The Safety and Efficacy of Citrus aurantium (Bitter Orange) Extracts and p-Synephrine: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Selection Criteria
2.2. Data Extraction and Endpoints
2.3. Quality Evaluations
2.4. Statistical Analysis
3. Results
3.1. Literature Search
3.1.1. Characteristics of the Trials
3.1.2. Demography of the Patients
3.2. Outcomes
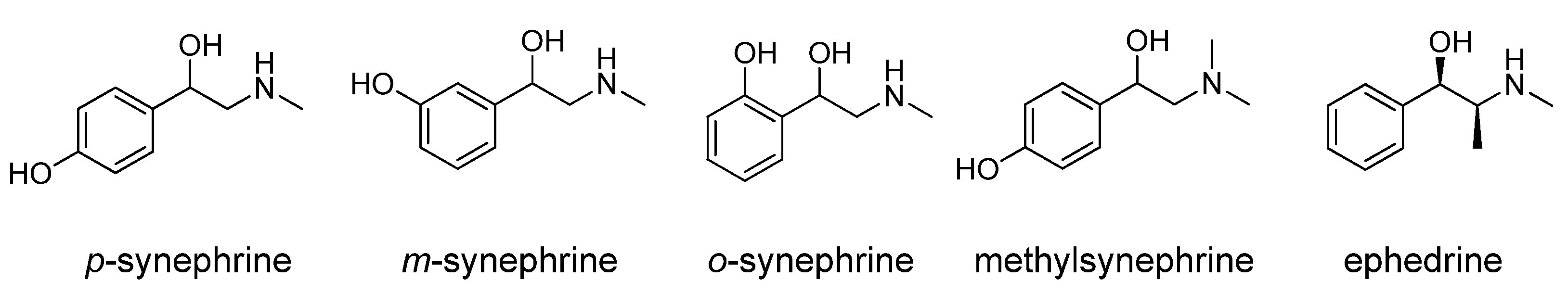
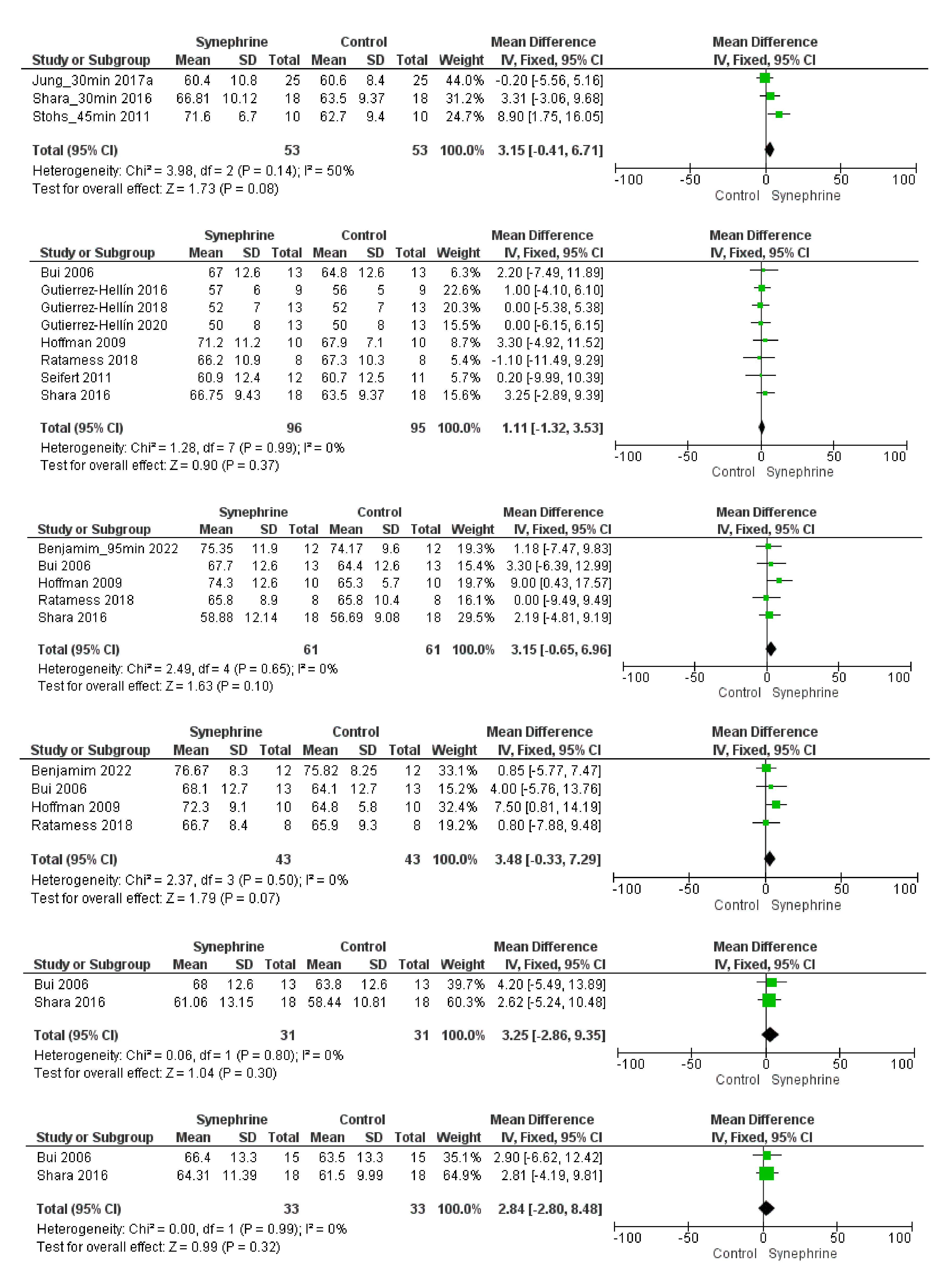
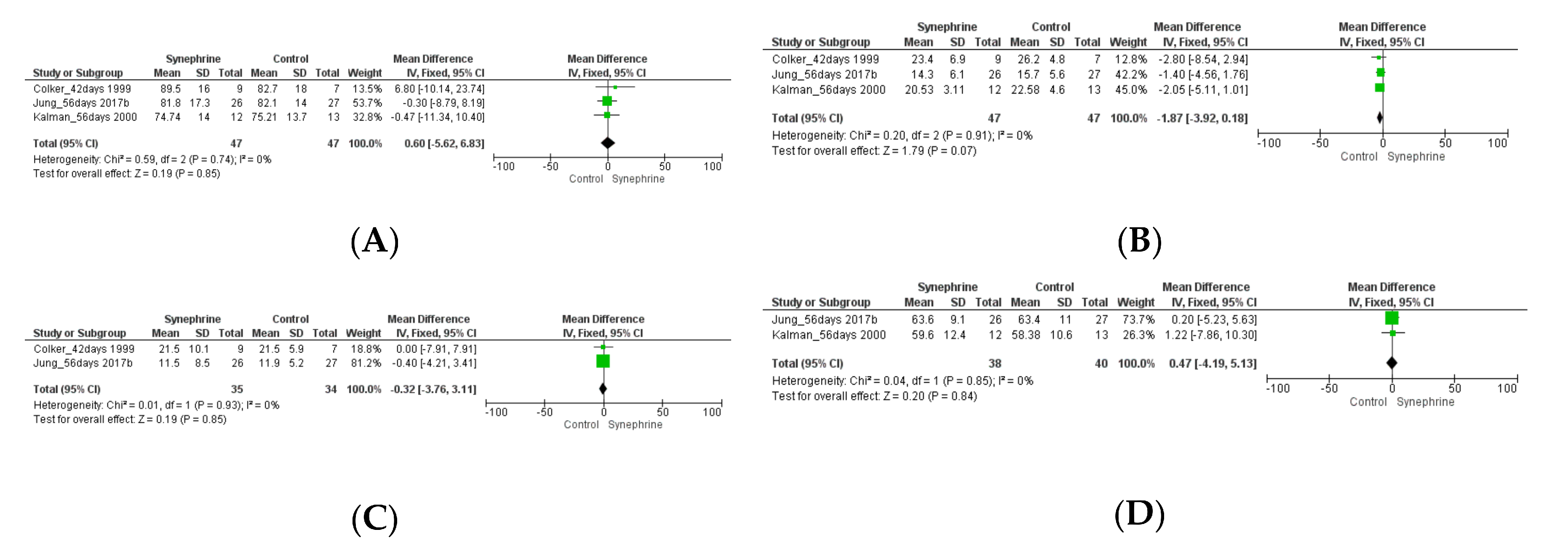
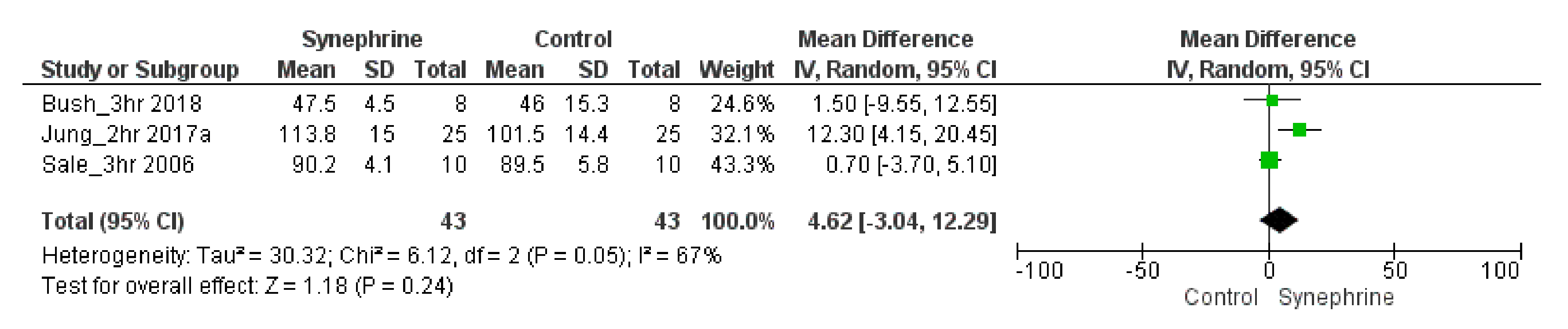
3.2.1. Cardiovascular Parameters
3.2.2. Weight Loss and Body Composition
3.2.3. Other Outcomes
3.3. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANSES | The French Agency for Food, Environmental and Occupational Health & Safety |
| BP | Blood pressure |
| CA | Citrus aurantium |
| CI | Confidence interval |
| DBP | Diastolic blood pressure |
| HCL | Hydrochloride |
| HR | Heart rate |
| E | Epinephrine |
| EC | European Commission |
| EE | Energy expenditure |
| EU | European Union |
| FDA | U.S. Food and Drug Administration |
| MD | Mean difference |
| NA | Noradrenaline |
| NE | Norepinephrine |
| NK | Not known |
| mmHg | Millimeters of mercury |
| m-synephrine | Meta-synephrine (phenylephrine) |
| p-synephrine | Para-synephrine |
| QT | Measurement made on electrocardiogram. It is calculated as the time from the start of the Q wave to the end of the T wave. |
| RASFF | Rapid Alert System for Food and Feed |
| RCT | Randomized clinical trial |
| RER | Respiratory exchange ratio |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| VO2 | Oxygen consumption |
| 4-HMP | Methyl-synephrine HCl, oxiflorine |
References
- Stoner, L.; Rowlands, D.; Morrison, A.; Credeur, D.; Hamlin, M.; Gaffney, K.; Lambrick, D.; Matheson, A. Efficacy of Exercise Intervention for Weight Loss in Overweight and Obese Adolescents: Meta-Analysis and Implications. Sports Med. 2016, 46, 1737–1751. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, Regional, and Global Trends in Body-Mass Index since 1980: Systematic Analysis of Health Examination Surveys and Epidemiological Studies with 960 Country-Years and 9·1 Million Participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a Multisystem Disease: Trends in Obesity Rates and Obesity-Related Complications. Diabetes Obes. Metab. 2021, 23, 3–16. [Google Scholar] [CrossRef]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Tronieri, J.S.; Butryn, M.L. Lifestyle Modification Approaches for the Treatment of Obesity in Adults. Am. Psychol. 2020, 75, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Koncz, D.; Tóth, B.; Kiss, T.; Roza, O.; Csupor, D. Acacia Rigidula versus Other Acacia Taxa: An Alarming Issue in the European Novel Food Regulation and Food Supplement Industry. Acta Pharm. Hung. 2021, 91, 67–74. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Shara, M. The Safety of Citrus aurantium (Bitter Orange) and Its Primary Protoalkaloid p-Synephrine. Phyther. Res. 2011, 25, 1421–1428. [Google Scholar] [CrossRef]
- Ruiz-Moreno, C.; Del Coso, J.; Giráldez-Costas, V.; González-García, J.; Gutiérrez-Hellín, J. Effects of P-Synephrine during Exercise: A Brief Narrative Review. Nutrients 2021, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Haaz, S.; Fontaine, K.R.; Cutter, G.; Limdi, N.; Perumean-Chaney, S.; Allison, D.B. Citrus aurantium and Synephrine Alkaloids in the Treatment of Overweight and Obesity: An Update. Obes. Rev. 2006, 7, 79–88. [Google Scholar] [CrossRef]
- Andrade, A.S.; Schmitt, G.C.; Rossato, L.G.; Russowsky, D.; Limberger, R.P. Gas Chromatographic Method for Analysis of P-Synephrine in Citrus aurantium L. Products. Chromatographia 2009, 69, S225–S229. [Google Scholar] [CrossRef]
- Arbo, M.D.; Larentis, E.R.; Linck, V.M.; Aboy, A.L.; Pimentel, A.L.; Henriques, A.T.; Dallegrave, E.; Garcia, S.C.; Leal, M.B.; Limberger, R.P. Concentrations of P-Synephrine in Fruits and Leaves of Citrus Species (Rutaceae) and the Acute Toxicity Testing of Citrus aurantium Extract and p-Synephrine. Food Chem. Toxicol. 2008, 46, 2770–2775. [Google Scholar] [CrossRef] [PubMed]
