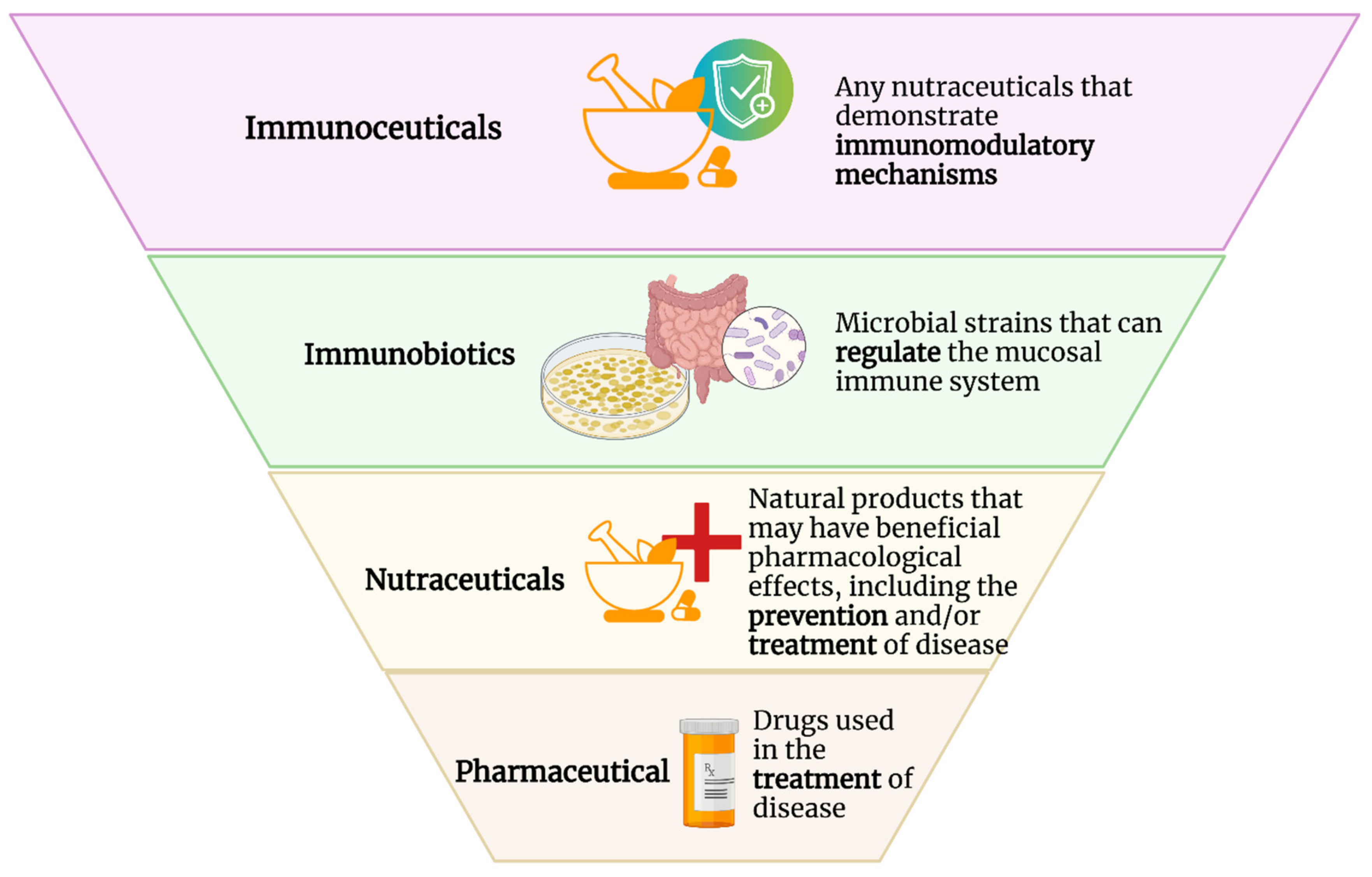
Immunoceuticals: Harnessing Their Immunomodulatory Potential to Promote Health and Wellness
Abstract
1. Introduction
1.1. Brief Overview of the Immune System and Its Regulation
1.2. Inflammation and Immunopathology
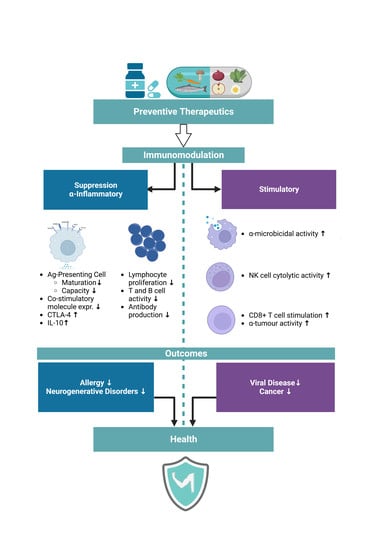
1.3. The Importance of Immunomodulation
1.4. Traditional Immunomodulators
1.5. Clinical Benefits of Immunoceuticals
1.6. Economic Considerations for Nutraceuticals
1.7. Defining and Testing for Immunocompetence
1.8. Nutritional Immunology
2. Key Immunoceuticals and Their Immunomodulatory Properties
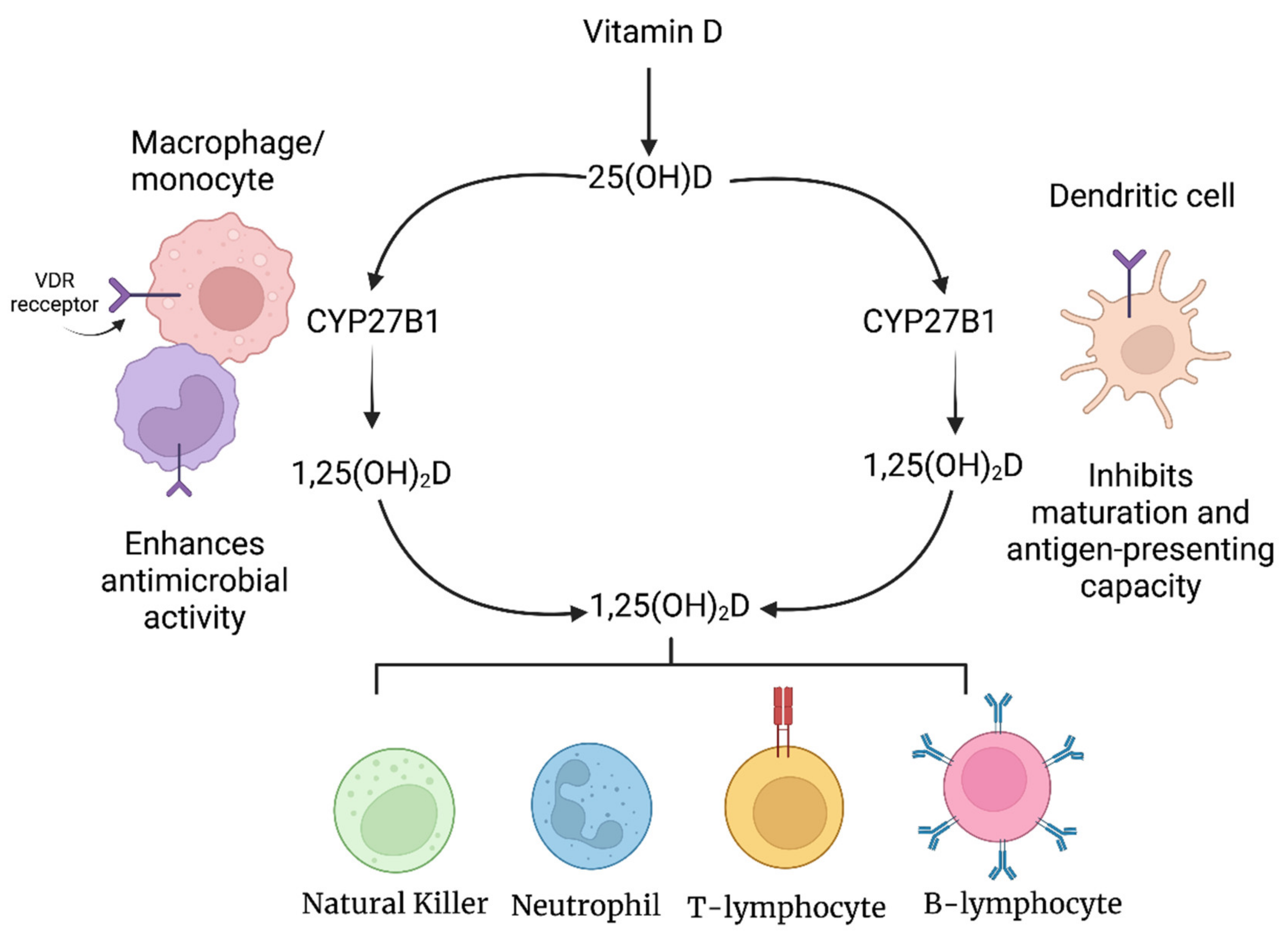
2.1. Vitamin D and Immunomodulation
2.1.1. Vitamin D and the Innate Immune System
2.1.2. Vitamin D and the Adaptive Immune System
2.1.3. Vitamin D and Autoimmune Disease
2.1.4. Vitamin D Dosing
2.2. Medicinal Mushrooms and Their Immunomodulatory Properties
2.3. Immunomodulatory Properties of Quercetin and Kaempferol
2.4. Immunomodulatory Properties of Curcumin and Resveratrol
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalra, E.K. Nutraceutical—Definition and Introduction. AAPS PharmSci. 2003, 5, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Nutraceuticals/Functional Foods and Health Claims on Foods. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/nutraceuticals-functional-foods-health-claims-foods-policy-paper.html (accessed on 28 August 2022).
- Sahoo, C.K.; Rao, S.R.M.; Sudhakar, M. A Review on Human Immunity System and HIV Infection. IJCPR 2015, 6, 262–268. [Google Scholar]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. 2), 49. [Google Scholar] [CrossRef]
- Hunt, S.E.; McLaren, W.; Gil, L.; Thormann, A.; Schuilenburg, H.; Sheppard, D.; Parton, A.; Armean, I.M.; Trevanion, S.J.; Flicek, P.; et al. Ensembl variation resources. Database 2018, 2018, bay119. [Google Scholar] [CrossRef]
- Brodin, P.; Davis, M.M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef]
- Ross, M.; Atalla, H.; Karrow, N.; Mallard, B.A. The bioactivity of colostrum and milk exosomes of high, average, and low immune responder cows on human intestinal epithelial cells. J. Dairy Sci. 2021, 104, 2499–2510. [Google Scholar] [CrossRef]
- Morovic, W.; Budinoff, C.R. Epigenetics: A New Frontier in Probiotic Research. Trends Microbiol. 2021, 29, 117–126. [Google Scholar] [CrossRef]
- Russo, G.L.; Ungaro, P. Epigenetic Mechanisms of Quercetin and Other Flavonoids in Cancer Therapy and Prevention. In Epigenetics of Cancer Prevention; Bishayee, A., Bhatia, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 8, pp. 187–202. [Google Scholar]
- Venturelli, S.; Leuschner, C.; Burkard, M. Natural Polyphenol Kaempferol and Its Epigenetic Impact on Histone Deacetylases: Focus on Human Liver Cells. In Handbook of Nutrition, Diet, and Epigenetics, 1st ed.; Patel, V., Preedy, V., Eds.; Springer: Cham, Swizterland, 2017; pp. 1–17. [Google Scholar]
- Hassan, F.U.; Rehman, M.S.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef]
- De la Rocha, C.; Rodríguez-Ríos, D.; Ramírez-Chávez, E.; Molina-Torres, J.; de Jesús Flores-Sierra, J.; Orozco-Castellanos, L.M.; Galván-Chía, J.P.; Sánchez, A.V.; Zaina, S.; Lund, G. Cumulative Metabolic and Epigenetic Effects of Paternal and/or Maternal Supplementation with Arachidonic Acid across Three Consecutive Generations in Mice. Cells 2022, 11, 1057. [Google Scholar] [CrossRef]
