Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Exercise Intervention Scheme
2.3. Morris Water Maze Experiment
2.4. Material Collection
2.5. Nissl Staining
2.6. HE Staining
2.7. ELISA Assay
2.8. 16S rDNA Sequencing
2.9. Liquid Chromatography–Mass Spectrometry Detection
2.10. Real-Time PCR Assay
2.11. Immunohistochemical Assays
2.12. Immunofluorescence Assay
2.13. Western Blotting Assay
2.14. Statistical Analysis
3. Results
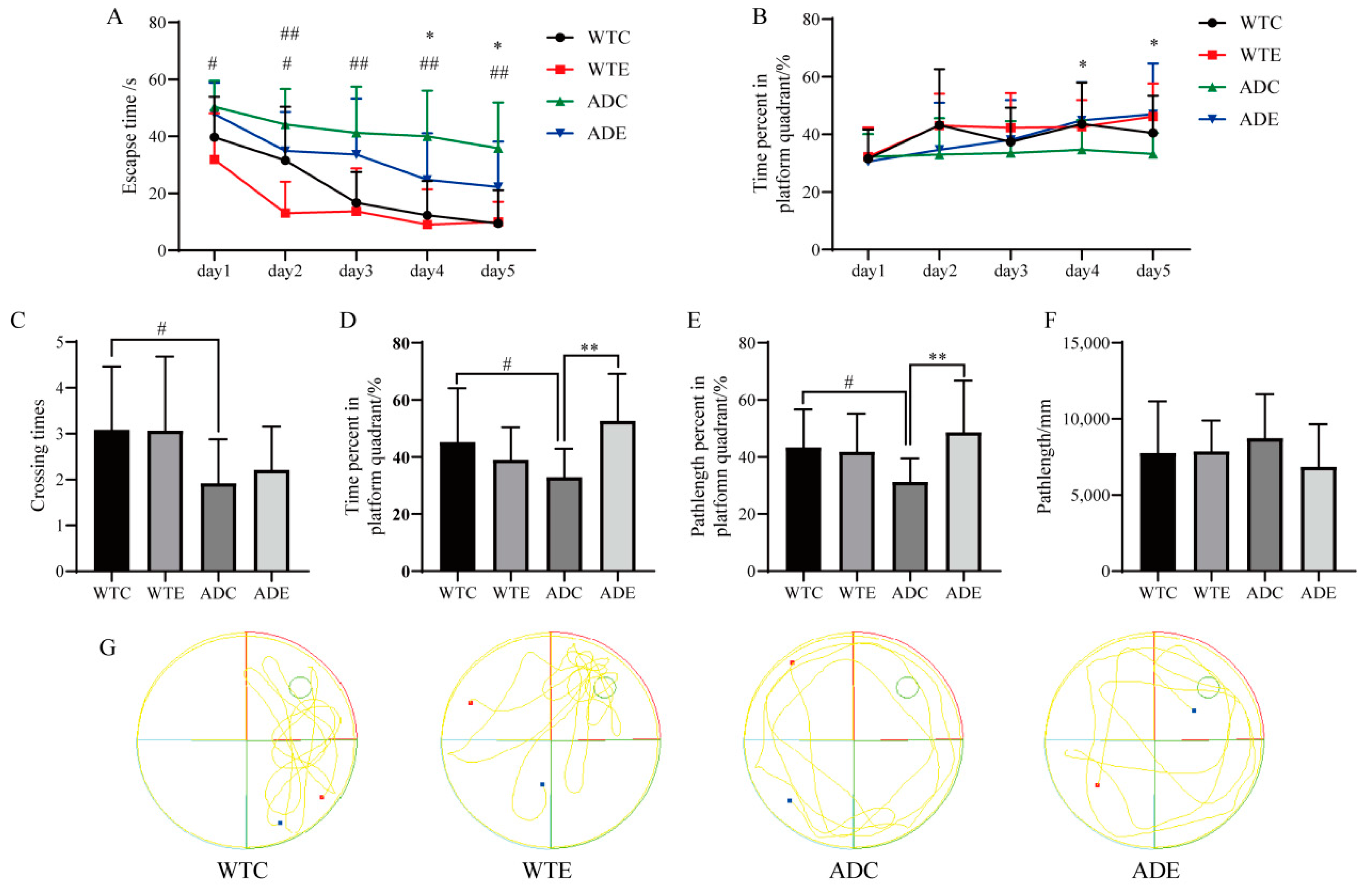
3.1. Exercise Improves the Performance of Learning Memory for Mice
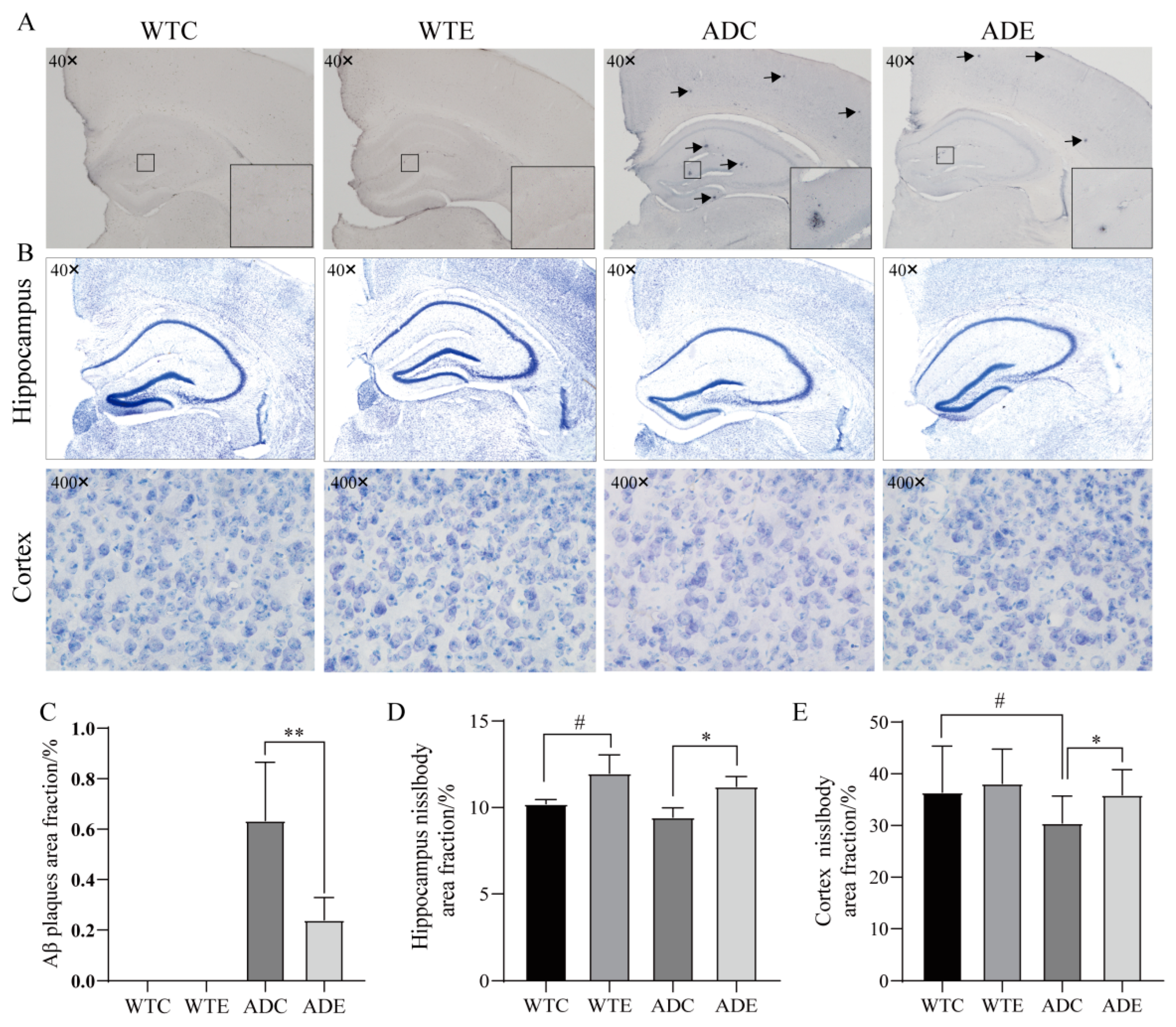
3.2. Exercise Reduces Aβ Pathology and Neuronal Loss in Mice
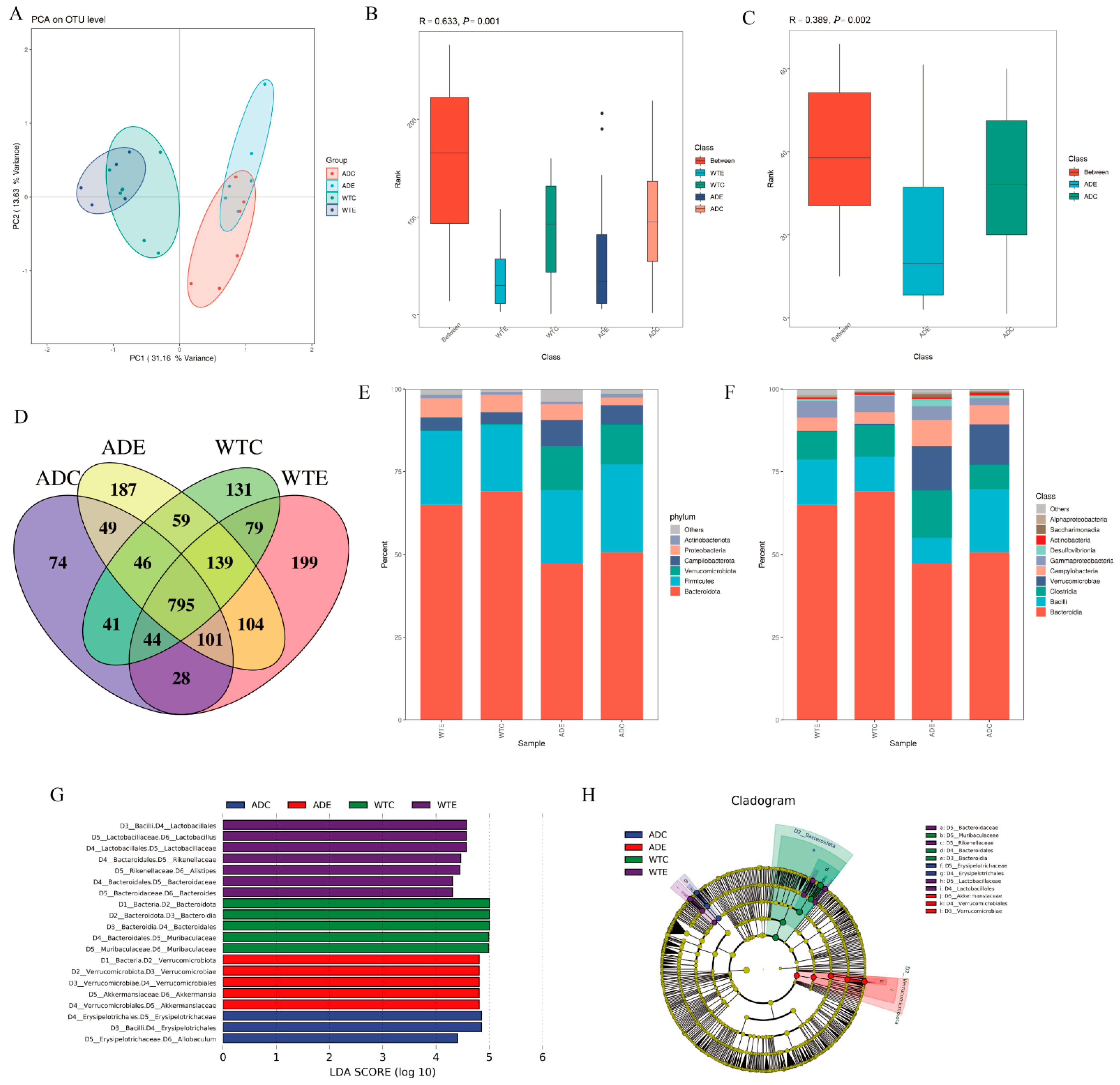
3.3. Significant Effect of Treadmill Exercise on Intestinal Microorganisms
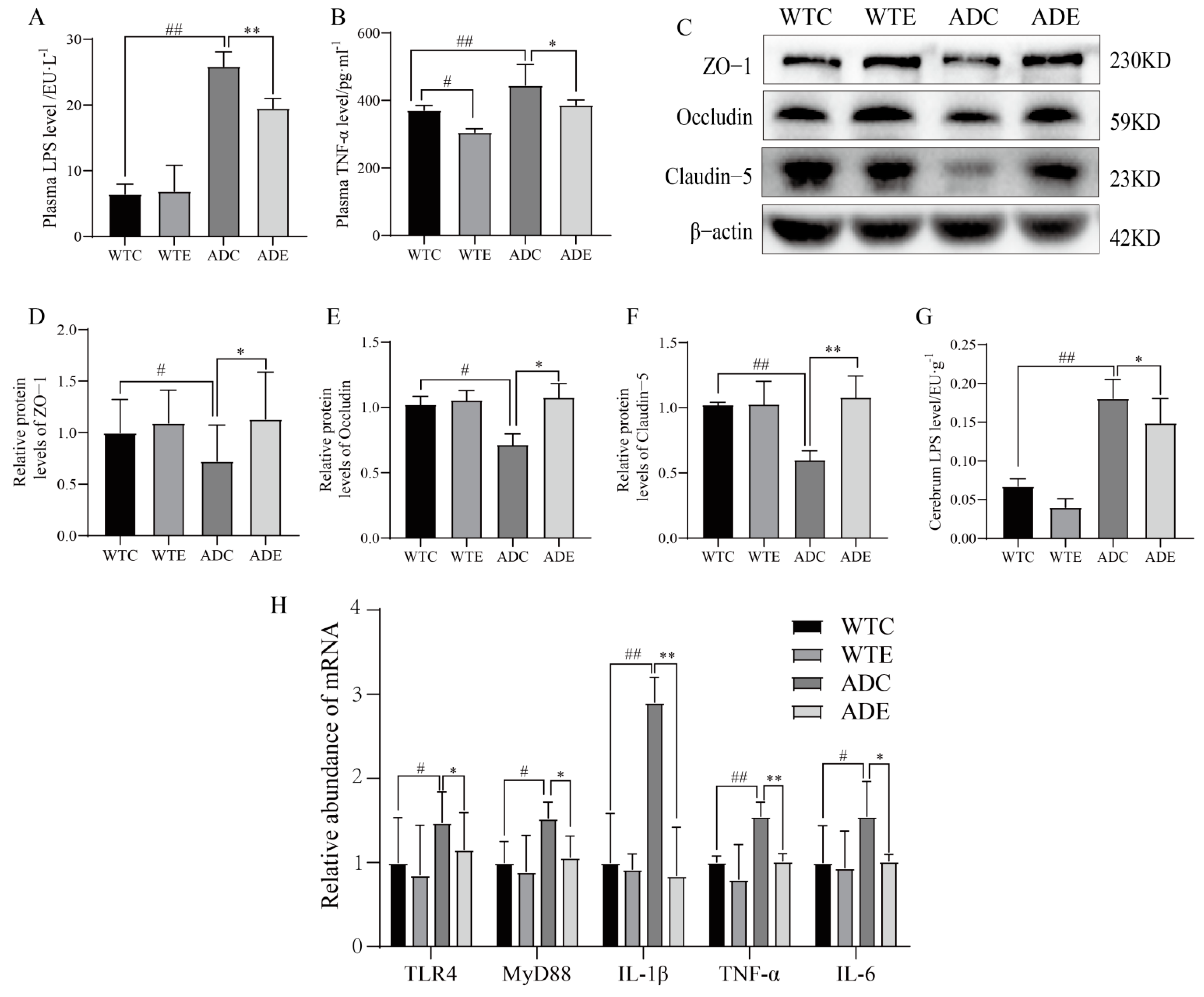
3.4. Treadmill Exercise Protects the Intestinal Barrier
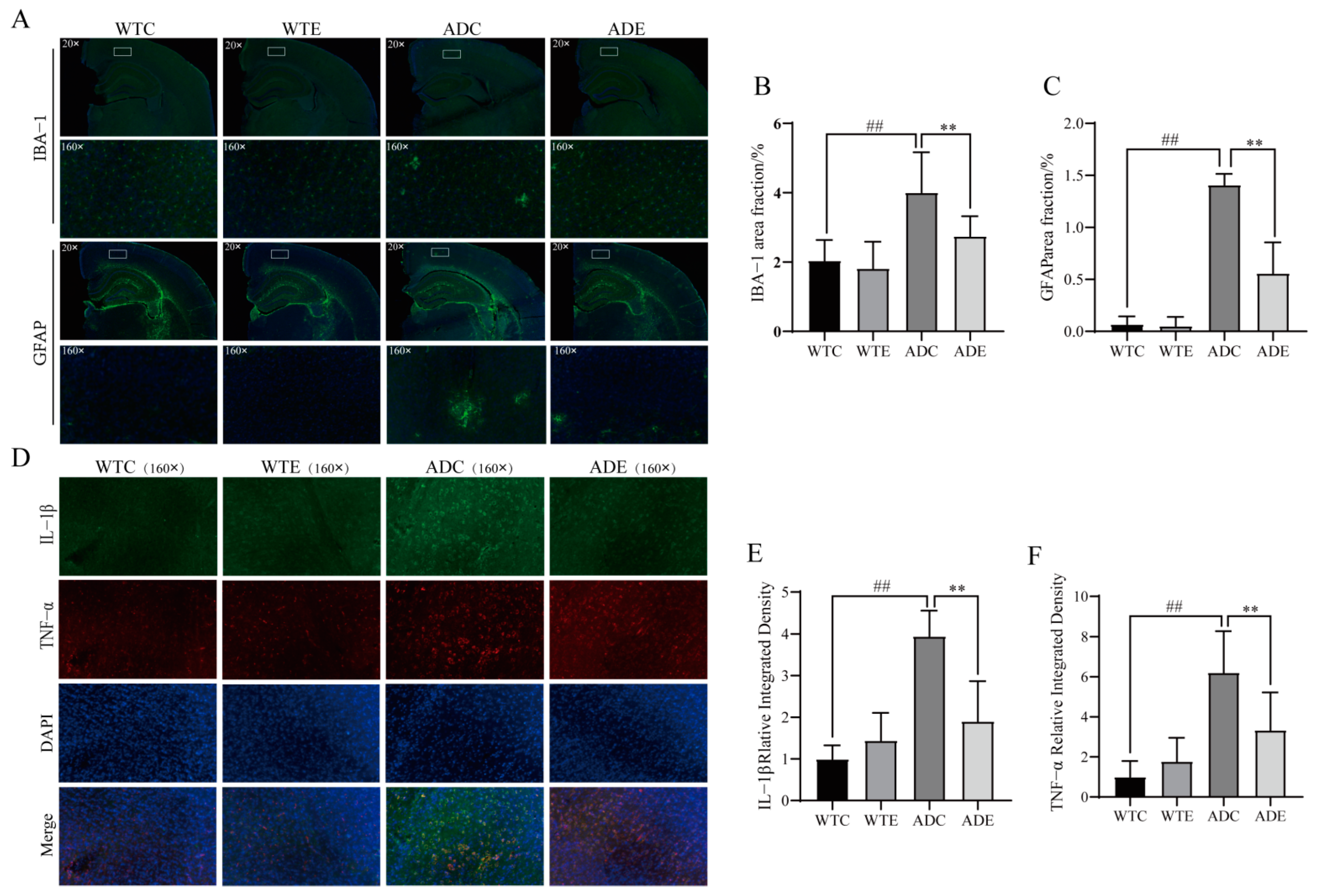
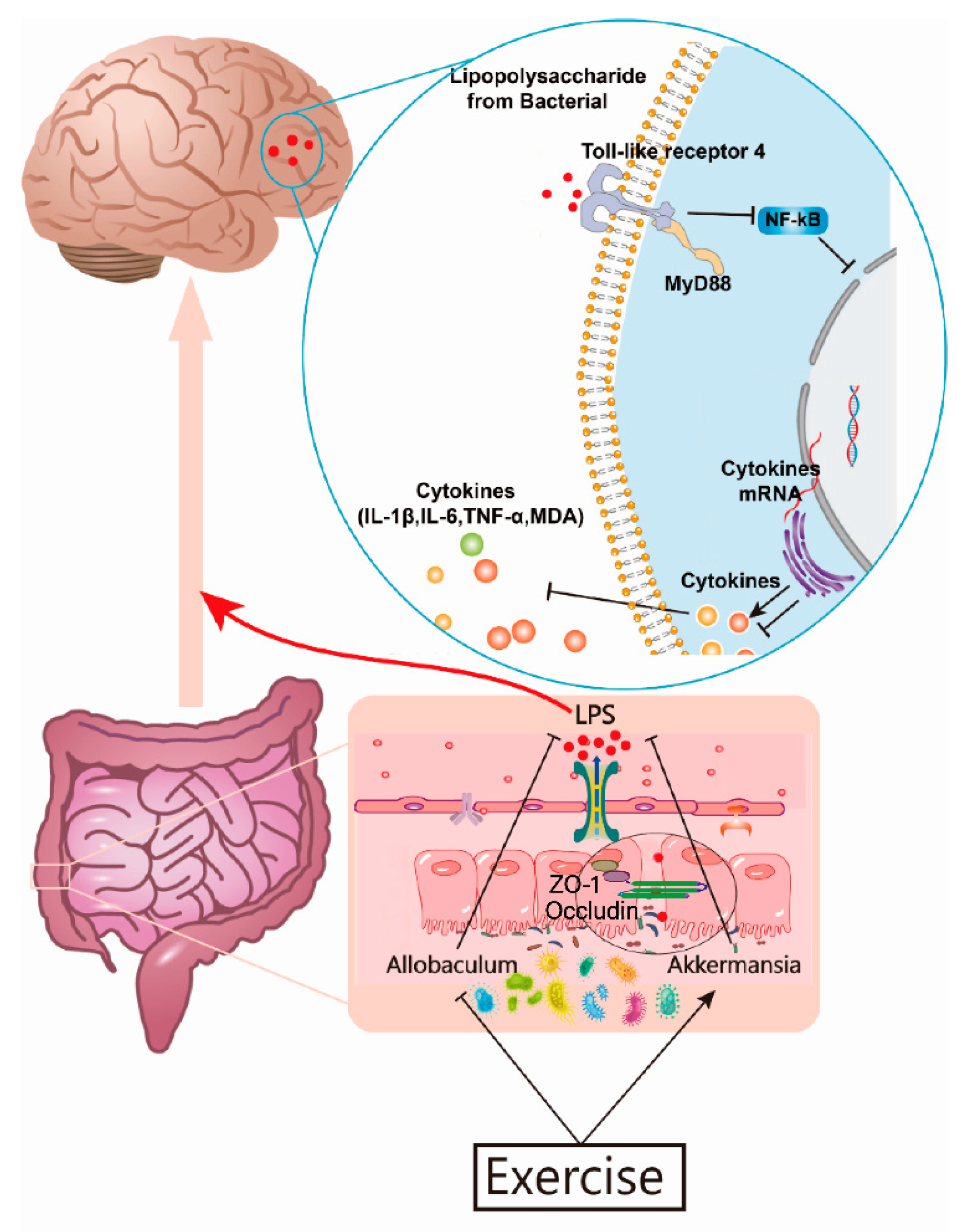
3.5. Exercise Attenuates Displacement of LPS to Alleviate Neuroinflammation in the Brains of AD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soria Lopez, J.A.; Gonzalez, H.M.; Leger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [PubMed]
- Eratne, D.; Loi, S.M.; Farrand, S.; Kelso, W.; Velakoulis, D.; Looi, J.C. Alzheimer’s disease: Clinical update on epidemiology, pathophysiology and diagnosis. Australas Psychiatry 2018, 26, 347–357. [Google Scholar] [CrossRef]
- Kulczynska-Przybik, A.; Slowik, A.; Mroczko, P.; Borawski, B.; Groblewska, M.; Borawska, R.; Mroczko, B. Cerebrospinal Fluid and Blood CX3CL1 as a Potential Biomarker in Early Diagnosis and Prognosis of Dementia. Curr. Alzheimer Res. 2020, 17, 709–721. [Google Scholar] [CrossRef]
- Li, X.; Zhou, T.; Qiu, S. Alzheimer’s Disease Analysis Algorithm Based on No-threshold Recurrence Plot Convolution Network. Front. Aging Neurosci. 2022, 14, 888577. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Liu, X.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Tortora, F.; Rendina, A.; Angiolillo, A.; Di Costanzo, A.; Aniello, F.; Donizetti, A.; Febbraio, F.; Vitale, E. CD33 rs2455069 SNP: Correlation with Alzheimer’s Disease and Hypothesis of Functional Role. Int. J. Mol. Sci. 2022, 23, 3629. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Provenzano, F.A.; Small, S.A. Alzheimer’s Disease Neuroimaging Initiative. A deep learning MRI approach outperforms other biomarkers of prodromal Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 45. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, T.; Xi, Y.; Li, L.; Mo, F.; Liu, X.; Liu, Z.; Gao, J.M.; Yuan, T. Sesamol Attenuates Amyloid Peptide Accumulation and Cognitive Deficits in APP/PS1 Mice: The Mediating Role of the Gut-Brain Axis. J. Agric. Food Chem. 2021, 69, 12717–12729. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Kareem, O.; Khushtar, M.; Akbar, M.; Haque, M.R.; Iqubal, A.; Haider, M.F.; Pottoo, F.H.; Abdulla, F.S.; Al-Haidar, M.B.; et al. Neuroinflammation: A Potential Risk for Dementia. Int. J. Mol. Sci. 2022, 23, 616. [Google Scholar] [CrossRef] [PubMed]
- Shadfar, S.; Hwang, C.J.; Lim, M.S.; Choi, D.Y.; Hong, J.T. Involvement of inflammation in Alzheimer’s disease pathogenesis and therapeutic potential of anti-inflammatory agents. Arch. Pharm. Res. 2015, 38, 2106–2119. [Google Scholar] [CrossRef]
- Shen, H.; Guan, Q.; Zhang, X.; Yuan, C.; Tan, Z.; Zhai, L.; Hao, Y.; Gu, Y.; Han, C. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109884. [Google Scholar] [CrossRef]
- Giovannini, M.G.; Lana, D.; Traini, C.; Vannucchi, M.G. The Microbiota-Gut-Brain Axis and Alzheimer Disease. From Dysbiosis to Neurodegeneration: Focus on the Central Nervous System Glial Cells. J. Clin. Med. 2021, 10, 2358. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Shu, C.C.; Chen, Y.M.; Lu, J.J.; Wu, T.S.; Lai, W.F.; Tzeng, C.M.; Lai, H.C.; Lu, C.C. Like Cures Like: Pharmacological Activity of Anti-Inflammatory Lipopolysaccharides from Gut Microbiome. Front. Pharmacol. 2020, 11, 554. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, A.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Khan, M.S.; Ikram, M.; Park, J.S.; Park, T.J.; Kim, M.O. Gut Microbiota, Its Role in Induction of Alzheimer’s Disease Pathology, and Possible Therapeutic Interventions: Special Focus on Anthocyanins. Cells 2020, 9, 853. [Google Scholar] [CrossRef]