- Fugh-Berman, A.; Myers, A. Citrus aurantium, an Ingredient of Dietary Supplements Marketed for Weight Loss: Current Status of Clinical and Basic Research. Exp. Biol. Med. 2004, 229, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.G.; de Pinho, P.G.; Silva, R.; Carmo, H.; Carvalho, F.; Bastos, M.D.L.; Costa, V.M.; Remião, F. Development and Validation of a GC/IT-MS Method for Simultaneous Quantitation of Para and Meta-Synephrine in Biological Samples. J. Pharm. Biomed. Anal. 2010, 52, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.; Sharpless, K.E.; Nelson, B.C. Determination of Para-Synephrine and Meta-Synephrine Positional Isomers in Bitter Orange-Containing Dietary Supplements by LC/UV and LC/MS/MS. Food Chem. 2008, 109, 675–682. [Google Scholar] [CrossRef]
- Pawar, R.S.; Grundel, E. Overview of Regulation of Dietary Supplements in the USA and Issues of Adulteration with Phenethylamines (PEAs). Drug Test. Anal. 2017, 9, 500–517. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J. Safety, Efficacy, and Mechanistic Studies Regarding Citrus aurantium (Bitter Orange) Extract and p-Synephrine. Phyther. Res. 2017, 31, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Vaysse, J.; Balayssac, S.; Gilard, V.; Desoubdzanne, D.; Malet-Martino, M.; Martino, R. Analysis of Adulterated Herbal Medicines and Dietary Supplements Marketed for Weight Loss by DOSY 1H-NMR. Food Addit. Contam.—Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Avula, B.; Venhuis, B.; Travis, J.C.; Wang, Y.-H.; Khan, I.A. Pharmaceutical Doses of the Banned Stimulant Oxilofrine Found in Dietary Supplements Sold in the USA. Drug Test. Anal. 2017, 9, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.E.; Munir, J.A.; McIntyre, P.Z.; Ferguson, M.A. STEMI in a 24-Year-Old Man after Use of a Synephrine-Containing Dietary Supplement: A Case Report and Review of the Literature. Tex. Heart Inst. J. 2009, 36, 586–590. [Google Scholar]