- Kleeman, E.A.; Gubert, C.; Hannan, A.J. Transgenerational epigenetic impacts of parental infection on offspring health and disease susceptibility. Trends Genet. 2022, 38, 662–675. [Google Scholar] [CrossRef]
- Shanker, A.; Marincola, F.M. Cooperativity of adaptive and innate immunity: Implications for cancer therapy. Cancer Immunol. Immunother. 2011, 60, 1061–1074. [Google Scholar] [CrossRef]
- Fitzpatrick, Z.; Frazer, G.; Ferro, A.; Clare, S.; Bouladoux, N.; Ferdinand, J.; Tuong, Z.K.; Negro-Demontel, M.L.; Kumar, N.; Suchanek, O.; et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 2020, 587, 472–476. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Rivest, S. The HPA—Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Fietta, P.; Delsante, G. The inflammasomes: The key regulators of inflammation. Riv. Biol. 2009, 102, 365–384. [Google Scholar]
- Fernández, D.J.; Lamkanfi, M. Inflammatory caspases: Key regulators of inflammation and cell death. Biol. Chem. 2015, 396, 193–203. [Google Scholar] [CrossRef]
- Brennan, E.; Kantharidis, P.; Cooper, M.E.; Godson, C. Pro-resolving lipid mediators: Regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 2021, 17, 725–739. [Google Scholar] [CrossRef]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Gea-Banacloche, J.C. Immunomodulation. In Principles of Molecular Medicine, 2nd ed.; Runge, M.S., Patterson, C., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 893–904. [Google Scholar]
- Mallard, B.A.; Emam, M.; Paibomesai, M.; Thompson-Crispi, K.; Wagter-Lesperance, L. Genetic selection of cattle for improved immunity and health. Jpn. J. Vet. Res. 2015, 63 (Suppl. 1), S37–S44. [Google Scholar]
- Liston, A.; Humblet-Baron, S.; Duffy, D.; Goris, A. Human immune diversity: From evolution to modernity. Nat. Immunol. 2021, 22, 1479–1489. [Google Scholar] [CrossRef]
- Mallard, B.A.; Atalla, H.; Cartwright, S.; Hine, B.; Hussey, B.; Paibomesai, M.; Thompson-Crispi, K.; Wagter-Lesperance, L. Genetic and epigenetic regulation of the bovine immune system: Practical implications of the high immune response technology. In Proceedings of the 50th Annual National Mastitis Council Meeting, Arlington, VA, USA, 23–26 January 2011. [Google Scholar]
- Thompson-Crispi, K.A.; Miglior, F.; Mallard, B.A. Incidence rates of clinical mastitis among Canadian Holsteins classified as high, average, or low immune responders. Clin. Vaccine Immunol. 2013, 20, 106–112. [Google Scholar] [CrossRef]
- O’Flynn, D.; Lawler, J.; Yusuf, A.; Parle-McDermott, A.; Harold, D.; Mc Cloughlin, T.; Holland, L.; Regan, F.; White, B. A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic. Anal. Methods 2021, 13, 575–594. [Google Scholar] [CrossRef]
- Huang, L.C.; Wu, X.; Chen, J.Y. Predicting adverse side effects of drugs. BMC Genom. 2011, 12 (Suppl. 5), 11. [Google Scholar] [CrossRef]
- Üstün, N.Ş.; Bulam, S.; Pekşen, A. The use of Mushrooms and Their Extracts and Compounds in Functional Foods and Nutraceuticals. In Proceedings of the International Technology Sciences and Design Symposium, Giresun, Turkey, 27–29 June 2018. [Google Scholar]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef]