- Lukiw, W.J. Bacteroides fragilis Lipopolysaccharide and Inflammatory Signaling in Alzheimer’s Disease. Front. Microbiol. 2016, 7, 1544. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; Garcia-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Ribaric, S. Physical Exercise, a Potential Non-Pharmacological Intervention for Attenuating Neuroinflammation and Cognitive Decline in Alzheimer’s Disease Patients. Int. J. Mol. Sci. 2022, 23, 3245. [Google Scholar] [CrossRef]
- Li, G.; He, H. Hormesis, allostatic buffering capacity and physiological mechanism of physical activity: A new theoretic framework. Med. Hypotheses 2009, 72, 527–532. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, J.L.; Valldecabres, R.; Villarrasa-Sapina, I.; Monfort-Torres, G.; Marco-Ahullo, A.; Ribeiro Do Couto, B. Increased cortisol levels caused by acute resistance physical exercise impair memory and learning ability. PeerJ 2022, 10, e13000. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Steele, J.; Smith, D. High- and Low-Load Resistance Training: Interpretation and Practical Application of Current Research Findings. Sports Med. 2017, 47, 393–400. [Google Scholar] [CrossRef]
- Fernando, P.; Bonen, A.; Hoffman-Goetz, L. Predicting submaximal oxygen consumption during treadmill running in mice. Can. J. Physiol. Pharmacol. 1993, 71, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Schefer, V.; Talan, M.I. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp. Gerontol. 1996, 31, 387–392. [Google Scholar] [CrossRef]
- Chenfei, Z.; Haizhen, Y.; Jie, X.; Na, Z.; Bo, X. Effects of aerobic exercise on hippocampal SUMOylation in APP/PS1 transgenic mice. Neurosci. Lett. 2022, 767, 136303. [Google Scholar] [CrossRef]
- Shinohara, M.; Sato, N.; Shimamura, M.; Kurinami, H.; Hamasaki, T.; Chatterjee, A.; Rakugi, H.; Morishita, R. Possible modification of Alzheimer’s disease by statins in midlife: Interactions with genetic and non-genetic risk factors. Front. Aging Neurosci. 2014, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Liang, W.; Wei, Z.Z.; Li, X.; Tian, Y.; Qiao, S.; Yang, Y.; Yang, L.; Wu, D.; et al. The identification of PSEN1 p.Tyr159Ser mutation in a non-canonic early-onset Alzheimer’s disease family. Mol. Cell Neurosci. 2022, 120, 103715. [Google Scholar]