- Palamar, J. How Ephedrine Escaped Regulation in the United States: A Historical Review of Misuse and Associated Policy. Health Policy 2011, 99, 1–9. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Safety Evaluation of Ephedra Species for Use in Food. EFSA J. 2013, 11, 3467. [Google Scholar] [CrossRef]
- Colker, C.M.; Kalman, D.S.; Torina, G.C.; Perlis, T.; Street, C. Effects of Citrus aurantium Extract, Caffeine, and St. John’s Wort on Body Fat Loss, Lipid Levels, and Mood States in Overweight Healthy Adults. Curr. Ther. Res. Exp. 1999, 60, 145–153. [Google Scholar] [CrossRef]
- Arch, J.R.S. Β3-Adrenoceptor Agonists: Potential, Pitfalls and Progress. Eur. J. Pharmacol. 2002, 440, 99–107. [Google Scholar] [CrossRef]
- Jones, D. Citrus and Ephedra. Whole Foods 2004, 40, 40–41. [Google Scholar]
- Haller, C.A.; Benowitz, N.L. Adverse Cardiovascular and Central Nervous System Events Associated with Dietary Supplements Containing Ephedra Alkaloids. N. Engl. J. Med. 2000, 343, 1833–1838. [Google Scholar] [CrossRef]
- Inchiosa, M.A. Experience (Mostly Negative) with the Use of Sympathomimetic Agents for Weight Loss. J. Obes. 2011, 2011, 764584. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.G.; Costa, V.M.; Limberger, R.P.; de Lourdes Bastos, M.; Remião, F. Synephrine: From Trace Concentrations to Massive Consumption in Weight-Loss. Food Chem. Toxicol. 2011, 49, 1472–1473. [Google Scholar] [CrossRef]
- Haller, C.A.; Duan, M.; Jacob III, P.; Benowitz, N. Human Pharmacology of a Performance-Enhancing Dietary Supplement under Resting and Exercise Conditions. Br. J. Clin. Pharmacol. 2008, 65, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.R. Pharmacology of Stimulants Prohibited by the World Anti-Doping Agency (WADA). Br. J. Pharmacol. 2008, 154, 606–622. [Google Scholar] [CrossRef] [PubMed]
- WADA. The Prohibited List. Available online: https://www.wada-ama.org/en/prohibited-list (accessed on 20 August 2022).
- Piattoly, T.J. Dietary Supplement Safety: Risk vs Reward for Athletes. Oper. Tech. Sports Med. 2022, 30, 150891. [Google Scholar] [CrossRef]