- Annweiler, C.; Hanotte, B.; Grandin de l’Eprevier, C.; Sabatier, J.M.; Lafaie, L.; Célarier, T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J. Steroid Biochem. Mol. Biol. 2020, 204, 105771. [Google Scholar] [CrossRef]
- Sánchez-Zuno, G.A.; González-Estevez, G.; Matuz-Flores, M.G.; Macedo-Ojeda, G.; Hernández-Bello, J.; Mora-Mora, J.C.; Pérez-Guerrero, E.E.; García-Chagollán, M.; Vega-Magaña, N.; Turrubiates-Hernández, F.J.; et al. Vitamin D Levels in COVID-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation. J. Clin. Med. 2021, 10, 2378. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef]
- Torisu, M.; Hayashi, Y.; Ishimitsu, T.; Fujimura, T.; Iwasaki, K.; Katano, M.; Yamamoto, H.; Kimura, Y.; Takesue, M.; Kondo, M. Significant prolongation of disease-free period gained by oral polysaccharide K (PSK) administration after curative surgical operation of colorectal cancer. Cancer Immunol. Immunother. 1990, 31, 261–268. [Google Scholar] [CrossRef]
- Mitomi, T.; Tsuchiya, S.; Iijima, N.; Aso, K.; Suzuki, K.; Nishiyama, K.; Amano, T.; Takahashi, T.; Murayama, N.; Oka, H. Randomized, controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. Dis. Colon Rectum 1992, 35, 123–130. [Google Scholar] [CrossRef]
- Tsang, K.W.; Lam, C.L.; Yan, C.; Mak, J.C.; Ooi, G.C.; Ho, J.C.; Lam, B.; Man, R.; Sham, J.S.; Lam, W.K. Coriolus versicolor polysaccharide peptide slows progression of advanced non-small cell lung cancer. Respir. Med. 2003, 97, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Guo, Y.; Li, M.; Zhu, H.; Wang, S.; Shen, X.; He, M.; Huang, C.; He, F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin. Interv. Aging 2017, 12, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Sazawal, S.; Dhingra, U.; Hiremath, G.; Sarkar, A.; Dhingra, P.; Dutta, A.; Verma, P.; Menon, V.P.; Black, R.E. Prebiotic and probiotic fortified milk in prevention of morbidities among children: Community-based, randomized, double-blind, controlled trial. PLoS ONE 2010, 5, e12164. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Fryburg, D.A.; Mark, R.J.; Griffith, B.P.; Askenase, P.W.; Patterson, T.F. The effect of supplemental beta-carotene on immunologic indices in patients with AIDS: A pilot study. Yale J. Biol. Med. 1995, 68, 19–23. [Google Scholar]
- Sedighiyan, M.; Abdollahi, H.; Karimi, E.; Badeli, M.; Erfanian, R.; Raeesi, S.; Hashemi, R.; Vahabi, Z.; Asanjarani, B.; Mansouri, F.; et al. Omega-3 polyunsaturated fatty acids supplementation improve clinical symptoms in patients with COVID-19: A randomised clinical trial. Int. J. Clin. Pract. 2021, 75, e14854. [Google Scholar] [CrossRef]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaie, E.; Ghaleh, H.; Aghamollaei, H.; Fasihi-Ramandi, M.; et al. Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial. Arch. Med. Res. 2022, 53, 79–85. [Google Scholar] [CrossRef]
- Nutraceuticals Market Size, Share and Trends Analysis Report by Product (Dietary Supplements, Functional Food, Functional Beverages), By Region (North America, Europe, APAC, CSA, MEA), And Segment Forecasts, 2021–2030. Available online: https://www.grandviewresearch.com/industry-analysis/nutraceuticals-market (accessed on 27 August 2022).
- Costagliola, G.; Nuzzi, G.; Spada, E.; Comberiati, P.; Verduci, E.; Peroni, D.G. Nutraceuticals in Viral Infections: An Overview of the Immunomodulating Properties. Nutrients 2021, 13, 2410. [Google Scholar] [CrossRef]
- Weisse, C.S. Depression and immunocompetence: A review of the literature. Psychol. Bull. 1992, 111, 475–489. [Google Scholar] [CrossRef]
- Claus, M.; Dychus, N.; Ebel, M.; Damaschke, J.; Maydych, V.; Wolf, O.T.; Kleinsorge, T.; Watzl, C. Measuring the immune system: A comprehensive approach for the analysis of immune functions in humans. Arch. Toxicol. 2016, 90, 2481–2495. [Google Scholar] [CrossRef]
- Glaser, R.; Gotlieb-Stematsky, T. Human Herpesvirus Infections: Clinical Aspects; Taylor & Francis Inc.: New York, NY, USA, 1982; ISBN 978-08-2471-536-6. [Google Scholar]
- Saeed, F.; Nadeem, M.; Ahmed, R.S.; Nadeem, M.T.; Arshad, M.S.; Ullah, A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016, 27, 205–229. [Google Scholar] [CrossRef]
- Bhaskaram, P. Micronutrient malnutrition, infection, and immunity: An overview. Nutr. Rev. 2002, 60, S40–S45. [Google Scholar] [CrossRef]
- Welch, R.M. Breeding strategies for biofortified staple plant foods to reduce micronutrient malnutrition globally. J. Nutr. 2002, 132, 495S–499S. [Google Scholar] [CrossRef]
- Merriam-Webster. Available online: https://www.merriam-webster.com/dictionary/nutritional%20deficiency (accessed on 26 August 2022).
- Parrow, N.L.; Fleming, R.E.; Minnick, M.F. Sequestration and scavenging of iron in infection. Infect. Immun. 2013, 81, 3503–3514. [Google Scholar] [CrossRef]
- Musavi, H.; Abazari, O.; Barartabar, Z.; Kalaki-Jouybari, F.; Hemmati-Dinarvand, M.; Esmaeili, P.; Mahjoub, S. The benefits of Vitamin D in the COVID-19 pandemic: Biochemical and immunological mechanisms. Arch. Physiol. Biochem. 2020, 1–9. [Google Scholar] [CrossRef]
- Topilski, I.; Flaishon, L.; Naveh, Y.; Harmelin, A.; Levo, Y.; Shachar, I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur. J. Immunol. 2004, 34, 1068–1076. [Google Scholar] [CrossRef]
- Abu-Amer, Y.; Bar-Shavit, Z. Regulation of TNF-alpha release from bone marrow-derived macrophages by vitamin D. J. Cell. Biochem. 1994, 55, 435–444. [Google Scholar] [CrossRef]
- Panfili, F.M.; Roversi, M.; D’Argenio, P.; Rossi, P.; Cappa, M.; Fintini, D. Possible role of vitamin D in COVID-19 infection in pediatric population. J. Endocrinol. Investig. 2021, 44, 27–35. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutri. 2004, 80 (Suppl. 6), 1678S–1688S. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory Properties of Coriolus versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef]
- Sims, J.E.; Smith, D.E. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef]
- Chu, K.K.; Ho, S.S.; Chow, A.H. Coriolus versicolor: A medicinal mushroom with promising immunotherapeutic values. J. Clin. Pharmacol. 2002, 42, 976–984. [Google Scholar]
- Liu, F.; Ooi, V.E.; Fung, M.C. Analysis of immunomodulating cytokine mRNAs in the mouse induced by mushroom polysaccharides. Life Sci. 1999, 64, 1005–1011. [Google Scholar] [CrossRef]
- Sakagami, H.; Takeda, M. Diverse biological activity of PSK (Krestin), a protein-bound polysaccharide from Coriolus versicolor (Fr.) Quel. In Mushroom Biology and Mushroom Products; Chang, S.T., Buswell, J.A., Chiu, S.W., Eds.; Chinese University Press: Hong Kong, China, 1993; pp. 237–245. [Google Scholar]
- Gu, Z.L.; Liang, Z.Q.; Wang, X.X. Effect of Coriolus versicolor polysaccharopeptide on production of IL-6 from human peripheral blood lymphocytes. In Advanced Research in PSP; Yang, Q.Y., Ed.; Hong Kong Association for Health Care: Hong Kong, China, 1999; pp. 99–103. [Google Scholar]
- Saakre, M.; Mathew, D.; Ravisankar, V. Perspectives on plant flavonoid quercetin-based drugs for novel SARS-CoV-2. Beni Suef Univ. J. Basic. Appl. Sci. 2021, 10, 21. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Mujwar, S.; Sun, L.; Fidan, O. In silico evaluation of food-derived carotenoids against SARS-CoV-2 drug targets: Crocin is a promising dietary supplement candidate for COVID-19. J. Food Biochem. 2022, 46, e14219. [Google Scholar] [CrossRef]
- Kurian, S.J.; Unnikrishnan, M.K.; Miraj, S.S.; Bagchi, D.; Banerjee, M.; Reddy, B.S.; Rodrigues, G.S.; Manu, M.K.; Saravu, K.; Mukhopadhyay, C.; et al. Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. Arch. Med. Res. 2021, 52, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, D.; Pandav, K.; Patel, M.; Riva-Moscoso, A.; Singh, B.M.; Patel, A.; Min, Z.C.; Singh-Makkar, S.; Sana, M.K.; Sanchez-Dopazo, R.; et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect. Chemother. 2020, 52, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J. Mechanisms and clinical evidence to support melatonin’s use in severe COVID-19 patients to lower mortality. Life Sci. 2020, 294, 120368. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef]
- Vitamin D—Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Vitamind-HealthProfessional/#h3 (accessed on 28 August 2022).
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. North Am. 2010, 39, 243–253. [Google Scholar] [CrossRef]
- Heaney, R.P. Vitamin D—Baseline status and effective dose. N. Engl. J. Med. 2012, 367, 77–78. [Google Scholar] [CrossRef]
- Weisman, Y.; Harell, A.; Edelstein, S.; David, M.; Spirer, Z.; Golander, A. 1 alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature 1979, 281, 317–319. [Google Scholar] [CrossRef]
- Gray, T.K.; Lester, G.E.; Lorenc, R.S. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science 1979, 204, 1311–1313. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Bouillon, R.; Mathieu, C. Immune regulation of 1alpha-hydroxylase in murine peritoneal macrophages: Unravelling the IFNgamma pathway. J. Steroid Biochem. Mol. Biol. 2007, 103, 567–571. [Google Scholar] [CrossRef]
- Esteban, L.; Vidal, M.; Dusso, A. 1alpha-Hydroxylase transactivation by gamma-interferon in murine macrophages requires enhanced C/EBPbeta expression and activation. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 131–137. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef]
- Deluca, H.F.; Cantorna, M.T. Vitamin D: Its role and uses in immunology. FASEB J. 2001, 15, 2579–2585. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Lowry, M.B.; Guo, C.; Borregaard, N.; Gombart, A.F. Regulation of the human cathelicidin antimicrobial peptide gene by 1α,25-dihydroxyvitamin D3 in primary immune cells. J. Steroid Biochem. Mol. Biol. 2014, 143, 183–191. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Ferreira, G.B.; Gysemans, C.A.; Demengeot, J.; da Cunha, J.P.; Vanherwegen, A.S.; Overbergh, L.; Van Belle, T.L.; Pauwels, F.; Verstuyf, A.; Korf, H.; et al. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J. Immunol. 2014, 192, 4210–4220. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G. Dendritic cell tolerogenicity: A key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009, 70, 345–352. [Google Scholar] [CrossRef]
- Van Halteren, A.G.; van Etten, E.; de Jong, E.C.; Bouillon, R.; Roep, B.O.; Mathieu, C. Redirection of human autoreactive T-cells Upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3). Diabetes 2002, 51, 2119–2125. [Google Scholar] [CrossRef]
- Van Halteren, A.G.; Tysma, O.M.; van Etten, E.; Mathieu, C.; Roep, B.O. 1alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J. Autoimmun. 2004, 23, 233–239. [Google Scholar] [CrossRef]
- Penna, G.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Berti, E.; Colonna, M.; Adorini, L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 2005, 106, 3490–3497. [Google Scholar] [CrossRef]
- Unger, W.W.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef]
- Van Belle, P.A.; Elenitsas, R.; Satyamoorthy, K.; Wolfe, J.T.; Guerry, D.; Schuchter, L.; Van Belle, T.J.; Albelda, S.; Tahin, P.; Herlyn, M.; et al. Progression-related expression of beta3 integrin in melanomas and nevi. Hum. Pathol. 1999, 30, 562–567. [Google Scholar] [CrossRef]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Bergman, P.; Henriques-Normark, B. Vitamin D Promotes Pneumococcal Killing and Modulates Inflammatory Responses in Primary Human Neutrophils. J. Innate Immun. 2017, 9, 375–386. [Google Scholar] [CrossRef]
- Xu, H.; Soruri, A.; Gieseler, R.K.; Peters, J.H. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand. J. Immunol. 1993, 38, 535–540. [Google Scholar] [CrossRef]
- Baeke, F.; Etten, E.V.; Overbergh, L.; Mathieu, C. Vitamin D3 and the immune system: Maintaining the balance in health and disease. Nutr. Res. Rev. 2007, 20, 106–118. [Google Scholar] [CrossRef]
- Takiishi, T.; Van Belle, T.; Gysemans, C.; Mathieu, C. Effects of vitamin D on antigen-specific and non-antigen-specific immune modulation: Relevance for type 1 diabetes. Pediatr. Diabetes 2013, 14, 81–89. [Google Scholar] [CrossRef]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984, 74, 657–661. [Google Scholar] [CrossRef]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zugel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef]
- Drozdenko, G.; Scheel, T.; Heine, G.; Baumgrass, R.; Worm, M. Impaired T cell activation and cytokine production by calcitriol-primed human B cells. Clin. Exp. Immunol. 2014, 178, 364–372. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Vitamin D and Calcium: Updated Dietary Reference Intakes. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/vitamins-minerals/vitamin-calcium-updated-dietary-reference-intakes-nutrition.html#shr-pg0 (accessed on 29 August 2022).
- Vitamin D. Available online: https://www.canada.ca/en/health-canada/services/nutrients/vitamin-d.html (accessed on 29 August 2022).
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Grant, W.B.; Karras, S.N.; Bischoff-Ferrari, H.A.; Annweiler, C.; Boucher, B.J.; Juzeniene, A.; Garland, C.F.; Holick, M.F. Do studies reporting ‘U’-shaped serum 25-hydroxyvitamin D-health outcome relationships reflect adverse effects. Dermatoendocrinology 2016, 8, e1187349. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Global epidemic of coronavirus- COVID-19: What can we do to minimize risks. Eur. J. Biomed. Pharm. Sci. 2020, 7, 432–438. [Google Scholar]
- Bleizgys, A. Vitamin D and COVID-19: It is time to act. Int. J. Clin. Pract. 2021, 75, e13748. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland-Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel with Participation of National Specialist Consultants and Representatives of Scientific Societies-2018 Update. Front. Endocrinol. 2018, 9, 246. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef]
- Lopez, A.G.; Kerlan, V.; Desailloud, R. Non-classical effects of vitamin D: Non-bone effects of vitamin D. Ann. Endocrinol. 2021, 82, 43–51. [Google Scholar] [CrossRef]
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. J. Am. Geriatr. Soc. 2014, 62, 147–152. [Google Scholar] [CrossRef]
- Yisak, H.; Ewunetei, A.; Kefale, B.; Mamuye, M.; Teshome, F.; Ambaw, B.; Yideg Yitbarek, G. Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review. Risk Manag. Healthc. Policy 2021, 14, 31–38. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Kimball, S.M.; Holick, M.F. Official recommendations for vitamin D through the life stages in developed countries. Eur. J. Clin. Nutr. 2020, 74, 1514–1518. [Google Scholar] [CrossRef]
- Ebadi, M.; Montano-Loza, A.J. Perspective: Improving vitamin D status in the management of COVID-19. Eur. J. Clin. Nutr. 2020, 74, 856–859. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Holick, M.F. The coronavirus disease (COVID-19)—A supportive approach with selected micronutrients. Int. J. Vitam. Nutr. Res. 2022, 92, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D and Its Potential Benefit for the COVID-19 Pandemic. Endocr. Pract. 2021, 27, 484–493. [Google Scholar] [CrossRef]
- Smith, J.E.; Sullivan, R.; Rowan, N. Mushrooms and cancer therapy. Biologist 2005, 52, 328–336. [Google Scholar]
- Mushroom Market Size, Share & Trends Analysis Report by Product (Button, Shiitake, Oyster), by Form, by Distribution Channel, By Application (Food, Pharmaceuticals, Cosmetics), By Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/mushroom-market (accessed on 29 August 2022).
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anticancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef]
- Chen, J.; Seviour, R. Medicinal importance of fungal beta-(1-->3), (1-->6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal beta-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Hahn, P.Y.; Evans, S.E.; Kottom, T.J.; Standing, J.E.; Pagano, R.E.; Limper, A.H. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 2003, 278, 2043–2050. [Google Scholar] [CrossRef]
- Evans, S.E.; Hahn, P.Y.; McCann, F.; Kottom, T.J.; Pavlovic, Z.V.; Limper, A.H. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am. J. Respir. Cell. Mol. Biol. 2005, 32, 490–497. [Google Scholar] [CrossRef]
- Wakshull, E.; Brunke-Reese, D.; Lindermuth, J.; Fisette, L.; Nathans, R.S.; Crowley, J.J.; Tufts, J.C.; Zimmerman, J.; Mackin, W.; Adams, D.S. PGG-glucan, a soluble beta-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-kappa B-like factor in human PMN: Evidence for a glycosphingolipid beta-(1,3)-glucan receptor. Immunopharmacology 1999, 41, 89–107. [Google Scholar] [CrossRef]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef]
- Chan, S.L.; Yeung, J.H. Polysaccharide peptides from COV-1 strain of Coriolus versicolor induce hyperalgesia via inflammatory mediator release in the mouse. Life Sci. 2006, 78, 2463–2470. [Google Scholar] [CrossRef]
- Li, K.-Y. Advances in immunomodulating studies of PSP. In Advanced Research in PSP; Yang, Q.-Y., Ed.; Hong Kong Association for Health Care Ltd.: Hong Kong, China, 1999; pp. 39–46. [Google Scholar]
- Ehrke, M.J.; Reino, J.M.; Eppolito, C.; Mihich, E. The effect of PS-K, a protein bound polysaccharide, on immune responses against allogeneic antigens. Int. J. Immunopharmacol. 1983, 5, 35–42. [Google Scholar] [CrossRef]
- Tsukagoshi, S.; Hashimoto, Y.; Fujii, G.; Kobayashi, H.; Nomoto, K.; Orita, K. Krestin (PSK). Cancer Treat. Rev. 1984, 11, 131–155. [Google Scholar] [CrossRef]
- Dong, Y.; Kwan, C.Y.; Chen, Z.N.; Yang, M.M. Antitumor effects of a refined polysaccharide peptide fraction isolated from Coriolus versicolor: In vitro and in vivo studies. Res. Commun. Mol. Pathol. Pharmacol. 1996, 92, 140–148. [Google Scholar]
- Dong, Y.; Yang, M.M.; Kwan, C.Y. In vitro inhibition of proliferation of HL-60 cells by tetrandrine and coriolus versicolor peptide derived from Chinese medicinal herbs. Life Sci. 1997, 60, PL135–PL140. [Google Scholar] [CrossRef]
- Harada, M.; Matsunaga, K.; Oguchi, Y.; Iijima, H.; Tamada, K.; Abe, K.; Takenoyama, M.; Ito, O.; Kimura, G.; Nomoto, K. Oral administration of PSK can improve the impaired anti-tumor CD4+ T-cell response in gut-associated lymphoid tissue (GALT) of specific-pathogen-free mice. Int. J. Cancer 1997, 70, 362–372. [Google Scholar] [CrossRef]
- Kato, M.; Hirose, K.; Hakozaki, M.; Ohno, M.; Saito, Y.; Izutani, R.; Noguchi, J.; Hori, Y.; Okumoto, S.; Kuroda, D. Induction of gene expression for immunomodulating cytokines in peripheral blood mononuclear cells in response to orally administered PSK, an immunomodulating protein-bound polysaccharide. Cancer Immunol. Immunother. 1995, 40, 152–156. [Google Scholar] [CrossRef]
- Liu, F.; Fung, M.C.; Ooi, V.E.; Chang, S.T. Induction in the mouse of gene expression of immunomodulating cytokines by mushroom polysaccharide-protein complexes. Life Sci. 1996, 58, 1795–1803. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Krawczyk, M.; Zgoła-Grześkowiak, A. Determination of antioxidant activity, rutin, quercetin, phenolic acids and trace elements in tea infusions: Influence of citric acid addition on extraction of metals. J. Food Compost. Anal. 2015, 40, 70–77. [Google Scholar] [CrossRef]
- Martelo-Vidal, M.J.; Vázquez, M. Determination of polyphenolic compounds of red wines by UV-VIS-NIR spectroscopy and chemometrics tools. Food Chem. 2014, 158, 28–34. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Manjunath, S.H.; Thimmulappa, R.K. Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: Potential role in prevention and management of COVID-19. J. Pharm. Anal. 2022, 12, 29–34. [Google Scholar] [CrossRef]
- Metodiewa, D.; Kochman, A.; Karolczak, S. Evidence for antiradical and antioxidant properties of four biologically active N,N-diethylaminoethyl ethers of flavanone oximes: A comparison with natural polyphenolic flavonoid (rutin) action. Biochem. Mol. Biol. Int. 1997, 41, 1067–1075. [Google Scholar] [CrossRef]
- Hayashi, T.; Sawa, K.; Kawasaki, M.; Arisawa, M.; Shimizu, M.; Morita, N. Inhibition of cow’s milk xanthine oxidase by flavonoids. J. Nat. Prod. 1988, 51, 345–348. [Google Scholar] [CrossRef]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fatty Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J. Agric. Food Chem. 2010, 58, 5815–5820. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.G.; Kim, H.K.; Lee, T.H.; Jeong, Y.I.; Lee, C.M.; Yoon, M.S.; Na, Y.J.; Suh, D.S.; Park, N.C.; et al. Silibinin polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells. J. Cell. Physiol. 2007, 210, 385–397. [Google Scholar] [CrossRef]
- Huang, R.Y.; Yu, Y.L.; Cheng, W.C.; OuYang, C.N.; Fu, E.; Chu, C.L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef]
- Yoon, M.S.; Lee, J.S.; Choi, B.M.; Jeong, Y.I.; Lee, C.M.; Park, J.H.; Moon, Y.; Sung, S.C.; Lee, S.K.; Chang, Y.H.; et al. Apigenin inhibits immunostimulatory function of dendritic cells: Implication of immunotherapeutic adjuvant. Mol. Pharmacol. 2006, 70, 1033–1044. [Google Scholar] [CrossRef]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [CrossRef]
- Chirumbolo, S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef]
- Finn, D.F.; Walsh, J.J. Twenty-first century mast cell stabilizers. Br. J. Pharmacol. 2013, 170, 23–37. [Google Scholar] [CrossRef]
- Thornhill, S.M.; Kelly, A.M. Natural treatment of perennial allergic rhinitis. Altern. Med. Rev. 2000, 5, 448–454. [Google Scholar]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, B.; Asadi, S.; Sismanopoulos, N.; Butcher, A.; Fu, X.; Katsarou-Katsari, A.; Antoniou, C.; Theoharides, T.C. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS ONE 2012, 7, e33805. [Google Scholar] [CrossRef]
- Chirumbolo, S. Quercetin as a potential anti-allergic drug: Which perspectives? Iran. J. Allergy Asthma Immunol. 2011, 10, 139–140. [Google Scholar]
- Zhang, R.; Ai, X.; Duan, Y.; Xue, M.; He, W.; Wang, C.; Xu, T.; Xu, M.; Liu, B.; Li, C.; et al. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed. Pharmacother. 2017, 89, 660–672. [Google Scholar] [CrossRef]
- Tang, X.L.; Liu, J.X.; Dong, W.; Li, P.; Li, L.; Hou, J.C.; Zheng, Y.Q.; Lin, C.R.; Ren, J.G. Protective effect of kaempferol on LPS plus ATP-induced inflammatory response in cardiac fibroblasts. Inflammation 2015, 38, 94–101. [Google Scholar] [CrossRef]
- Saw, C.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Nam, S.Y.; Jeong, H.J.; Kim, H.M. Kaempferol impedes IL-32-induced monocyte-macrophage differentiation. Chem. Biol. Interact. 2017, 274, 107–115. [Google Scholar] [CrossRef]
- Lin, M.K.; Yu, Y.L.; Chen, K.C.; Chang, W.T.; Lee, M.S.; Yang, M.J.; Cheng, H.C.; Liu, C.H.; Chen, D.; Chu, C.L. Kaempferol from Semen cuscutae attenuates the immune function of dendritic cells. Immunobiology 2011, 216, 1103–1109. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007, 2007, 1–10. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.J.; Kwon, S.; Lee, Y.; Choi, S.Y.; Park, J.; Kwon, H.J. Inhibitory effects of flavonoids on TNF-alpha-induced IL-8 gene expression in HEK 293 cells. BMB Rep. 2009, 42, 265–270. [Google Scholar] [CrossRef]
- Lee, E.J.; Ji, G.E.; Sung, M.K. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 2010, 59, 847–854. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, C.L.; Lei, H.; Zhang, S.D.; Zheng, J.; Wei, Q. Kaempferol: A new immunosuppressant of calcineurin. IUBMB Life 2008, 60, 549–554. [Google Scholar] [CrossRef]
- Liang, Y.C.; Tsai, S.H.; Tsai, D.C.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001, 496, 12–18. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Shindikar, A.; Singh, A.; Nobre, M.; Kirolikar, S. Curcumin and Resveratrol as Promising Natural Remedies with Nanomedicine Approach for the Effective Treatment of Triple Negative Breast Cancer. J. Oncol. 2016, 2016, 9750785. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Zhou, X.; Afzal, S.; Zheng, Y.F.; Münch, G.; Li, C.G. Synergistic Protective Effect of Curcumin and Resveratrol against Oxidative Stress in Endothelial EAhy926 Cells. Evid. Based Complement. Alternat. Med. 2021, 2021, 2661025. [Google Scholar] [CrossRef]
- Mazzanti, G.; Di Giacomo, S. Curcumin and Resveratrol in the Management of Cognitive Disorders: What is the Clinical Evidence? Molecules 2016, 21, 1243. [Google Scholar] [CrossRef]
- Teng, S.; Joseph, M.J.; Yu, H.; Hu, C.; Li, X.; Hu, C. A narrative review of the protective effects of curcumin in treating ischemia-reperfusion injury. Ann. Transl. Med. 2022, 10, 807. [Google Scholar] [CrossRef]
