, potentially decreasing the risk of reverse causality. This is relevant as eating behaviors change over time, given the nutritional intervention given to participants in the PREDIMED-Plus trial [64].
Our study also has limitations. First, its observational nature enabled the identifications of associations only. Second, the participants were older individuals with overweight/obesity and MetS from a Mediterranean area, which limits the generalizability of our findings. However, the described health profile is quite common in modern societies. Third, measurement error is unavoidable when using self-reported dietary data, even though we undertook some actions to improve measurement precision; namely, the FFQ was previously validated in a Spanish population and administrated repeatedly (each 6 months) to the participants by trained dietitians during face-to-face interviews. Moreover, participants with implausible total energy intake values were a priori excluded from analyses, and models were adjusted for total energy intake changes in sensitivity analyses. Fourth, some misclassification in NOVA groups cannot be ruled out, as the FFQ used was not designed to capture details on food processing, and the definition of UPF in the NOVA system is rather broad, allowing multiple interpretations. However, the FFQ items were classified into processing groups with caution and consensus was reached between experts in nutrition and epidemiology. Last but not least, liver fat was estimated based on two surrogate indices, but was not directly measured using imaging techniques. However, both NAFLD algorithms have been validated and have shown good agreement with ultrasonography [37,38].
5. Conclusions
In this prospective study we revealed that in older adults with chronic health conditions, consumption of UPF was directly and robustly associated with FLI and HSI scores. Furthermore, these associations were only to a lesser extent explained by the nutritional characteristics of UPF, pointing out the potential and uncovered role of factors related to the processing itself (i.e., non-nutritional chemicals and food matrix breakdown). Future prospective studies in different contexts and with more precise imaging techniques are warranted to confirm our findings on liver fat accumulation, as well as future toxicological, technological, and human experimental studies to clarify underlying mechanisms and develop detection methods for components generated through food processing. With this study we provide novel insights into the recently growing body of evidence on food processing and health risk. The accumulation of firm and high-quality evidence would help global health authorities to update dietary recommendations and food policies by considering criteria of food processing, imposing restrictions to marketing, use of additives, and types of packaging in food technology and trade. Discouragement of UPF consumption and favoring instead fresh or minimally processed foods should be considered by health care providers as a valid preventive and treatment strategy for NAFLD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14194142/s1, Figure S1: Flow chart for the selection of participants for analysis; Figure S2: Directed acyclic graph (DAG); Table S1: Examples of food and beverage items considered as NOVA processing groups; Table S2: Characteristics of the study participants at baseline, 6 months and 12 months of follow-up; Table S3: Association between concurrent changes in UPF consumption (% of g/day) and changes in NAFLD indices during 1-year of follow-up; Table S4: Sensitivity analysis for the association between concurrent changes in UPF consumption (% of g/day) and changes in NAFLD indices during 1-year of follow-up; Table S5: Mediation analysis for the association between concurrent changes in UPF consumption (% of g/day, continuous variable) and changes in NAFLD indices during 1-year of follow-up, through nutritional factors and NAFLD-related biomarkers; Table S6: Association between concurrent changes in UPF consumption (% of g/day) and changes in NAFLD indices during 1-year of follow-up by subgroups; Table S7: Association between concurrent changes in consumption of specific food subgroups within UPF (% of g/day) and changes in NAFLD indices during 1-year of follow-up; Text S1: Procedure for mediation analysis; File S1: Group information.
Author Contributions
Conceptualization, J.K. and D.R.; Methodology, J.K. and D.R.; Validation, J.K. and D.R.; Formal Analysis, J.K. and D.R.; Investigation, J.K., M.F., A.C., M.Á.M.-G., J.S.-S., D.C., M.T.S.-F., J.A.M., Á.M.A.-G., J.W., J.V. (Jesus Vioque), J.L.-M., R.E., M.R.B.-L., J.L., L.S.-M., A.B.-C., J.A.T., V.M.S., X.P., J.J.G., P.M.-M., J.V. (Josep Vidal), C.V., L.D., E.R., M.B.-R., M.P., J.V.S., A.G., M.Á.Z., A.M.-R., F.J.C.G., R.V.-E., J.M.J., A.G.-R., R.C., A.M.G.-P., J.M.S.-L., F.J.B.-G., M.Á.M., C.O.-A., J.B., I.A., I.S.-L., M.R.-C., N.B., L.C. and D.R.; Resources, J.K., M.F., A.C., M.Á.M.-G., J.S.-S., D.C., M.T.S.-F., J.A.M., Á.M.A.-G., J.W., J.V. (Jesus Vioque), J.L.-M., R.E., M.R.B.-L., J.L., L.S.-M., A.B.-C., J.A.T., V.M.S., X.P., J.J.G., P.M.-M., J.V. (Josep Vidal), C.V., L.D., E.R., M.B.-R., M.P., J.V.S., A.G., M.Á.Z., A.M.-R., F.J.C.G., R.V.-E., J.M.J., A.G.-R., R.C., A.M.G.-P., J.M.S.-L., F.J.B.-G., M.Á.M., C.O.-A., J.B., I.A., I.S.-L., M.R.-C., N.B., L.C. and D.R.; Data Curation, J.K., M.F., A.C., M.Á.M.-G., J.S.-S., D.C., M.T.S.-F., J.A.M., Á.M.A.-G., J.W., J.V. (Jesus Vioque), J.L.-M., R.E., M.R.B.-L., J.L., L.S.-M., A.B.-C., J.A.T., V.M.S., X.P., J.J.G., P.M.-M., J.V. (Josep Vidal), C.V., L.D., E.R., M.B.-R., M.P., J.V.S., A.G., M.Á.Z., A.M.-R., F.J.C.G., R.V.-E., J.M.J., A.G.-R., R.C., A.M.G.-P., J.M.S.-L., F.J.B.-G., M.Á.M., C.O.-A., J.B., I.A., I.S.-L., M.R.-C., N.B., L.C. and D.R.; Writing—Original Draft Preparation, J.K. and D.R.; Writing—Review & Editing, J.K., M.F., A.C., M.Á.M.-G., J.S.-S., D.C., M.T.S.-F., J.A.M., Á.M.A.-G., J.W., J.V. (Jesus Vioque), J.L.-M., R.E., M.R.B.-L., J.L., L.S.-M., A.B.-C., J.A.T., V.M.S., X.P., J.J.G., P.M.-M., J.V. (Josep Vidal), C.V., L.D., E.R., M.B.-R., M.P., J.V.S., A.G., M.Á.Z., A.M.-R., F.J.C.G., R.V.-E., J.M.J., A.G.-R., R.C., A.M.G.-P., J.M.S.-L., F.J.B.-G., M.Á.M., C.O.-A., J.B., I.A., I.S.-L., M.R.-C., N.B., L.C. and D.R.; Visualization, J.K., M.F., A.C., M.Á.M.-G., J.S.-S., D.C., M.T.S.-F., J.A.M., Á.M.A.-G., J.W., J.V.(Jesus Vioque), J.L.-M., R.E., M.R.B.-L., J.L., L.S.-M., A.B.-C., J.A.T., V.M.S., X.P., J.J.G., P.M.-M., J.V. (Josep Vidal), C.V., L.D., E.R., M.B.-R., M.P., J.V.S., A.G., M.Á.Z., A.M.-R., F.J.C.G., R.V.-E., J.M.J., A.G.-R., R.C., A.M.G.-P., J.M.S.-L., F.J.B.-G., M.Á.M., C.O.-A., J.B., I.A., I.S.-L., M.R.-C., N.B., L.C. and D.R.; Supervision, D.R.; Project Administration, J.S.-S., M.Á.M.-G., R.E., E.R., D.C.; Funding Acquisition, J.K., M.F., A.C., M.Á.M.-G., J.S.-S., D.C., M.T.S.-F., J.A.M., Á.M.A.-G., J.W., J.V. (Jesus Vioque), J.L.-M., R.E., M.R.B.-L., J.L., L.S.-M., A.B.-C., J.A.T., V.M.S., X.P., J.J.G., P.M.-M., J.V. (Josep Vidal), C.V., L.D., E.R., M.B.-R., M.P., J.V.S., A.G., M.Á.Z., A.M.-R., F.J.C.G., R.V.-E., J.M.J., A.G.-R., R.C., A.M.G.-P., J.M.S.-L., F.J.B.-G., M.Á.M., C.O.-A., J.B., I.A., I.S.-L., M.R.-C., N.B., L.C. and D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Research Council (Advanced Research Grant 2014–2019; agreement #340918) granted to MÁM-G and the Spanish National Health Institute of Health Carlos III (ISCIII), through CIBEROBN and “Fondo de Investigación para la Salud” (FIS), which is co-funded by the European Regional Development Fund (six coordinated FIS projects led by JS-S and JVi, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, PI20/01158, PI21/00465); the Especial Action Project entitled: Implementación y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus grant to JS-S; the Recercaixa (number 2013ACUP00194) grant to JS-S; grants from the Consejería de Salud de la Junta de Andalucía (PI0458/2013, PS0358/2016, PI0137/2018, RH-0024-2021); the PROMETEO/2017/017 grant from the Generalitat Valenciana; and the SEMERGEN grant; Juan de la Cierva-Incorporación research grant (IJC2019-042420-I) of the Spanish Ministry of Economy, Industry and Competitiveness and European Social Funds to JK; Miguel Servet Tipo 2 research grant (CPII20/00014) of the Spanish National Health Institute of Health Carlos III (ISCIII) to MRBL This work was also partially supported by ICREA under the ICREA Academia programme. None of the funding sources took part in the design, collection, analysis, interpretation of the data, or writing the report, or in the decision to submit the manuscript for publication.
Institutional Review Board Statement
The study protocol was approved by the Research Ethics Committees from all recruiting centers according to the ethical standards of the Declaration of Helsinki: CEI Provincial de Málaga-Servicio Andaluz de Salud (O01_feb_PR2); CEI de los Hospitales Universitarios Virgen Macarena y Virgen del Rocío-Servicio Andaluz de Salud (PI13/00673); CEIC Universidad de Navarra (053/2013); CEI de las Illes Balears—Conselleria de Salut Direcció General de Salut Publica i Consum (IB 2242/14 PI); CEIC del Hospital Clínic de Barcelona (HCB/2016/0287); CEIC Parc de Salut Mar y IDIAP Jordi Gol (PI13/120); CEIC del Hospital Universitari Sant Joan de Reus y IDIAB Jordi Gol (13-07-25/7proj2); CEI de la Provincia de Granada- Servicio Andaluz de Salud (MAB/BGP/pg); CEIC de la Fundacion Jiménez Díaz (EC 26-14/IIS-FJD); CEIC Universidad de Navarra (053/2013); CEIC Euskadi (PI2014044); CEIC Corporativo de Atención Primaria de la Comunitat Valenciana (2011-005398-22); CEI Humana de la Universidad de las Palmas de Gran Canaria (CEIH-2013-07); CEIC del Hospital de Bellvitge (PR240/13); CEI de Cordoba-Junta de Salud (3078); CEI de la Fundación IMDEA Alimentación (PI-012); CEIC Hospital Clínico San Carlos de Madrid-Piloto-CEIC Servicio Madrileño de salud-General (30/15); CEI Provincial de Málaga-Servicio Andaluz de Salud; CEI de las Illes Balears—Conselleria de Salut Direcció General de Salut Publica i Consum (IB 2251/14 PI); CEIC del Hospital Clínic de Barcelona (HCB/2017/0351); CEIC del Hospital General Universitario de Alicante (CEIC PI2017/02); CEIC de la Investigación Biomédica de Andalucía (CCEIBA); and CEI de la Universidad de León (ÉTICA-ULE-014-2015).
Informed Consent Statement
All participants provided written informed consent.
Data Availability Statement
There are restrictions on data availability for the PREDIMED-Plus trial due to the signed consent agreements around data sharing, which only allow access to external researchers for studies following the project purposes. Requestors wishing to access the PREDIMED-Plus trial data used in this study can make a request to the PREDIMED-Plus trial Steering Committee chair: jordi.salas@urv.cat. The request will then be passed to members of the PREDIMED-Plus Steering Committee for deliberation.
Trial Registration
The trial was registered at the International Standard Randomized Controlled Trial (ISRCTN: http://www.isrctn.com/ISRCTN89898870) with number 89898870 and registration date of 24 July 2014, retrospectively registered.
Acknowledgments
We are grateful to the PREDIMED-Plus volunteers for their participation and to the personnel, investigators, and primary care centers for their contribution. (Supplementary File S1).
Conflicts of Interest
E Ros reports grants, personal fees, non-financial support, and other support from the California Walnut Commission during the conduct of the study; grants, personal fees, non-financial support and other support from Alexion; personal fees, non-financial support and other support from Ferrer International and Danone; and personal fees and other from Amarin, outside the submitted work. R Estruch reports grants from Fundación Dieta Mediterránea, Spain and Cerveza y Salud, Spain. In addition, personal fees for lectures given from Brewers of Europe, Belgium, Fundación Cerveza y Salud, Spain, Pernaud-Ricard, Mexico, Instituto Cervantes, Alburquerque, USA, Instituto Cervantes, Milan, Italy, Instituto Cervantes, Tokyo, Japan, Lilly Laboratories, Spain, and Wine and Culinary International Forum, Spain, and non-financial support to organize a National Congress on Nutrition. In addition, feeding trials with product from Grand Fountain and Uriach Laboratories, Spain. J Salas-Salvadó reports receiving research support from the Instituto de Salud Carlos III, Ministerio de Educación y Ciencia, the European Commission, the USA National Institutes of Health; receiving consulting fees or travel expenses from Eroski Foundation, Instituto Danone, Nestle, and Abbott Laboratories; receiving nonfinancial support from Hojiblanca, Patrimonio Comunal Olivarero, the California Walnut Commission, Almond Board of California, La Morella Nuts, Pistachio Growers, and Borges S.A; serving on the board of and receiving grant support through his institution from the International Nut and Dried Foundation and the Eroski Foundation; personal fees from Instituto Danone; and serving in the Board of Danone Institute International. R Casas reports receiving fees of educational conferences from Fundación para la investigación del Vino y la Nutrición (FIVIN), Spain. The other authors declare no conflicts of interest.
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CVD | cardiovascular disease |
| er | energy-restricted |
| FA | fatty acids |
| FFQ | food frequency questionnaire |
| FLI | fatty liver Index |
| GGT | gamma-glutamyl transferase |
| HbA1c | glycated hemoglobin |
| HDL-c | high-density lipoprotein cholesterol |
| HSI | hepatic steatosis index |
| LOCF | last observation carried forward |
| MedDiet | Mediterranean diet |
| MetS | metabolic syndrome |
| NAFLD | non-alcoholic fatty liver disease |
| PA | physical activity |
| PREDIMED-Plus | PREvención con DIeta MEDiterránea Plus |
| SB | sedentary behavior |
| SSB | sugar-sweetened beverages |
| TCLSIH | Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study |
| UPF | ultra-processed foods |
References
- Ofosu, A.; Ramai, D.; Reddy, M. Non-alcoholic fatty liver disease: Controlling an emerging epidemic, challenges, and future directions. Ann. Gastroenterol. 2018, 31, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Non-alcoholic fatty liver disease in 2015. World J. Hepatol. 2015, 7, 1450. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Bastarrika, G. Mediterranean diet as the ideal model for preventing non-alcoholic fatty liver disease (NAFLD). Hepatobiliary Surg. Nutr. 2020, 9, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C.; Jaime, P.; Paula Martins, A.; Canella, D.; Louzada, M.; Parra, D. NOVA. The star shines bright. [Food classification. Public health]. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Morales, F.J.; Mesías, M.; Delgado-Andrade, C. Association between Heat-Induced Chemical Markers and Ultra-Processed Foods: A Case Study on Breakfast Cereals. Nutrients 2020, 12, 1418. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.-C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Martín-Calvo, N. Ultraprocessed Foods and Public Health: A Need for Education. Mayo Clin. Proc. 2019, 94, 2156–2157. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Konieczna, J.; Morey, M.; Abete, I.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Vioque, J.; Gonzalez-Palacios, S.; Daimiel, L.; Salas-Salvadó, J.; Fiol, M.; et al. Contribution of ultra-processed foods in visceral fat deposition and other adiposity indicators: Prospective analysis nested in the PREDIMED-Plus trial. Clin. Nutr. 2021, 40, 4290–4300. [Google Scholar] [CrossRef]
- Mendonça, R.D.; Pimenta, A.M.; Gea, A.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Lopes, A.C.S.; Bes-Rastrollo, M. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2016, 104, 1433–1440. [Google Scholar] [CrossRef]
- Canhada, S.L.; Luft, V.C.; Giatti, L.; Duncan, B.B.; Chor, D.; da Fonseca, M.d.J.M.; Matos, S.M.A.; Molina, M.D.C.B.; Barreto, S.M.; Levy, R.B.; et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr. 2020, 23, 1076–1086. [Google Scholar] [CrossRef]
- Beslay, M.; Srour, B.; Méjean, C.; Allès, B.; Fiolet, T.; Debras, C.; Chazelas, E.; Deschasaux, M.; Wendeu-Foyet, M.G.; Hercberg, S.; et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: A prospective analysis of the French NutriNet-Santé cohort. PLoS Med. 2020, 17, e1003256. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern. Med. 2020, 180, 283. [Google Scholar] [CrossRef]
- Llavero-Valero, M.; Escalada-San Martín, J.; Martínez-González, M.A.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Bes-Rastrollo, M. Ultra-processed foods and type-2 diabetes risk in the SUN project: A prospective cohort study. Clin. Nutr. 2021, 40, 2817–2824. [Google Scholar] [CrossRef]
- Rey-García, J.; Donat-Vargas, C.; Sandoval-Insausti, H.; Bayan-Bravo, A.; Moreno-Franco, B.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Ultra-Processed food consumption is associated with renal function decline in older adults: A prospective cohort study. Nutrients 2021, 13, 428. [Google Scholar] [CrossRef]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef]
- Alonso-Pedrero, L.; Ojeda-Rodríguez, A.; Martínez-González, M.A.; Zalba, G.; Bes-Rastrollo, M.; Marti, A. Ultra-processed food consumption and the risk of short telomeres in an elderly population of the Seguimiento Universidad de Navarra (SUN) Project. Am. J. Clin. Nutr. 2020, 111, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hu, E.A.; Rebholz, C.M. Ultra-processed food intake and mortality in the USA: Results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr. 2019, 22, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Webb, M.; Bentov, I.I.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int. 2021, 41, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, S.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Gu, Y.; Wang, Y.; Zhang, T.; et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study. Int. J. Epidemiol. 2021, 51, 237–249. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Buil-Cosiales, P.; Corella, D.; Bulló, M.; Fitó, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; López-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019, 48, 387–388. [Google Scholar] [CrossRef]
- Sayón-Orea, C.; Razquin, C.; Bulló, M.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; Wärnberg, J.; Martínez, J.A.; et al. Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence among Patients with Metabolic Syndrome. JAMA 2019, 322, 1486. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; International Diabetes Federation Task Force on Epidemiology and Prevention; et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: Oxford, UK, 2013; 529p. [Google Scholar]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef]
- de la Fuente-Arrillaga, C.; Vázquez Ruiz, Z.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos “Food Composition Table”, 16th ed.; Pirámide: Madrid, Spain, 2013. [Google Scholar]
- Salmerón, J. Dietary Fiber, Glycemic Load, and Risk of Non—Insulin-dependent Diabetes Mellitus in Women. JAMA J. Am. Med. Assoc. 1997, 277, 472. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Molina, L.; Sarmiento, M.; Peñafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schroder, H.; et al. Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.; Yoon, J.; Cho, S.; Sung, M.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package “dagitty”. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef]
- Iacobucci, D.; Saldanha, N.; Deng, X. A Meditation on Mediation: Evidence That Structural Equations Models Perform Better Than Regressions. J. Consum. Psychol. 2007, 17, 139–153. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Musio, A.; Marchesini, G. Moderate Alcohol Intake in Non-Alcoholic Fatty Liver Disease: To Drink or Not to Drink? Nutrients 2019, 11, 3048. [Google Scholar] [CrossRef]
- Mendonça, R.d.D.; Lopes, A.C.S.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens. 2016, 30, hpw137. [Google Scholar] [CrossRef]
- De Oliveira da Silva Scaranni, P.; De Oliveira Cardoso, L.; Chor, D.; Prates Melo, E.C.; Alvim Matos, S.M.; Giatti, L.; Barreto, S.M.; da Fonseca, M.J.M. Ultra-processed foods, changes in blood pressure and incidence of hypertension: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr. 2021, 24, 3352–3360. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fox, C.S.; Jacques, P.F.; Speliotes, E.K.; Hoffmann, U.; Smith, C.E.; Saltzman, E.; McKeown, N.M. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J. Hepatol. 2015, 63, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. Technology, Diet, and the Burden of Chronic Disease. JAMA 2011, 305, 1352. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Banach, M. Link between plasma trans-fatty acid and fatty liver is moderated by adiposity. Int. J. Cardiol. 2018, 272, 316–322. [Google Scholar] [CrossRef]
- World Health Organization. Countdown to 2023: WHO Report on Global Trans-Fat Elimination 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, S.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Bao, X.; Gu, Y.; Sun, S.; Wang, X.; et al. Insoluble dietary fibre intake is associated with lower prevalence of newly-diagnosed non-alcoholic fatty liver disease in Chinese men: A large population-based cross-sectional study. Nutr. Metab. 2020, 17, 4. [Google Scholar] [CrossRef]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which Foods May Be Addictive? The Roles of Processing, Fat Content, and Glycemic Load. Weir TL, editor. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef]
- Parker, A.; Kim, Y. The Effect of Low Glycemic Index and Glycemic Load Diets on Hepatic Fat Mass, Insulin Resistance, and Blood Lipid Panels in Individuals with Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord. 2019, 17, 389–396. [Google Scholar] [CrossRef]
- Valtueña, S.; Pellegrini, N.; Ardigò, D.; Del Rio, D.; Numeroso, F.; Scazzina, F.; Monti, L.; Zavaroni, I.; Brighenti, F. Dietary glycemic index and liver steatosis. Am. J. Clin. Nutr. 2006, 84, 136–142. [Google Scholar] [CrossRef]
- Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M. Dietary Additives and Supplements Revisited: The Fewer, the Safer for Gut and Liver Health. Curr. Pharmacol. Rep. 2019, 5, 303–316. [Google Scholar] [CrossRef]
- Hassan, M.M.; Elrrigieg, M.A.A.; Sabahelkhier, M.; Idris, O. Impacts of the food additive benzoic acid on liver function of Wistar rats. Int. J. Adv. Res. 2016, 4, 568–575. [Google Scholar] [CrossRef]
- Elbassuoni, E.A.; Ragy, M.M.; Ahmed, S.M. Evidence of the protective effect of l-arginine and vitamin D against monosodium glutamate-induced liver and kidney dysfunction in rats. Biomed. Pharmacother. 2018, 108, 799–808. [Google Scholar] [CrossRef]
- Singh, R.K. Food additive P-80 impacts mouse gut microbiota promoting intestinal inflammation, obesity and liver dysfunction. SOJ Microbiol. Infect. Dis. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Xia, M.-F.; Bian, H.; Gao, X. NAFLD and Diabetes: Two Sides of the Same Coin? Rationale for Gene-Based Personalized NAFLD Treatment. Front. Pharmacol. 2019, 10, 877. [Google Scholar] [CrossRef]
- Lonardo, A.; Leoni, S.; Alswat, K.A.; Fouad, Y. History of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 5888. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Juul, F.; Neri, D.; Rauber, F.; Monteiro, C.A. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev. Med. 2019, 125, 40–48. [Google Scholar] [CrossRef]
- Long, M.T.; Pedley, A.; Colantonio, L.D.; Massaro, J.M.; Hoffmann, U.; Muntner, P.; Fox, C.S. Development and Validation of the Framingham Steatosis Index to Identify Persons With Hepatic Steatosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1172–1180.e2. [Google Scholar] [CrossRef]
- Shimony, M.K.; Schliep, K.C.; Schisterman, E.F.; Ahrens, K.A.; Sjaarda, L.A.; Rotman, Y.; Perkins, N.J.; Pollack, A.Z.; Wactawski-Wende, J.; Mumford, S.L.; et al. The relationship between sugar-sweetened beverages and liver enzymes among healthy premenopausal women: A prospective cohort study. Eur. J. Nutr. 2016, 55, 569–576. [Google Scholar] [CrossRef]
- Mirmiran, P.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Elevated serum levels of aminotransferases in relation to unhealthy foods intake: Tehran lipid and glucose study. BMC Endocr. Disord. 2019, 19, 100. [Google Scholar] [CrossRef]
- Smith, J.D.; Hou, T.; Hu, F.B.; Rimm, E.B.; Spiegelman, D.; Willett, W.C.; Mozaffarian, D. A Comparison of different methods for evaluating diet, physical activity, and long-term weight gain in 3 prospective cohort studies. J. Nutr. 2015, 145, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).