- Arbo, M.D.; Schmitt, G.C.; Limberger, M.F.; Charão, M.F.; Moro, A.M.; Ribeiro, G.L.; Dallegrave, E.; Garcia, S.C.; Leal, M.B.; Limberger, R.P. Subchronic Toxicity of Citrus aurantium L. (Rutaceae) Extract and p-Synephrine in Mice. Regul. Toxicol. Pharmacol. 2009, 54, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Upparapalli, S.K.; Navarrete, A.; Khan, I.A. Simultaneous Quantification of Adrenergic Amines and Flavonoids in C. Aurantium, Various Citrus Species, and Dietary Supplements by Liquid Chromatography. J. AOAC Int. 2005, 88, 1593–1606. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.S.; Comar, J.F.; Moreira, C.T.; Soares, A.A.; de Oliveira, A.L.; Bracht, A.; Peralta, R.M. Effects of Citrus aurantium (Bitter Orange) Fruit Extracts and p-Synephrine on Metabolic Fluxes in the Rat Liver. Molecules 2012, 17, 5854–5869. [Google Scholar] [CrossRef] [PubMed]
- ANSES. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the Risks Associated with the Presence in Food Supplements of P-synephrine or Ingredients Obtained from Citrus Spp. Fruits Containing This Substance. Available online: https://www.anses.fr/en/content/opinion-french-agency-food-environmental-and-occupational-health-safety-risks-associated-1 (accessed on 21 August 2022).
- Bakhyia, N.; Dusemund, B.; Richter, K.; Lindtner, O.; Hirsch-Ernst, K.I.; Schäfer, B.; Lampen, A. Risk assessment of synephrine in dietary supplements. Bundesgesundheitsblatt. Gesundheitsforschung. Gesundheitsschutz 2017, 60, 323–331. [Google Scholar] [CrossRef]
- European Parliament. Directive 2002/46/CE. Off. J. Eur. Communities 2002, 45, 51–57. [Google Scholar]
- Haššo, M.; Sarakhman, O.; Stanković, D.M.; Švorc, Ĺ. A New Voltammetric Platform for Reliable Determination of the Sport Performance-Enhancing Stimulant Synephrine in Dietary Supplements Using a Boron-Doped Diamond Electrode. Anal. Methods 2020, 12, 4749–4758. [Google Scholar] [CrossRef]
- RASFF. Food and Feed Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff/how_does_rasff_work/legal_basis_en (accessed on 31 December 2019).
- Penzak, S.R.; Jann, M.W.; Cold, J.A.; Hori, Y.Y.; Desai, H.D.; Gurley, B.J. Seville (Sour) Orange Juice: Synephrine Content and Cardiovascular Effects in Normotensive Adults. J. Clin. Pharmacol. 2001, 41, 1059–1063. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Bechke, E.; Williamson, C.; Bailey, P.; Hoffstetter, W.; McLester, J.; McLester, C. The Influence of Citrus aurantium and Caffeine Complex versus Placebo on the Cardiac Autonomic Response: A Double Blind Crossover Design. J. Int. Soc. Sports Nutr. 2018, 15, 34. [Google Scholar] [CrossRef]
- Rashti, S.L.; Ratamess, N.A.; Kang, J.; Faigenbaum, A.D.; Chilakos, A.; Hoffman, J.R. Thermogenic Effect of Meltdown RTD Energy Drink in Young Healthy Women: A Double Blind, Cross-over Design Study. Lipids Health Dis. 2009, 8, 57. [Google Scholar] [CrossRef]
- Bui, L.T.; Nguyen, D.T.T.; Ambrose, P.J. Blood Pressure and Heart Rate Effects Following a Single Dose of Bitter Orange. Ann. Pharmacother. 2006, 40, 53–57. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Kang, J.; Ratamess, N.A.; Rashti, S.L.; Tranchina, C.P.; Faigenbaum, A.D. Thermogenic Effect of an Acute Ingestion of a Weight Loss Supplement. J. Int. Soc. Sports Nutr. 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Galvan, E.; Dalton, R.; Walker, D.; Rasmussen, C.; Murano, P.S.; Greenwood, M.; Kreider, R.B. Effects of Acute Ingestion of a Pre-Workout Dietary Supplement with and without p-Synephrine on Resting Energy Expenditure, Cognitive Function and Exercise Performance. J. Int. Soc. Sports Nutr. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Cios, D.; Kluger, J.; White, C.M. Absence of QTc-Interval-Prolonging or Hemodynamic Effects of a Single Dose of Bitter-Orange Extract in Healthy Subjects. Pharmacotherapy 2005, 25, 1719–1724. [Google Scholar] [CrossRef]
- Seifert, J.G.; Nelson, A.; Devonish, J.; Burke, E.R.; Stohs, S.J. Effect of Acute Administration of an Herbal Preparation on Blood Pressure and Heart Rate in Humans. Int. J. Med. Sci. 2011, 8, 192–197. [Google Scholar] [CrossRef]
- Shara, M.; Stohs, S.J.; Mukattash, T.L. Cardiovascular Safety of Oral P-Synephrine (Bitter Orange) in Healthy Subjects: A Randomized Placebo-Controlled Cross-over Clinical Trial. Phyther. Res. 2016, 30, 842–847. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; de Sousa Júnior, F.W.; Porto, A.A.; Rocha, É.M.B.; Santana, M.D.; Garner, D.M.; Valenti, V.E.; Bueno Júnior, C.R. Bitter Orange (Citrus aurantium L.) Intake Before Submaximal Aerobic Exercise Is Safe for Cardiovascular and Autonomic Systems in Healthy Males: A Randomized Trial. Front. Nutr. 2022, 9, 890388. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Cho, M.; Barringer, N.; Walker, D.; Rasmussen, C.; Greenwood, M.; Murano, P.S.; Kreider, R.B. Effects of Ingesting a Pre-Workout Dietary Supplement with and without Synephrine for 8 Weeks on Training Adaptations in Resistance-Trained Males. J. Int. Soc. Sports Nutr. 2017, 14, 1. [Google Scholar] [CrossRef]
- Kaats, G.R.; Miller, H.; Preuss, H.G.; Stohs, S.J. A 60day Double-Blind, Placebo-Controlled Safety Study Involving Citrus aurantium (Bitter Orange) Extract. Food Chem. Toxicol. 2013, 55, 358–362. [Google Scholar] [CrossRef]
- Kalman, D.S.; Colker, C.M.; Shi, Q.; Swain, M.A. Effects of a Weight-Loss Aid in Healthy Overweight Adults: Double-Blind, Placebo-Controlled Clinical Trial. Curr. Ther. Res.-Clin. Exp. 2000, 61, 199–205. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Keith, S.C.; Keith, P.L.; Miller, H.; Kaats, G.R. Effects of P-Synephrine Alone and in Combination with Selected Bioflavo-Noids on Resting Metabolism, Blood Pressure, Heart Rate and Self-Reported Mood Changes. Int. J. Med. Sci. 2011, 8, 295–301. [Google Scholar] [CrossRef]
- Bush, J.A.; Ratamess, N.A.; Stohs, S.J.; Ellis, N.L.; Vought, I.T.; O’Grady, E.A.; Kuper, J.D.; Kang, J.; Faigenbaum, A.D. Acute Hematological and Mood Perception Effects of Bitter Orange Extract (p-Synephrine) Consumed Alone and in Combination with Caffeine: A Placebo-Controlled, Double-Blind Study. Phyther. Res. 2018, 32, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Ratamess, N.A.; Bush, J.A.; Stohs, S.J.; Ellis, N.L.; Vought, I.T.; O’Grady, E.A.; Kuper, J.D.; Hasan, S.B.; Kang, J.; Faigenbaum, A.D. Acute Cardiovascular Effects of Bitter Orange Extract (p-Synephrine) Consumed Alone and in Combination with Caffeine in Human Subjects: A Placebo-Controlled, Double-Blind Study. Phyther. Res. 2018, 32, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Hellin, J.; Del Coso, J. Dose-Response Effects of p-Synephrine on Fat Oxidation Rate During Exercise of Increasing Intensity. Phyther. Res. 2018, 32, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Hellin, J.; Ruiz-Moreno, C.; Del Coso, J. Acute P-Synephrine Ingestion Increases Whole-Body Fat Oxidation during 1-h of Cycling at Fatmax. Eur. J. Nutr. 2020, 59, 3341–3345. [Google Scholar] [CrossRef] [PubMed]
- Sale, C.; Harris, R.C.; Delves, S.; Corbett, J. Metabolic and Physiological Effects of Ingesting Extracts of Bitter Orange, Green Tea and Guarana at Rest and during Treadmill Walking in Overweight Males. Int. J. Obes. 2006, 30, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hellín, J.; Del Coso, J. Acute P-Synephrine Ingestion Increases Fat Oxidation Rate during Exercise. Br. J. Clin. Pharmacol. 2016, 82, 362–368. [Google Scholar] [CrossRef]
- Stohs, S.J.; Shara, M.; Ray, S.D. P-Synephrine, Ephedrine, p-Octopamine and m-Synephrine: Comparative Mechanistic, Physiological and Pharmacological Properties. Phytother. Res. 2020, 34, 1838–1846. [Google Scholar] [CrossRef]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview on Citrus aurantium L.: Its Functions as Food Ingredient and Therapeutic Agent. Oxid. Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef]
- Samenuk, D.; Link, M.S.; Homoud, M.K.; Contreras, R.; Theoharides, T.C.; Wang, P.J.; Estes, N.A.M., 3rd. Adverse Cardiovascular Events Temporally Associated with Ma Huang, an Herbal Source of Ephedrine. Mayo Clin. Proc. 2002, 77, 12–16. [Google Scholar] [CrossRef]
- Park, D.; Lee, D.; Lee, Y. P-Synephrine Stimulates Glucose Consumption via AMPK in L6 Skeletal Muscle Cells. Diabetes 2012, 61, A469–A470. [Google Scholar] [CrossRef]
- Kratz, A.; Lewandrowski, K.B.; Siegel, A.J.; Chun, K.Y.; Flood, J.G.; Van Cott, E.M.; Lee-Lewandrowski, E. Effect of Marathon Running on Hematologic and Biochemical Laboratory Parameters, Including Cardiac Markers. Am. J. Clin. Pathol. 2002, 118, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Simonson, D.C.; DeFronzo, R.A. