Ketoanalogues Supplemental Low Protein Diet Safely Decreases Short-Term Risk of Dialysis among CKD Stage 4 Patients
Abstract
1. Introduction
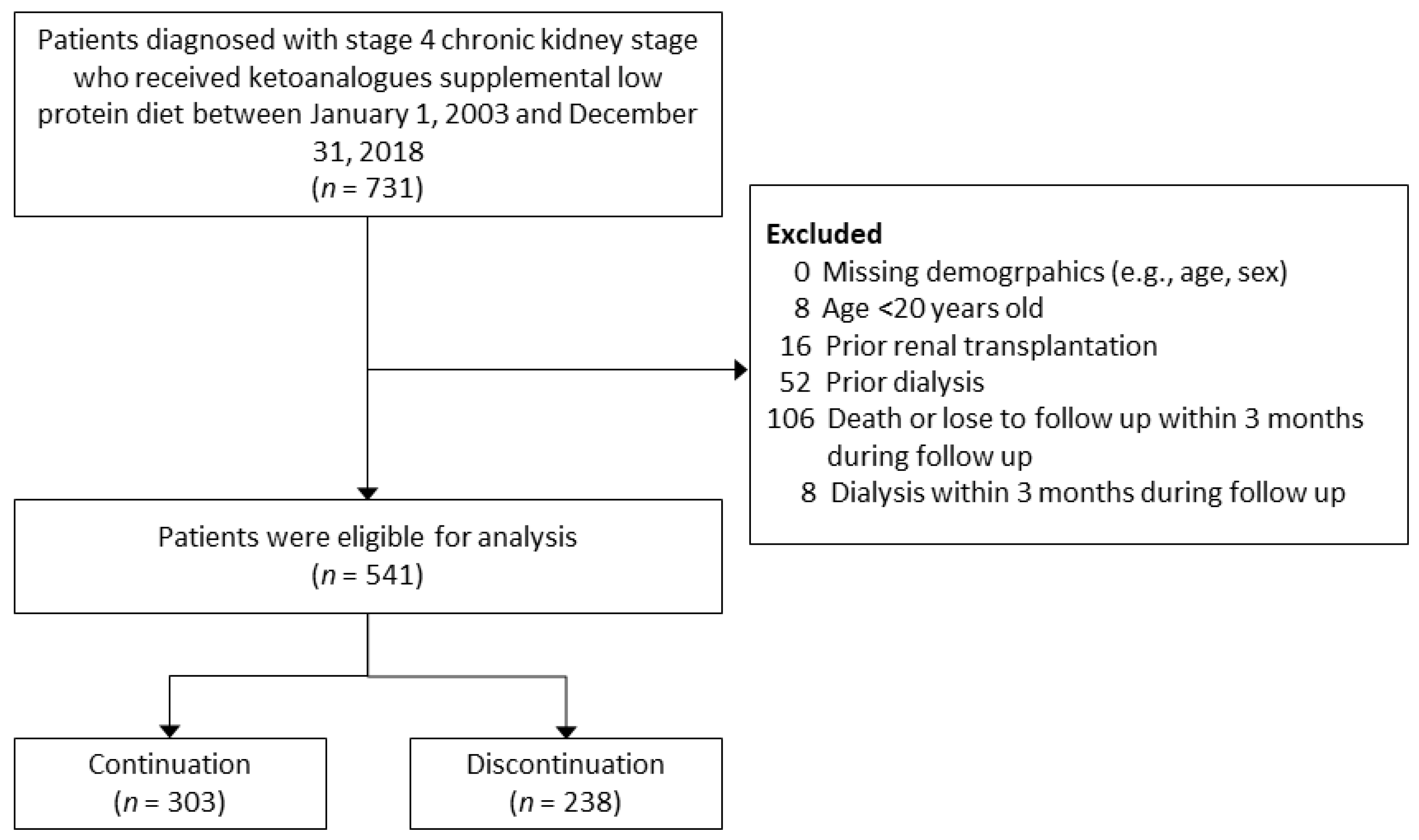
2. Materials and Methods
2.1. Data Source
2.2. Study Design
2.3. Assessment of Covariates
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
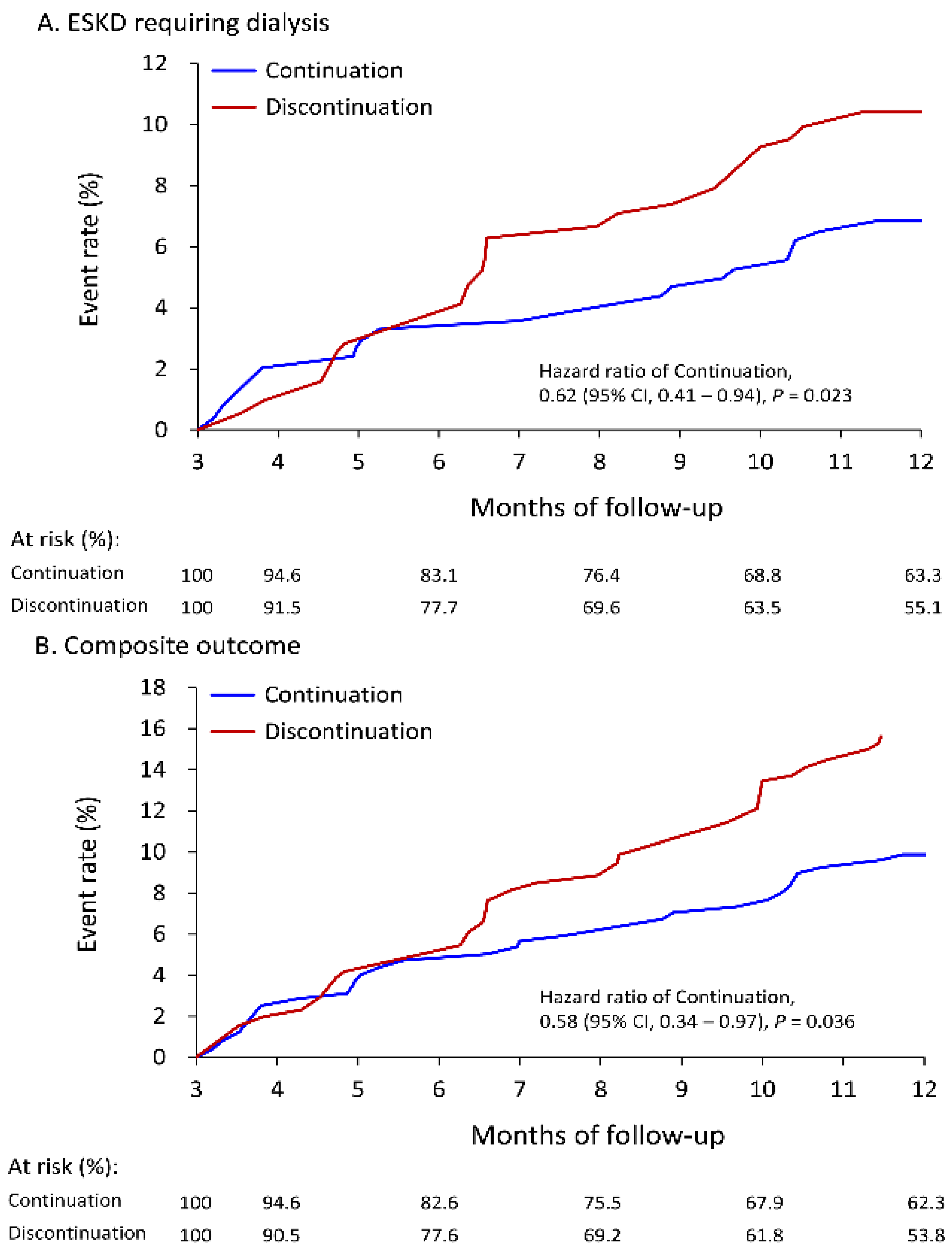
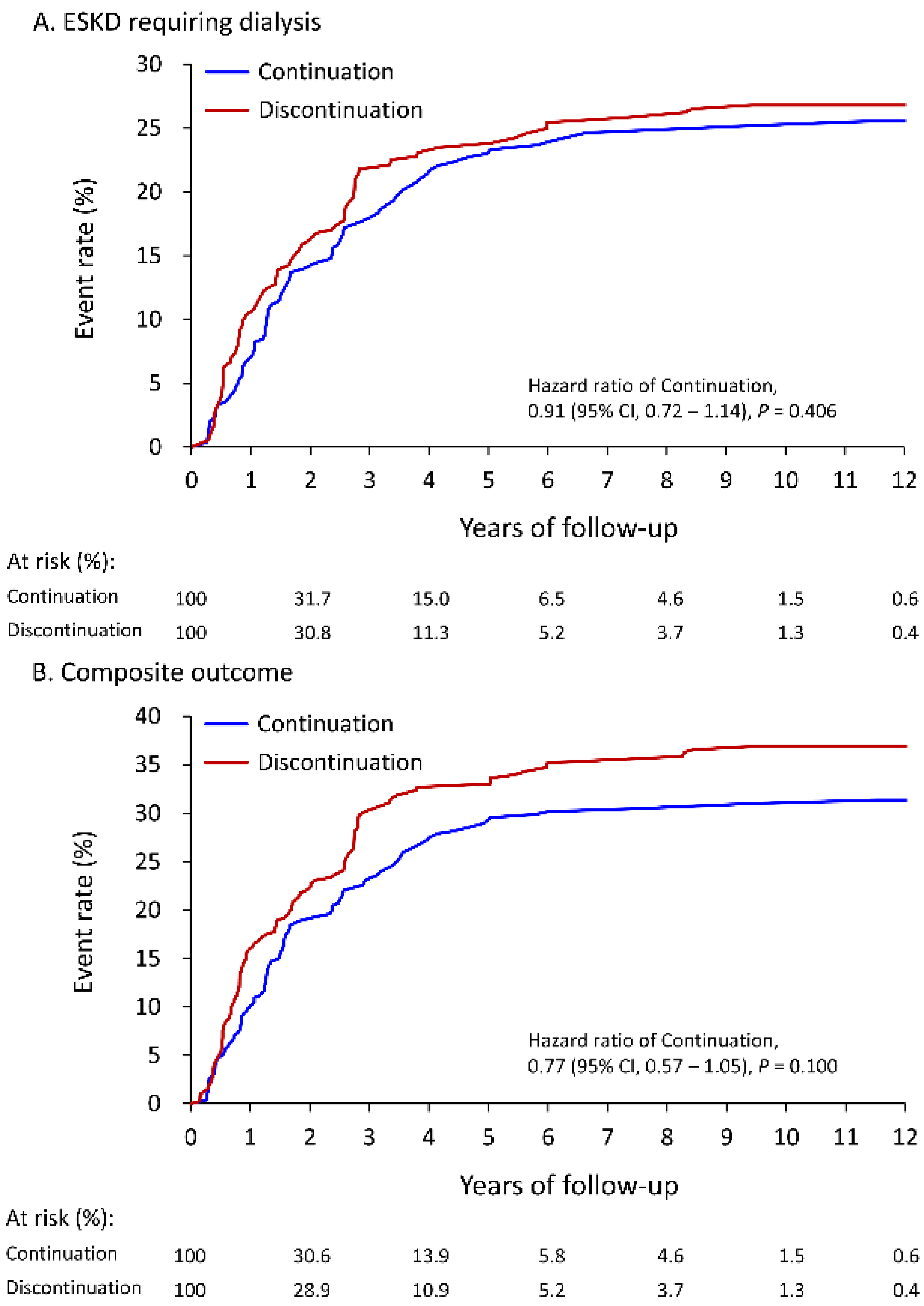
3.2. Follow-Up Outcomes
3.3. Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2018, 378, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Addis, T.; Lew, W. Diet and Death in Acute Uremia. J. Clin. Investig. 1939, 18, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.S. On the Influence of a Diet with High Protein Content on the Kidney. Can. Med. Assoc. J. 1921, 11, 682–683. [Google Scholar] [PubMed]
- Cuppari, L.; Meireles, M.S.; Ramos, C.I.; Kamimura, M.A. Subjective global assessment for the diagnosis of protein-energy wasting in nondialysis-dependent chronic kidney disease patients. J. Ren. Nutr. 2014, 24, 385–389. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef]
- Walser, M. Ketoacids in the treatment of uremia. Clin. Nephrol. 1975, 3, 180–186. [Google Scholar]
- Walser, M.; Lund, P.; Ruderman, N.B.; Coulter, A.W. Synthesis of essential amino acids from their alpha-keto analogues by perfused rat liver and muscle. J. Clin. Investig. 1973, 52, 2865–2877. [Google Scholar] [CrossRef]
- Kang, C.W.; Tungsanga, K.; Walser, M. Effect of the level of dietary protein on the utilization of alpha-ketoisocaproate for protein synthesis. Am. J. Clin. Nutr. 1986, 43, 504–509. [Google Scholar] [CrossRef]
- Jiang, N.; Qian, J.; Sun, W.; Lin, A.; Cao, L.; Wang, Q.; Ni, Z.; Wan, Y.; Linholm, B.; Axelsson, J.; et al. Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: A prospective, randomized trial. Nephrol. Dial. Transpl. 2009, 24, 2551–2558. [Google Scholar] [CrossRef]
- Yen, C.L.; Fan, P.C.; Lee, C.C.; Kuo, G.; Tu, K.H.; Chen, J.J.; Lee, T.H.; Hsu, H.H.; Tian, Y.C.; Chang, C.H. Advanced Chronic Kidney Disease with Low and Very Low GFR: Can a Low-Protein Diet Supplemented with Ketoanalogues Delay Dialysis? Nutrients 2020, 12, 3358. [Google Scholar] [CrossRef] [PubMed]
- Satirapoj, B.; Vongwattana, P.; Supasyndh, O. Very low protein diet plus ketoacid analogs of essential amino acids supplement to retard chronic kidney disease progression. Kidney Res. Clin. Pract. 2018, 37, 384–392. [Google Scholar] [CrossRef]
- Bolasco, P. Very low protein plus ketoacid analogs of essential aminoacids do not confirm superiority of a low protein diet to retard chronic kidney disease progression. Kidney Res. Clin. Pract. 2019, 38, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, H.; Fang, M.; Wang, K.; Chen, J.; Sun, W.; Yang, L.; Lin, H. Keto-supplemented Low Protein Diet: A Valid Therapeutic Approach for Patients with Steroid-resistant Proteinuria during Early-stage Chronic Kidney Disease. J. Nutr. Health Aging 2016, 20, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Marzocco, S.; Bellasi, A.; De Simone, E.; Dal Piaz, F.; Rocchetti, M.T.; Cosola, C.; Di Micco, L.; Gesualdo, L. Nutritional therapy reduces protein carbamylation through urea lowering in chronic kidney disease. Nephrol. Dial. Transpl. 2018, 33, 804–813. [Google Scholar] [CrossRef]
- David, C.; Peride, I.; Niculae, A.; Constantin, A.M.; Checherita, I.A. Very low protein diets supplemented with keto-analogues in ESRD predialysis patients and its effect on vascular stiffness and AVF Maturation. BMC Nephrol. 2016, 17, 131. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Bellizzi, V.; Signoriello, S.; Minutolo, R.; Di Iorio, B.; Nazzaro, P.; Garofalo, C.; Calella, P.; Chiodini, P.; De Nicola, L.; The ERIKA Study Group Investigators of the Italian Society of Nephrology-Conservative Therapy of CKD Work Group. No additional benefit of prescribing a very low-protein diet in patients with advanced chronic kidney disease under regular nephrology care: A pragmatic, randomized, controlled trial. Am. J. Clin. Nutr. 2022, 115, 1404–1417. [Google Scholar] [CrossRef]
- Ikizler, T.A. Very low-protein diets in advanced kidney disease: Safe, effective, but not practical. Am. J. Clin. Nutr. 2022, 115, 1266–1267. [Google Scholar] [CrossRef]
- Lin, M.Y.; Cheng, L.J.; Chiu, Y.W.; Hsieh, H.M.; Wu, P.H.; Lin, Y.T.; Wang, S.L.; Jian, F.X.; Hsu, C.C.; Yang, S.A.; et al. Effect of national pre-ESRD care program on expenditures and mortality in incident dialysis patients: A population-based study. PLoS ONE 2018, 13, e0198387. [Google Scholar] [CrossRef]
- Wu, C.H.; Yang, Y.W.; Hung, S.C.; Kuo, K.L.; Wu, K.D.; Wu, V.C.; Hsieh, T.C. National Taiwan University Study Group on Acute Renal, F. Ketoanalogues supplementation decreases dialysis and mortality risk in patients with anemic advanced chronic kidney disease. PLoS ONE 2017, 12, e0176847. [Google Scholar] [CrossRef]
- Yen, C.L.; Fan, P.C.; Kuo, G.; Chen, C.Y.; Cheng, Y.L.; Hsu, H.H.; Tian, Y.C.; Chatrenet, A.; Piccoli, G.B.; Chang, C.H. Supplemented Low-Protein Diet May Delay the Need for Preemptive Kidney Transplantation: A Nationwide Population-Based Cohort Study. Nutrients 2021, 13, 3002. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Tu, K.H.; Lin, M.S.; Chang, S.W.; Fan, P.C.; Hsiao, C.C.; Chen, C.Y.; Hsu, H.H.; Tian, Y.C.; Chang, C.H. Does a Supplemental Low-Protein Diet Decrease Mortality and Adverse Events After Commencing Dialysis? A Nationwide Cohort Study. Nutrients 2018, 10, 1035. [Google Scholar] [CrossRef]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Mircescu, G.; Garneata, L.; Stancu, S.H.; Capusa, C. Effects of a supplemented hypoproteic diet in chronic kidney disease. J. Ren. Nutr. 2007, 17, 179–188. [Google Scholar] [CrossRef]
- Cianciaruso, B.; Capuano, A.; D’Amaro, E.; Ferrara, N.; Nastasi, A.; Conte, G.; Bellizzi, V.; Andreucci, V.E. Dietary compliance to a low protein and phosphate diet in patients with chronic renal failure. Kidney Int. Suppl. 1989, 27, S173–S176. [Google Scholar]
- Cupisti, A.; Gallieni, M.; Avesani, C.M.; D’Alessandro, C.; Carrero, J.J.; Piccoli, G.B. Medical Nutritional Therapy for Patients with Chronic Kidney Disease not on Dialysis: The Low Protein Diet as a Medication. J. Clin. Med. 2020, 9, 3644. [Google Scholar] [CrossRef]
- Chiang, H.H.; Hong, R.M.; Tsai, J.P.; Chen, M.Y. The Lived Experiences of Living with LPD among CKD Patients in Southern Taiwan. Neuropsychiatry 2017, 7, 48–56. [Google Scholar] [CrossRef]
- Bellizzi, V.; Calella, P.; Hernandez, J.N.; Gonzalez, V.F.; Lira, S.M.; Torraca, S.; Arronte, R.U.; Cirillo, P.; Minutolo, R.; Montufar Cardenas, R.A. Safety and effectiveness of low-protein diet supplemented with ketoacids in diabetic patients with chronic kidney disease. BMC Nephrol. 2018, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Milovanova, L.; Fomin, V.; Moiseev, S.; Taranova, M.; Milovanov, Y.; Lysenko Kozlovskaya, L.; Kozlov, V.; Kozevnikova, E.; Milovanova, S.; Lebedeva, M.; et al. Effect of essential amino acid small ka, Cyrillicetoanalogues and protein restriction diet on morphogenetic proteins (FGF-23 and capital KA, Cyrilliclotho) in 3b-4 stages chronic small ka, Cyrillicidney disease patients: A randomized pilot study. Clin. Exp. Nephrol. 2018, 22, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Laville, S.M.; Couturier, A.; Lambert, O.; Metzger, M.; Mansencal, N.; Jacquelinet, C.; Laville, M.; Frimat, L.; Fouque, D.; Combe, C.; et al. Urea levels and cardiovascular disease in patients with chronic kidney disease. Nephrol. Dial. Transpl. 2022, 1–9. [Google Scholar] [CrossRef]
| Variables | Available Number | Continuation (n = 303) | Discontinuation (n = 238) | STD | p Value |
|---|---|---|---|---|---|
| Age, years | 541 | 67.1 ± 14.1 | 68.5 ± 14.4 | −0.10 | 0.24 |
| Age ≥ 65 years | 541 | 171 (56.4) | 157 (66.0) | −0.20 | 0.02 |
| Male | 541 | 187 (61.7) | 147 (61.8) | <0.01 | 0.99 |
| Body mass index, kg/m2 | 438 | 26.4 ± 24.2 | 24.8 ± 4.7 | 0.09 | 0.34 |
| eGFR at index, mL/min/1.73 m2 | 541 | 20.9 ± 4.8 | 20.9 ± 4.6 | <0.01 | 0.97 |
| Comorbidities | |||||
| Coronary artery disease | 541 | 87 (28.7) | 62 (26.1) | 0.06 | 0.49 |
| Hypertension | 541 | 248 (81.8) | 197 (82.8) | −0.02 | 0.78 |
| Diabetes mellitus | 541 | 152 (50.2) | 131 (55.0) | −0.10 | 0.26 |
| Atrial fibrillation | 541 | 18 (5.9) | 16 (6.7) | −0.03 | 0.71 |
| Liver cirrhosis | 541 | 26 (8.6) | 17 (7.1) | 0.05 | 0.54 |
| Peripheral artery disease | 541 | 29 (9.6) | 23 (9.7) | <0.01 | 0.97 |
| Dementia | 541 | 15 (5.0) | 18 (7.6) | −0.11 | 0.21 |
| Systemic lupus erythematosus | 541 | 5 (1.7) | 3 (1.3) | 0.03 | 0.71 |
| Hepatitis B infection | 541 | 20 (6.6) | 11 (4.6) | 0.09 | 0.33 |
| Hepatitis C infection | 541 | 12 (4.0) | 9 (3.8) | 0.01 | 0.92 |
| Heart failure hospitalization | 541 | 24 (7.9) | 20 (8.4) | −0.02 | 0.84 |
| Myocardial infarction | 541 | 25 (8.3) | 15 (6.3) | 0.08 | 0.39 |
| Stroke | 541 | 29 (9.6) | 30 (12.6) | −0.10 | 0.26 |
| No. of outpatient visits on nephrology in the previous year | 541 | 0.52 | |||
| 0 | 39 (12.9) | 37 (15.5) | −0.08 | ||
| 1–5 | 174 (57.4) | 139 (58.4) | −0.02 | ||
| 6–10 | 74 (24.4) | 47 (19.7) | 0.11 | ||
| >10 | 16 (5.3) | 15 (6.3) | −0.04 | ||
| No. of outpatient visits on all departments in the previous year | 541 | 12.8 ± 9.5 | 13.1 ± 9.1 | −0.03 | 0.75 |
| Admission in the previous year | 541 | 99 (32.7) | 99 (41.6) | −0.19 | 0.03 |
| Follow-up years | 541 | 1.5 [0.8, 3.4] | 1.3 [0.6, 3.2] | 0.03 | 0.74 |
| Variables | Available Number | Continuation (n = 303) | Discontinuation (n = 238) | STD | p Value |
|---|---|---|---|---|---|
| Medication at baseline | |||||
| ACEi/ARB | 541 | 179 (59.1) | 138 (58.0) | 0.02 | 0.80 |
| Beta-blockers | 541 | 79 (26.1) | 67 (28.2) | −0.05 | 0.59 |
| Calcium-channel blocker | 541 | 139 (45.9) | 106 (44.5) | 0.03 | 0.76 |
| Mineralocortocoid receptor antagonis | 541 | 23 (7.6) | 21 (8.8) | −0.04 | 0.60 |
| Loop diuretics | 541 | 99 (32.7) | 91 (38.2) | −0.12 | 0.18 |
| Nitrates | 541 | 40 (13.2) | 31 (13.0) | 0.01 | 0.95 |
| Vasodilator | 541 | 19 (6.3) | 17 (7.1) | −0.03 | 0.69 |
| Thiazide | 541 | 22 (7.3) | 22 (9.2) | −0.07 | 0.40 |
| Antiplatelet agents | 541 | 99 (32.7) | 72 (30.3) | 0.05 | 0.55 |
| NSAIDs | 541 | 31 (10.2) | 31 (13.0) | −0.09 | 0.31 |
| Steroid | 541 | 40 (13.2) | 39 (16.4) | −0.09 | 0.30 |
| Proton pump inhibitor | 541 | 48 (15.8) | 47 (19.7) | −0.10 | 0.24 |
| Insulin | 541 | 41 (13.5) | 35 (14.7) | −0.03 | 0.70 |
| Oral hypoglycemic agents | 541 | 107 (35.3) | 92 (38.7) | −0.07 | 0.42 |
| Pentoxyfillin | 541 | 136 (44.9) | 103 (43.3) | 0.03 | 0.71 |
| Sodium bicarbonate | 541 | 37 (12.2) | 20 (8.4) | 0.13 | 0.15 |
| Fibrate | 541 | 18 (5.9) | 13 (5.5) | 0.02 | 0.81 |
| Statin | 541 | 123 (40.6) | 93 (39.1) | 0.03 | 0.72 |
| Laboratory data at baseline | |||||
| Blood urine nitrogen, mg/dL | 497 | 43.6 ± 17.0 | 42.9 ± 19.2 | 0.04 | 0.67 |
| Creatinine, mg/dL | 541 | 2.9 ± 0.8 | 2.9 ± 0.8 | 0.06 | 0.46 |
| Proteinuria group, mg/dL | 335 | 0.02 | |||
| Negative (0–4) | 38 (20.2) | 14 (9.5) | 0.30 | ||
| Trace (5–29) | 14 (7.4) | 10 (6.8) | 0.03 | ||
| ≥1+ (≥30) | 136 (72.3) | 123 (83.7) | −0.28 | ||
| CO2 | 160 | 22.7 ± 3.8 | 22.5 ± 4.2 | 0.03 | 0.83 |
| Potassium, mg/dL | 496 | 4.4 ± 0.7 | 4.4 ± 0.7 | −0.05 | 0.55 |
| Sodium, mg/dL | 351 | 138.9 ± 4.2 | 138.6 ± 4.2 | 0.07 | 0.52 |
| Calcium, mg/dL | 429 | 8.9 ± 0.6 | 8.9 ± 0.7 | 0.07 | 0.46 |
| Phosphorus, mg/dL | 323 | 3.8 ± 0.7 | 4.0 ± 0.9 | −0.19 | 0.10 |
| HDL, mg/dL | 174 | 46.2 ± 13.2 | 43.4 ± 12.8 | 0.21 | 0.18 |
| LDL, mg/dL | 222 | 79.1 ± 56.7 | 79.3 ± 49.4 | <0.01 | 0.98 |
| Total cholesterol, mg/dL | 122 | 174.9 ± 41.2 | 176.3 ± 55.0 | −0.03 | 0.87 |
| HbA1C, % | 249 | 7.0 ± 1.5 | 7.1 ± 1.7 | −0.08 | 0.51 |
| Albumin, mg/dL | 400 | 3.8 ± 0.5 | 3.8 ± 0.6 | 0.05 | 0.62 |
| Hemoglobin, g/dL | 464 | 10.5 ± 1.8 | 10.4 ± 1.8 | 0.02 | 0.83 |
| Serum uric acid, mg/dL | 386 | 7.1 ± 2.0 | 7.5 ± 2.2 | −0.21 | 0.04 |
| Continuation | Discontinuation | HR (95% CI) of | ||||
|---|---|---|---|---|---|---|
| Follow Up/Outcome | Event Rate | Incidence (95% CI) * | Event Rate | Incidence (95% CI) * | Continuation | p Value |
| 1-year follow-up | ||||||
| Primary outcome: ESKD requiring dialysis | 6.8% | 8.2 (6.0–10.8) | 10.4% | 13.1 (9.7–16.5) | 0.62 (0.41–0.94) * | 0.023 |
| MACCE † | 3.2% | 3.8 (2.0–5.6) | 5.3% | 6.4 (4.0–8.8) | 0.59 (0.25–1.39) | 0.225 |
| Cardiovascular death | 2.0% | 2.3 (1.0–3.7) | 3.3% | 4.0 (2.1–5.8) | 0.57 (0.19–1.71) | 0.314 |
| Acute myocardial infarction | 0.9% | 1.1 (0.0–2.0) | 2.5% | 3.0 (1.4–4.7) | 0.36 (0.09–1.48) | 0.156 |
| Ischemic stroke | 0.3% | 0.4 (0.0–1.0) | 0.8% | 0.9 (0.0–1.8) | 0.44 (0.03–6.83) | 0.557 |
| All-cause death | 3.8% | 4.4 (3.0–6.4) | 5.6% | 6.7 (4.3–9.1) | 0.65 (0.27–1.54) | 0.329 |
| Infection related death | 3.1% | 3.6 (2.0–5.3) | 3.6% | 4.3 (2.4–6.2) | 0.82 (0.29–2.33) | 0.714 |
| Composite outcome # | 9.9% | 11.8 (9.0–15.0) | 15.9% | 20.2 (15.9–24.4) | 0.58 (0.34–0.97) * | 0.036 |
| At the end of follow-up | ||||||
| Primary outcome: ESKD requiring dialysis | 25.6% | 12.1 (10.0–14.2) | 26.8% | 13.9 (11.6–16.2) | 0.91 (0.72–1.14) | 0.406 |
| MACCE † | 11.5% | 4.6 (3.0–5.8) | 13.2% | 5.7 (4.4–7.1) | 0.81 (0.49–1.33) | 0.396 |
| Cardiovascular death | 8.6% | 3.3 (2.0–4.2) | 9.8% | 4.1 (3.0–5.2) | 0.79 (0.44–1.39) | 0.408 |
| Acute myocardial infarction | 3.3% | 1.3 (1.0–1.9) | 3.7% | 1.6 (0.9–2.3) | 0.83 (0.33–2.09) | 0.698 |
| Ischemic stroke | 2.9% | 1.2 (1.0–1.7) | 3.6% | 1.5 (0.8–2.2) | 0.75 (0.28–2.03) | 0.573 |
| All-cause death | 11.4% | 4.4 (3.0–5.4) | 13.9% | 5.8 (4.5–7.2) | 0.74 (0.45–1.21) | 0.228 |
| Infection related death | 8.4% | 3.2 (2.0–4.2) | 9.2% | 3.9 (2.8–4.9) | 0.82 (0.45–1.50) | 0.521 |
| Composite outcome # | 31.4% | 15.3 (13.0–17.6) | 36.9% | 19.6 (16.9–22.3) | 0.77 (0.57–1.05) | 0.100 |
| Event Rate | HR (95% CI) of Continuation | p for Interaction § | ||
|---|---|---|---|---|
| Subgroup | Continuation | Discontinuation | ||
| Age group | 0.375 | |||
| 20–65 | 8.3% | 9.0% | 0.88 (0.32–2.39) | |
| >65 | 5.9% | 11.3% | 0.48 (0.20–1.15) | |
| Gender | 0.186 | |||
| Female | 7.6% | 6.5% | 1.15 (0.38–3.45) | |
| Male | 6.4% | 12.7% | 0.46 (0.21–1.01) | |
| Body mass index, kg/m2 | 0.974 | |||
| <24 | 11.3% | 18.1% | 0.58 (0.27–1.27) | |
| ≥24 | 4.7% | 7.4% | 0.60 (0.18–2.01) | |
| eGFR at baseline | 0.843 | |||
| 15–20 mL/min/1.73 m2 | 10.3% | 16.4% | 0.60 (0.29–1.25) | |
| 21–30 mL/min/1.73 m2 | 3.1% | 4.0% | 0.71 (0.18–2.71) | |
| Hypertension | 0.934 | |||
| No | 4.8% | 7.1% | 0.67 (0.10–4.45) | |
| Yes | 7.3% | 11.1% | 0.61 (0.31–1.21) | |
| Diabetes mellitus | 0.430 | |||
| No | 5.7% | 10.8% | 0.46 (0.17–1.26) | |
| Yes | 7.9% | 10.1% | 0.78 (0.34–1.78) | |
| Cardiovascular disease * | 0.531 | |||
| No | 7.4% | 9.4% | 0.72 (0.31–1.71) | |
| Yes | 5.9% | 12.2% | 0.48 (0.18–1.29) | |
| ACEi/ARB | 0.514 | |||
| No | 7.2% | 7.7% | 0.82 (0.28–2.39) | |
| Yes | 6.6% | 12.3% | 0.53 (0.24–1.17) | |
| Loop diuretics | 0.412 | |||
| No | 5.6% | 10.0% | 0.50 (0.21–1.18) | |
| Yes | 9.4% | 11.3% | 0.86 (0.32–2.31) | |
| Pentoxyfilline | 0.719 | |||
| No | 7.0% | 10.1% | 0.69 (0.28–1.68) | |
| Yes | 6.7% | 10.8% | 0.54 (0.22–1.36) | |
| Proteinuria | 0.603 | |||
| Negative/Trace | 3.7% | 14.2% | 0.26 (0.02–3.46) | |
| ≥1+ | 7.7% | 13.7% | 0.53 (0.24–1.16) | |
| Albumin, mg/dL | 0.242 | |||
| <3.5 | 15.4% | 13.6% | 1.02 (0.35–2.95) | |
| ≥3.5 | 5.8% | 12.1% | 0.45 (0.18–1.10) | |
| Hemoglobin, g/dL | 0.388 | |||
| <10 | 10.4% | 14.1% | 0.66 (0.29–1.52) | |
| ≥10 | 4.1% | 10.7% | 0.36 (0.12–1.12) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-L.; Fan, P.-C.; Chen, J.-J.; Kuo, G.; Hsiao, C.-C.; Chen, C.-Y.; Tu, Y.-R.; Hsu, H.-H.; Chen, Y.-C.; Chang, C.-H. Ketoanalogues Supplemental Low Protein Diet Safely Decreases Short-Term Risk of Dialysis among CKD Stage 4 Patients. Nutrients 2022, 14, 4020. https://doi.org/10.3390/nu14194020
Yen C-L, Fan P-C, Chen J-J, Kuo G, Hsiao C-C, Chen C-Y, Tu Y-R, Hsu H-H, Chen Y-C, Chang C-H. Ketoanalogues Supplemental Low Protein Diet Safely Decreases Short-Term Risk of Dialysis among CKD Stage 4 Patients. Nutrients. 2022; 14(19):4020. https://doi.org/10.3390/nu14194020
Chicago/Turabian StyleYen, Chieh-Li, Pei-Chun Fan, Jia-Jin Chen, George Kuo, Ching-Chung Hsiao, Chao-Yu Chen, Yi-Ran Tu, Hsiang-Hao Hsu, Yung-Chang Chen, and Chih-Hsiang Chang. 2022. "Ketoanalogues Supplemental Low Protein Diet Safely Decreases Short-Term Risk of Dialysis among CKD Stage 4 Patients" Nutrients 14, no. 19: 4020. https://doi.org/10.3390/nu14194020
APA StyleYen, C.-L., Fan, P.-C., Chen, J.-J., Kuo, G., Hsiao, C.-C., Chen, C.-Y., Tu, Y.-R., Hsu, H.-H., Chen, Y.-C., & Chang, C.-H. (2022). Ketoanalogues Supplemental Low Protein Diet Safely Decreases Short-Term Risk of Dialysis among CKD Stage 4 Patients. Nutrients, 14(19), 4020. https://doi.org/10.3390/nu14194020