- Li, Y.H.; Niu, Y.B.; Sun, Y.; Zhang, F.; Liu, C.X.; Fan, L.; Mei, Q.B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015, 21, 9262–9272. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chopra, K.; Kulkarni, S.K.; Agrewala, J.N. Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin. Exp. Immunol. 2007, 147, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Couillard, C.; Paradis, M.-B.; Gigleux, I.; Marin, J.; Vohl, M.-C.; Couture, P.; Lamarche, B. Supplementation with Resveratrol and Curcumin Does Not Affect the Inflammatory Response to a High-Fat Meal in Older Adults with Abdominal Obesity: A Randomized, Placebo-Controlled Crossover Trial. J. Nutr. 2018, 148, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef]
- Karimi, K.; Sarir, H.; Mortaz, E.; Smit, J.J.; Hosseini, H.; De Kimpe, S.J.; Nijkamp, F.P.; Folkerts, G. Toll-like receptor-4 mediates cigarette smoke-induced cytokine production by human macrophages. Respir. Res. 2006, 7, 66. [Google Scholar] [CrossRef]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef]
- Gan, Z.; Wei, W.; Li, Y.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Zhong, X. Curcumin and Resveratrol Regulate Intestinal Bacteria and Alleviate Intestinal Inflammation in Weaned Piglets. Molecules 2019, 24, 1220. [Google Scholar] [CrossRef]
| Immunoceuticals | Authors | Study Design | Disease/Pathological Condition Addressed with Immunoceuticals | Dose | Results |
|---|---|---|---|---|---|
| Vitamin D3 (Cholecalciferol) | [31] | Multi-center, randomized clinical trial | Severe acute respiratory syndrome- coronavirus-2 (SARS-CoV-2) infection | 5000 IU or 1000 IU of Vit D3 once daily for two weeks |
|
| [32] | Quasi-experimental study | SARS-CoV-2 infection | Oral bolus of 80,000 IU Vit D3 during or just before infection with COVID-19 |
| |
| [33] | Randomized clinical trial | SARS-CoV-2 infection | Oral supplementation of 10,000 IU daily of Vit D3 for 14 days |
| |
| [34] | Multicenter, randomized, double-blind, placebo-controlled, parallel-group trial | Influenza A infection | 1200 IU of Vit D3 daily |
| |
| Polysaccharide K (PSK) | [35] | Randomized double-blind trial | Colorectal cancer | 3 g/day starting 10–15 days after surgery until two months after surgery, then 2 g daily until 24 months and 1 g daily thereafter |
|
| [36] | Randomized, controlled trial | Colorectal cancer | 3 g of PSK per day for over three years |
| |
| Polysaccharide-Peptides (PSP) | [37] | Double-blind placebo-controlled randomized study | Non-small cell lung cancer (NSCLC) | 340 mg of purified Yun-zhi PSP capsules three times daily for 28 days |
|
| Probiotics | [38] | Randomized controlled open-label trial | Acute upper respiratory tract infections (acute URTI) | 300 mL/day of yogurt supplemented with a probiotic strain, Lactobacillus paracasei N1115 (N1115), 3.6 × 107 CFU/mL for 12 weeks |
|
| Prebiotic and probiotic | [39] | Community based double-masked, randomized controlled trial | Diarrhea, respiratory infections and severe illnesses in children aged 1–4 years of age | milk fortified with 2.4 g/day of prebiotic oligosaccharide and 1.9 × 107 CFU of probiotic Bifidobacterium lactis HN019 |
|
| Quercetin | [40] | Prospective, randomized, controlled, and open-label study | SARS-CoV-2 infection | 1000 mg of Quercetin/day for 30 days |
|
| [41] | Second, pilot, randomized, controlled and open-label clinical trial | SARS-CoV-2 infection | 600 mg of Quercetin/day for seven days, followed by 400 mg of Quercetin/day for another seven days |
| |
| Beta-Carotene (Carotenoid) | [42] | Pilot study | AIDS | 60 mg/day for four weeks |
|
| Omega-3 fatty acids | [43] | Single-blind randomized controlled trial | SARS-CoV-2 infection | 2 g of docosahexaenoic acid (DHA) + eicosapentaenoic acid (EPA) for 2 weeks |
|
| [44] | Double-blind, randomized clinical trial | SARS-CoV-2 infection | One capsule of 1000 mg omega-3 daily containing 400 mg EPAs and 200 mg DHAs for 14 days |
| |
| Melatonin | [45] | Single-center, double-blind, randomized clinical trial | SARS-CoV-2 infection | 3 mg of melatonin three times daily for 14 days |
|
| Immunoceutical | Immunomodulatory Properties |
|---|---|
| Vitamin D3 (Cholecalciferol) |
|
| Coriolus versicolor extract (polysaccharide krestin (PSK), polysaccharide peptide (PSP)) |
|
| Quercetin |
|
| Carotenoids |
|
| Probiotics |
|
| Omega-3 fatty acids |
|
| Melatonin |
|
| Vitamin D Status | Serum 25(OH)D Concentrations (nmol/L) | Serum 25(OH)D Concentrations (ng/mL) |
|---|---|---|
| Severe deficiency | <25 | <10 |
| Moderate deficiency | 25–<50 | 10–<20 |
| Insufficiency | 50–<75 | 20–<30 |
| Sufficiency | 75–<100 | 30–<40 |
| Optimal concentration | 100–<150 | 40–<60 |
| Increased concentration | 150–<250 | 60–<100 |
| Overdose | ≥250 | ≥100 |
| Intoxication | ≥375 | ≥150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tieu, S.; Charchoglyan, A.; Wagter-Lesperance, L.; Karimi, K.; Bridle, B.W.; Karrow, N.A.; Mallard, B.A. Immunoceuticals: Harnessing Their Immunomodulatory Potential to Promote Health and Wellness. Nutrients 2022, 14, 4075. https://doi.org/10.3390/nu14194075
Tieu S, Charchoglyan A, Wagter-Lesperance L, Karimi K, Bridle BW, Karrow NA, Mallard BA. Immunoceuticals: Harnessing Their Immunomodulatory Potential to Promote Health and Wellness. Nutrients. 2022; 14(19):4075. https://doi.org/10.3390/nu14194075
Chicago/Turabian StyleTieu, Sophie, Armen Charchoglyan, Lauri Wagter-Lesperance, Khalil Karimi, Byram W. Bridle, Niel A. Karrow, and Bonnie A. Mallard. 2022. "Immunoceuticals: Harnessing Their Immunomodulatory Potential to Promote Health and Wellness" Nutrients 14, no. 19: 4075. https://doi.org/10.3390/nu14194075
APA StyleTieu, S., Charchoglyan, A., Wagter-Lesperance, L., Karimi, K., Bridle, B. W., Karrow, N. A., & Mallard, B. A. (2022). Immunoceuticals: Harnessing Their Immunomodulatory Potential to Promote Health and Wellness. Nutrients, 14(19), 4075. https://doi.org/10.3390/nu14194075