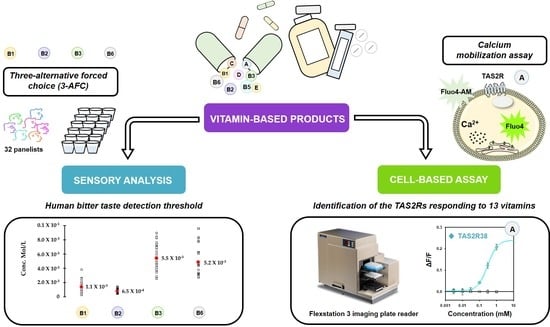
Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Taste Receptor Functional Assay
2.1.1. Chemical
2.1.2. Expression of TAS2Rs in Heterologous Cells
2.1.3. Calcium Mobilization Assay
2.1.4. Determination of Half-Maximal Effective Concentrations (EC50)
2.1.5. Determination of the Ideal Concentration
2.2. Bitter Taste Detection Threshold in Humans
2.2.1. Stimuli
2.2.2. Three-Alternative Forced Choice (3-AFC)
2.2.3. Statistical Analysis
3. Results
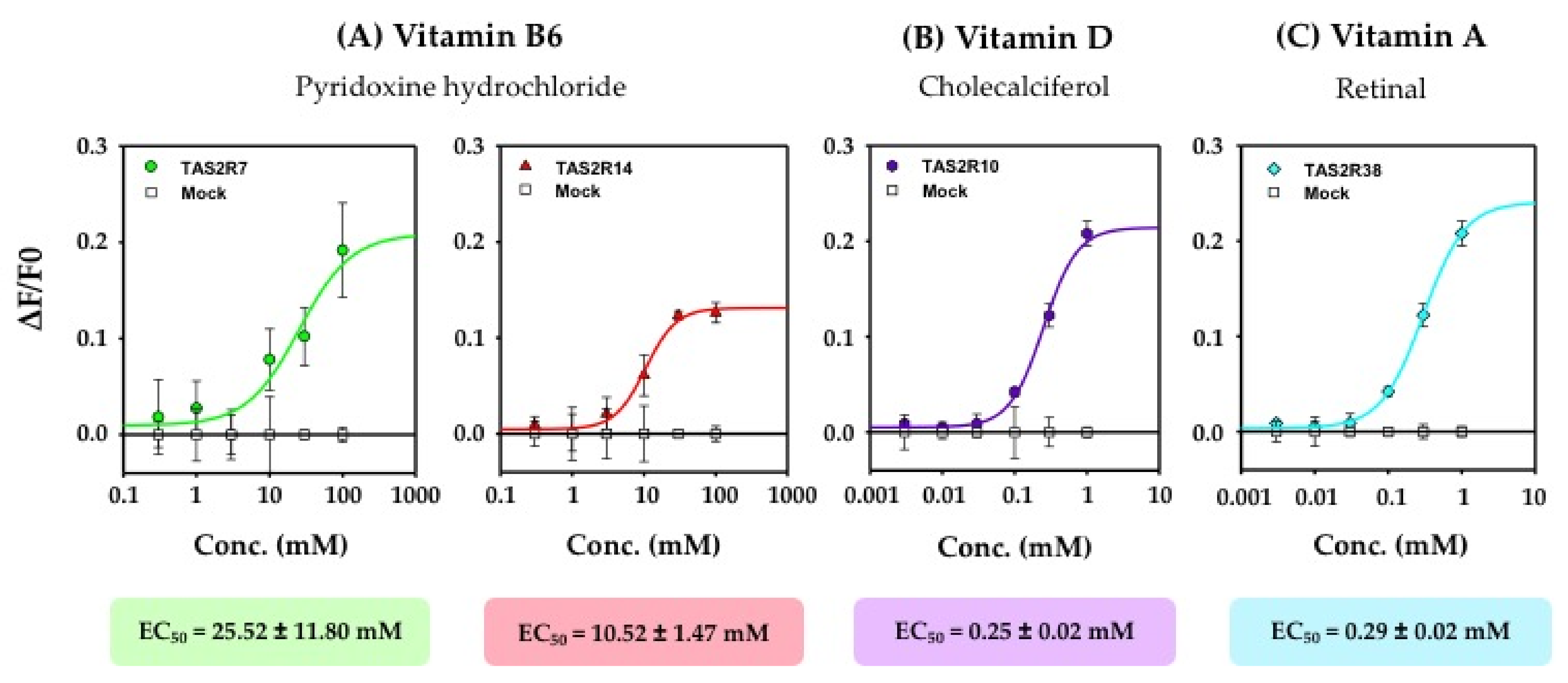
3.1. Identification of the TAS2Rs Responding to Vitamins
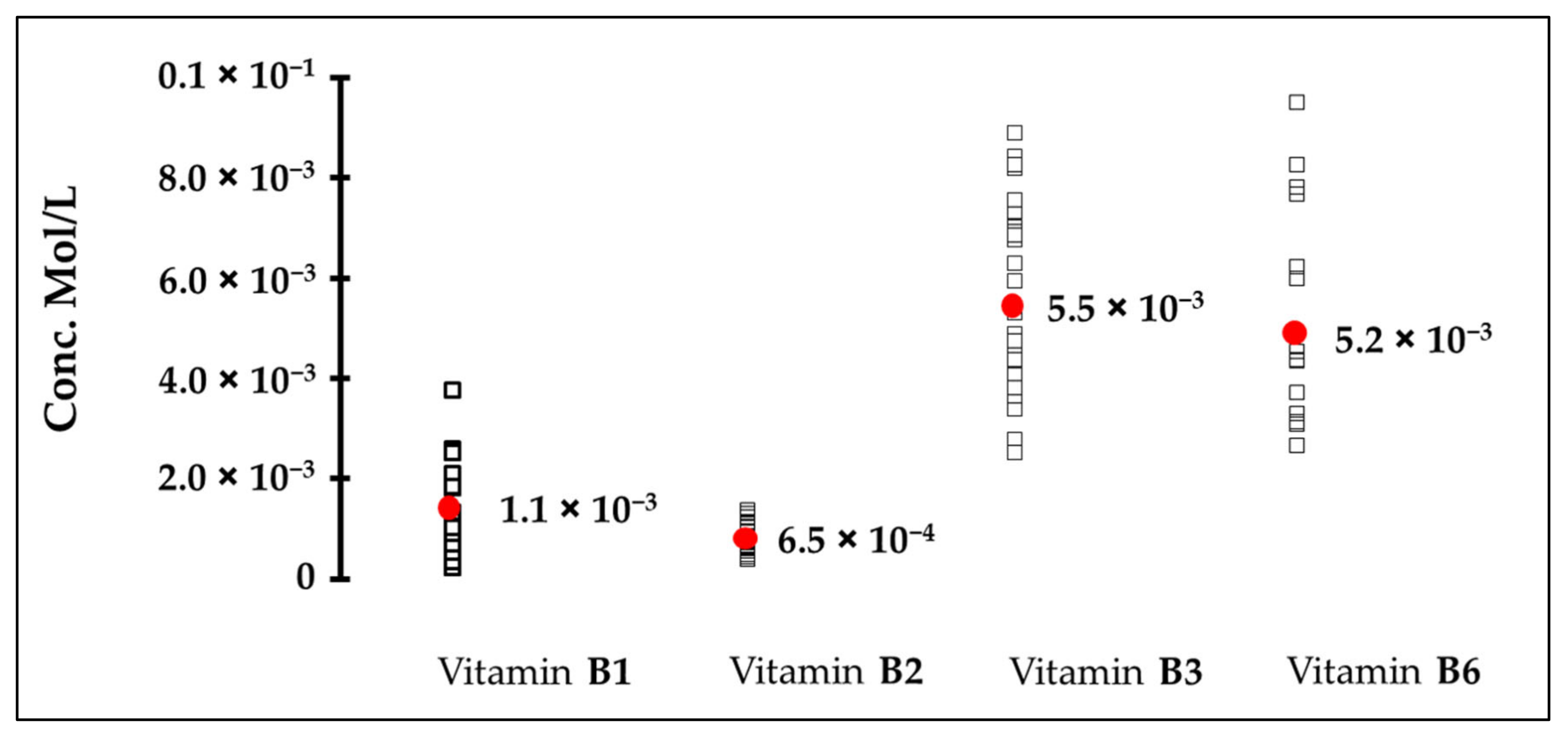
3.2. Human Bitter Taste Detection Threshold of Vitamins
4. Discussion
4.1. Comparison of Cellular-Based Assay and Sensory Analysis
4.2. Relationship between the Vitamin Functions and TAS2R Activation
4.3. Vitamins, Nutritional Supplements, and Bitter Taste
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briand, L.; Salles, C. 4—Taste perception and integration. In Flavor; Etiévant, P., Guichard, E., Salles, C., Voilley, A., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 101–119. ISBN 978-0-08-100295-7. [Google Scholar]
- Nissim, I.; Dagan-Wiener, A.; Niv, M.Y. The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 2017, 69, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste Perception of Nutrients Found in Nutritional Supplements: A Review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315S–1321S. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Dackis, C. Taste of nutrients: Amino acids, vitamins, and fatty acids. Percept. Psychophys. 1975, 17, 140–146. [Google Scholar] [CrossRef]
- Ozçağlı, T.G.; Stelling, J.; Stanford, J. A study in four European countries to examine the importance of sensory attributes of oral nutritional supplements on preference and likelihood of compliance. Turk J. Gastroenterol. 2013, 24, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Vraneš, M.; Tot, A.; Janković, N.; Gadžurić, S. What is the taste of vitamin-based ionic liquids? J. Mol. Liq. 2019, 276, 902–909. [Google Scholar] [CrossRef]
- Liu, A.; Huang, B.; Zuo, S.; Li, Z.; Zhou, J.-L.; Wong, W.-L.; Lu, Y.-J. Enzymatic glucosylation of citrus flavonoids to enhance their bioactivity and taste as new food additives. Mol. Catal. 2022, 528, 112467. [Google Scholar] [CrossRef]
- Matsunami, H.; Montmayeur, J.-P.; Buck, L.B. A family of candidate taste receptors in human and mouse. Nature 2000, 404, 601–604. [Google Scholar] [CrossRef]
- Meyerhof, W. Elucidation of mammalian bitter taste. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 37–72. ISBN 978-3-540-32431-7. [Google Scholar]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, A.; Behrens, M.; Massarotti, A.; Appendino, G.; Meyerhof, W. Broad Tuning of the Human Bitter Taste Receptor hTAS2R46 to Various Sesquiterpene Lactones, Clerodane and Labdane Diterpenoids, Strychnine, and Denatonium. J. Agric. Food Chem. 2007, 55, 6236–6243. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Brockhoff, A.; Batram, C.; Kuhn, C.; Appendino, G.; Meyerhof, W. The Human Bitter Taste Receptor hTAS2R50 Is Activated by the Two Natural Bitter Terpenoids Andrographolide and Amarogentin. J. Agric. Food Chem. 2009, 57, 9860–9866. [Google Scholar] [CrossRef]
- Intelmann, D.; Batram, C.; Kuhn, C.; Haseleu, G.; Meyerhof, W.; Hofmann, T. Three TAS2R Bitter Taste Receptors Mediate the Psychophysical Responses to Bitter Compounds of Hops (Humulus lupulus L.) and Beer. Chemosens. Percept. 2009, 2, 118–132. [Google Scholar] [CrossRef]
- Bufe, B.; Breslin, P.A.S.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.-K.; Drayna, D.; Meyerhof, W. The Molecular Basis of Individual Differences in Phenylthiocarbamide and Propylthiouracil Bitterness Perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Loutfy, M.A. Pyridoxine Hydrochloride. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1984; Volume 13, pp. 447–486. ISBN 0099-5428. [Google Scholar]
- Liu, K.; Jaggupilli, A.; Premnath, D.; Chelikani, P. Plasticity of the ligand binding pocket in the bitter taste receptor T2R7. Biochim. Et Biophys. Acta (BBA) Biomembr. 2018, 1860, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Verbeurgt, C.; Veithen, A.; Carlot, S.; Tarabichi, M.; Dumont, J.E.; Hassid, S.; Chatelain, P. The human bitter taste receptor T2R38 is broadly tuned for bacterial compounds. PLoS ONE 2017, 12, e0181302. [Google Scholar] [CrossRef]
- Behrens, M.; Brockhoff, A.; Kuhn, C.; Bufe, B.; Winnig, M.; Meyerhof, W. The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem. Biophys. Res. Commun. 2004, 319, 479–485. [Google Scholar] [CrossRef]
- Behrens, M.; Born, S.; Redel, U.; Voigt, N.; Schuh, V.; Raguse, J.-D.; Meyerhof, W. Immunohistochemical Detection of TAS2R38 Protein in Human Taste Cells. PLoS ONE 2012, 7, e40304. [Google Scholar] [CrossRef]
- Bufe, B.; Hofmann, T.; Krautwurst, D.; Raguse, J.-D.; Meyerhof, W. The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nat. Genet. 2002, 32, 397–401. [Google Scholar] [CrossRef]
- Kuhn, C.; Bufe, B.; Winnig, M.; Hofmann, T.; Frank, O.; Behrens, M.; Lewtschenko, T.; Slack, J.P.; Ward, C.D.; Meyerhof, W. Bitter Taste Receptors for Saccharin and Acesulfame K. J. Neurosci. 2004, 24, 10260. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ugawa, S.; Yamamura, H.; Imaizumi, Y.; Shimada, S. Functional Interaction between T2R Taste Receptors and G-Protein α Subunits Expressed in Taste Receptor Cells. J. Neurosci. 2003, 23, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Raliou, M.; Grauso, M.; Hoffmann, B.; Schlegel–Le-Poupon, C.; Nespoulous, C.; Débat, H.; Belloir, C.; Wiencis, A.; Sigoillot, M.; Preet Bano, S.; et al. Human Genetic Polymorphisms in T1R1 and T1R3 Taste Receptor Subunits Affect Their Function. Chem. Senses 2011, 36, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.O. Efficient estimation of sensory thresholds. Behav. Res. Methods Instrum. Comput. 1986, 18, 623–632. [Google Scholar] [CrossRef]
- Behrens, M.; Redel, U.; Blank, K.; Meyerhof, W. The human bitter taste receptor TAS2R7 facilitates the detection of bitter salts. Biochem. Biophys. Res. Commun. 2019, 512, 877–881. [Google Scholar] [CrossRef]
- Rafeeq, H.; Ahmad, S.; Tareen, M.B.K.; Shahzad, K.A.; Bashir, A.; Jabeen, R.; Shehzadi, I. Biochemistry of Fat Soluble Vitamins, Sources, Biochemical Functions and Toxicity. Haya Saudi J. Life Sci. 2020, 5, 188–196. [Google Scholar] [CrossRef]
- Soares, S.; Silva, M.S.; García-Estevez, I.; Groβmann, P.; Brás, N.; Brandão, E.; Mateus, N.; de Freitas, V.; Behrens, M.; Meyerhof, W. Human Bitter Taste Receptors Are Activated by Different Classes of Polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol. Behav. 2005, 85, 45–56. [Google Scholar] [CrossRef]
- Foster, S.R.; Porrello, E.R.; Purdue, B.; Chan, H.-W.; Voigt, A.; Frenzel, S.; Hannan, R.D.; Moritz, K.M.; Simmons, D.G.; Molenaar, P.; et al. Expression, Regulation and Putative Nutrient-Sensing Function of Taste GPCRs in the Heart. PLoS ONE 2013, 8, e64579. [Google Scholar] [CrossRef]
- Laffitte, A.; Neiers, F.; Briand, L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 379–385. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, C.-H.; Lifshitz, L.M.; ZhuGe, R. Extraoral bitter taste receptors in health and disease. J. Gen. Physiol. 2017, 149, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Prandi, S.; Meyerhof, W. Taste Receptor Gene Expression Outside the Gustatory System. In Taste and Smell; Krautwurst, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–34. ISBN 978-3-319-48927-8. [Google Scholar]
- Manson, M.L.; Säfholm, J.; Al-Ameri, M.; Bergman, P.; Orre, A.-C.; Swärd, K.; James, A.; Dahlén, S.-E.; Adner, M. Bitter taste receptor agonists mediate relaxation of human and rodent vascular smooth muscle. Eur. J. Pharmacol. 2014, 740, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Vrontakis, M.; Parkinson, F.; Chelikani, P. Functional bitter taste receptors are expressed in brain cells. Biochem. Biophys. Res. Commun. 2011, 406, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.F., III; Park, Y.; Lamers, Y.; Bandyopadhyay, N.; Chi, Y.-Y.; Lee, K.; Kim, S.; da Silva, V.; Hove, N.; Ranka, S.; et al. Metabolomic Analysis Reveals Extended Metabolic Consequences of Marginal Vitamin B-6 Deficiency in Healthy Human Subjects. PLoS ONE 2013, 8, e63544. [Google Scholar] [CrossRef]
- Keast, R.S.J.; Breslin, P.A.S. An overview of binary taste–taste interactions. Food Qual. Prefer. 2003, 14, 111–124. [Google Scholar] [CrossRef]
| Vitamins | Concentration (mM) | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | |
| Thiamine hydrochloride (B1) | 0.22 | 0.45 | 0.89 | 1.78 | 3.56 | 7.12 |
| Riboflavin phosphate (B2) | 0.16 | 0.24 | 0.37 | 0.55 | 0.83 | 1.25 |
| Niacinamide (B3) | 2.03 | 3.05 | 4.57 | 6.85 | 10.30 | 15.40 |
| Pyridoxine hydrochloride (B6) | 0.24 | 0.60 | 1.52 | 3.80 | 9.50 | 23.70 |
| Vitamin | M.A.C (mM) | TAS2R Receptor | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 5 | 7 | 8 | 10 | 13 | 14 | 16 | 20 | 30 | 31 | 38 | 39 | 39 | 40 | 41 | 43 | 46 | 50 | ||
| B1 | 1 | + | ||||||||||||||||||||
| B2 | 0.01 | |||||||||||||||||||||
| B3 | 3 | |||||||||||||||||||||
| B5 | 30 | |||||||||||||||||||||
| B6 | 100 | + | + | |||||||||||||||||||
| B8 | 3 | |||||||||||||||||||||
| B9 | 3 | |||||||||||||||||||||
| B12 | 3 | |||||||||||||||||||||
| C | 10 | |||||||||||||||||||||
| A | 1 | + | ||||||||||||||||||||
| E | 1 | |||||||||||||||||||||
| K | 1 | |||||||||||||||||||||
| D | 1 | + | ||||||||||||||||||||
| Vitamin | Cellular Bitter Taste Detection Threshold (mM) | Human Bitter Taste Detection Threshold (mM) | * Concentration in Nutritional Supplement (mM) |
|---|---|---|---|
| B1 | 0.10 | 1.10 | 0.44 |
| B2 | ND | 0.65 | 0.40 |
| B3 | ND | 5.50 | 4.06 |
| B6 | 1.00 | 5.20 | 0.73 |
| A | 0.05 | ND | 0.03 |
| D | 0.05 | ND | 0.01 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delompré, T.; Belloir, C.; Martin, C.; Salles, C.; Briand, L. Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors. Nutrients 2022, 14, 4141. https://doi.org/10.3390/nu14194141
Delompré T, Belloir C, Martin C, Salles C, Briand L. Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors. Nutrients. 2022; 14(19):4141. https://doi.org/10.3390/nu14194141
Chicago/Turabian StyleDelompré, Thomas, Christine Belloir, Christophe Martin, Christian Salles, and Loïc Briand. 2022. "Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors" Nutrients 14, no. 19: 4141. https://doi.org/10.3390/nu14194141
APA StyleDelompré, T., Belloir, C., Martin, C., Salles, C., & Briand, L. (2022). Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors. Nutrients, 14(19), 4141. https://doi.org/10.3390/nu14194141