- Wang, X.; Zhou, X.; Li, G.; Zhang, Y.; Wu, Y.; Song, W. Modifications and Trafficking of APP in the Pathogenesis of Alzheimer’s Disease. Front. Mol. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Mao, Q.; Zhao, N.; Xia, J.; Zhao, Y.; Xu, B. Treadmill exercise overcomes memory deficits related to synaptic plasticity through modulating ionic glutamate receptors. Behav. Brain Res. 2021, 414, 113502. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.F.; Loures, C.M.G.; Alves, L.C.V.; De Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Past, present and future of therapeutic strategies against amyloid-beta peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut Feelings on Parkinson’s and Depression. Cerebrum 2017, 2017, cer-04-17. [Google Scholar] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-Mcfarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Liu, W.; He, K.; Wu, D.; Zhou, L.; Li, G.; Lin, Z.; Yang, X.; Liu, J.; Pui Man Hoi, M. Natural Dietary Compound Xanthohumol Regulates the Gut Microbiota and Its Metabolic Profile in a Mouse Model of Alzheimer’s Disease. Molecules 2022, 27, 1281. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Wu, W.; Lv, L.; Shi, D.; Ye, J.; Fang, D.; Guo, F.; Li, Y.; He, X.; Li, L. Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model. Front. Microbiol. 2017, 8, 1804. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; De Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, S.; Nojoumi, S.A.; Fateh, A.; Siadat, S.D.; Sotoodehnejadnematalahi, F. Interaction between Clostridium species and microbiota to progress immune regulation. Acta Microbiol. Immunol. Hung. 2022, 69, 89–103. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, P.; Martorana, A.M.; Polissi, A. Lipopolysaccharide biogenesis and transport at the outer membrane of Gram-negative bacteria. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Paradis, T.; Begue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, F. Peripheral Inflammatory Biomarkers and Cognitive Decline in Older Adults With and Without Alzheimer’s Disease: A Systematic Review. J. Gerontol. Nurs. 2017, 43, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, H.; Zhang, S.; Lu, S.; Sun, J.; Qian, Y. Enhancement of LPS-induced microglial inflammation response via TLR4 under high glucose conditions. Cell Physiol. Biochem. 2015, 35, 1571–1581. [Google Scholar] [CrossRef]
- Fenton, L.; Weissberger, G.H.; Boyle, P.A.; Mosqueda, L.; Yassine, H.N.; Nguyen, A.L.; Lim, A.C.; Han, S.D. Cognitive and Neuroimaging Correlates of Financial Exploitation Vulnerability in Older Adults without Dementia: Implications for Early Detection of Alzheimer’s Disease. Neurosci. Biobehav. Rev. 2022, 140, 104773. [Google Scholar] [CrossRef]
- Andrade, A.; Siqueira, T.C.; D’oliveira, A.; Dominski, F.H. Effects of Exercise in the Treatment of Alzheimer’s Disease: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Aging Phys. Act. 2022, 30, 535–551. [Google Scholar] [CrossRef]
- Gaitan, J.M.; Moon, H.Y.; Stremlau, M.; Dubal, D.B.; Cook, D.B.; Okonkwo, O.C.; Van Praag, H. Effects of Aerobic Exercise Training on Systemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front. Endocrinol. 2021, 12, 660181. [Google Scholar] [CrossRef]
- Lee, S.M.; Song, H.S.; Chun, B.O.; Choi, M.; Sun, K.; Kim, K.S.; Jeon, H.; Seo, D.E.; Kwon, H.M.; Jeong, J.H.; et al. Feasibility of a 12 Week Physical Intervention to Prevent Cognitive Decline and Disability in the At-Risk Elderly Population in Korea. J. Clin. Med. 2020, 9, 3135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.T.; Zhu, Y.; Sun, Y.L.; Huang, J.; Li, Z.; Wang, Y.; Wu, J.C.; Qin, Z.H.; Lin, F. Long-term running exercise alleviates cognitive dysfunction in APP/PSEN1 transgenic mice via enhancing brain lysosomal function. Acta Pharmacol. Sin. 2022, 43, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Xie, P. Gut microbiota: A new insight into neurological diseases. Chin. Med. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients with Alzheimer’s Disease. Front. Cell Dev. Biol. 2020, 8, 634069. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Brandscheid, C.; Schuck, F.; Reinhardt, S.; Schafer, K.H.; Pietrzik, C.U.; Grimm, M.; Hartmann, T.; Schwiertz, A.; Endres, K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimers Dis. 2017, 56, 775–788. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, L.; Chen, S.; Zhou, H.; Fan, Y.; Lin, L.; Li, J.; Xu, J.; Chen, Y.; Ma, Y.; et al. Gut Microbiome Alterations Precede Cerebral Amyloidosis and Microglial Pathology in a Mouse Model of Alzheimer’s Disease. Biomed. Res. Int. 2020, 2020, 8456596. [Google Scholar] [CrossRef]
- Davidson, G.L.; Cooke, A.C.; Johnson, C.N.; Quinn, J.L. The gut microbiome as a driver of individual variation in cognition and functional behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170286. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Allen, J.M.; Pence, B.D.; Wallig, M.A.; Gaskins, H.R.; White, B.A.; Woods, J.A. Exercise and gut immune function: Evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol Cell Biol. 2016, 94, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Cerda, B.; Perez, M.; Perez-Santiago, J.D.; Tornero-Aguilera, J.F.; Gonzalez-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front Physiol. 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, B.C.; Martinez, M.E.; Ashbeck, E.L.; Roe, D.J.; Jacobs, E.T.; Alberts, D.S.; Thompson, P.A. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiol Biomarkers Prev. 2009, 18, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, N.; Diez, G.G.; Antunez-Almagro, C.; Bailen, M.; Bressa, C.; Gonzalez Soltero, R.; Perez, M.; Larrosa, M. A Critical Mutualism—Competition Interplay Underlies the Loss of Microbial Diversity in Sedentary Lifestyle. Front. Microbiol. 2019, 10, 3142. [Google Scholar] [CrossRef]
- Pei, Z.; Xiaowei, W.; Yajuan, L.; Yuanhong, X. Exploring the Characteristics of Intestinal Microbiota in Hematologic Malignancy Patients via 16s rDNA High-Throughput Sequencing. Clin. Lab. 2021, 67. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.K.; El Kasmi, K.C.; Anderson, A.L.; Devereaux, M.W.; Fillon, S.A.; Robertson, C.E.; Wagner, B.D.; Stevens, M.J.; Pace, N.R.; Sokol, R.J. Specific microbiome changes in a mouse model of parenteral nutrition associated liver injury and intestinal inflammation. PLoS ONE 2014, 9, e110396. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-diabetic Effects of Clostridium butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017, 7, 7046. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Lu, Y.; Tian, S.; Ma, S.; Sun, J.; Hu, Q.; Pang, X.; Li, X. Effects of L-arabinose by hypoglycemic and modulating gut microbiome in a high-fat diet- and streptozotocin-induced mouse model of type 2 diabetes mellitus. J. Food Biochem. 2021, 45, e13991. [Google Scholar] [CrossRef] [PubMed]
- De La Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Belforte, F.S.; Fernandez, N.; Tonin Monzon, F.; Rosso, A.D.; Quesada, S.; Cimolai, M.C.; Millan, A.; Cerrone, G.E.; Frechtel, G.D.; Burcelin, R.; et al. Getting to Know the Gut Microbial Diversity of Metropolitan Buenos Aires Inhabitants. Front. Microbiol. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef]
- Eikelenboom, P.; Hoogendijk, W.J.; Jonker, C.; Van Tilburg, W. Immunological mechanisms and the spectrum of psychiatric syndromes in Alzheimer’s disease. J. Psychiatr. Res. 2002, 36, 269–280. [Google Scholar] [CrossRef]
- Cowan, M.; Petri, W.A., Jr. Microglia: Immune Regulators of Neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Laferla, F.M. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp. Neurol. 2013, 239, 133–138. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Ferreira, A.C.; Sousa, J.C.; Correia-Neves, M.; Sousa, N.; Marques, F. Insights on the pathophysiology of Alzheimer’s disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci. Biobehav. Rev. 2016, 68, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ni, D.; Ribeiro, R.V.; Pinget, G.V.; Macia, L. How Changes in the Nutritional Landscape Shape Gut Immunometabolism. Nutrient 2021, 13, 823. [Google Scholar] [CrossRef]
- Shi, J.Z.; Zheng, X.M.; Zhou, Y.F.; Yun, L.Y.; Luo, D.M.; Hao, J.J.; Liu, P.F.; Zhang, W.K.; Xu, J.K.; Yan, Y.; et al. Cornuside Is a Potential Agent against Alzheimer’s Disease via Orchestration of Reactive Astrocytes. Nutrients 2022, 14, 3179. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sanchez, K.; Ariza-Salamanca, D.; Mora-Munoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Qiao, P.F.; Wan, C.Q.; Cai, M.; Zhou, N.K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimers. Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.S.; Kranjac, D.; Alonzo, C.A.; Haase, J.H.; Cedillos, R.O.; Mclinden, K.A.; Boehm, G.W.; Chumley, M.J. Prolonged elevation in hippocampal Abeta and cognitive deficits following repeated endotoxin exposure in the mouse. Behav. Brain Res. 2012, 229, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.W.; Zhao, N.; Xia, J.; Li, B.X.; Yin, L.Y. Effects of treadmill exercise on mitochondrial fusion and fission in the hippocampus of APP/PS1 mice. Neurosci. Lett. 2019, 701, 84–91. [Google Scholar] [CrossRef] [PubMed]


| Gene. | Forward Primer | Reverse Primer |
|---|---|---|
| TLR4 | ACAAACGCCGGAACTTTTCG | GTCGGACACACACAACTTAAGC |
| MyD88 | TCATGTTCTCCATACCCTTGGT | AAACTGCGAGTGGGGTCAG |
| TNF−α | TTAGAAAGGGGATTATGGCTCA | ACTCTCCCTTTGCAGAACTCAG |
| IL−6 | CATTTCCACGATTTCCCAGA | CGGAGAGGAGACTTCACAGAG |
| IL−1β | CTCACAAGCAGAGCACAAGC | AGCTGTCTGCTCATTCACGA |
| GAPDH | CATGGCCTTCCGTGTTCCTA | CCTGCTTCACCACCTTCTTGAT |
| Group | Sobs | Shannon | Simpson | ACE | Chao |
|---|---|---|---|---|---|
| WTC | 566.5 | 3.746 | 0.059 | 768.326 | 720.746 |
| WTE | 684.5 | 4.242 | 0.032 | 852.780 | 829.374 |
| ADC | 473.833 | 3.695 | 0.066 | 614.769 | 578.869 |
| ADE | 657.667 | 4.012 | 0.051 | 824.108 | 811.765 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Yang, J.; Jian, Y.; Lei, Y.; Yao, S.; Hu, Z.; Liu, X.; Tang, C.; Liu, W. Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice. Nutrients 2022, 14, 4134. https://doi.org/10.3390/nu14194134
Yuan S, Yang J, Jian Y, Lei Y, Yao S, Hu Z, Liu X, Tang C, Liu W. Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice. Nutrients. 2022; 14(19):4134. https://doi.org/10.3390/nu14194134
Chicago/Turabian StyleYuan, Shunling, Jialun Yang, Ye Jian, Yong Lei, Sisi Yao, Zelin Hu, Xia Liu, Changfa Tang, and Wenfeng Liu. 2022. "Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice" Nutrients 14, no. 19: 4134. https://doi.org/10.3390/nu14194134
APA StyleYuan, S., Yang, J., Jian, Y., Lei, Y., Yao, S., Hu, Z., Liu, X., Tang, C., & Liu, W. (2022). Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice. Nutrients, 14(19), 4134. https://doi.org/10.3390/nu14194134






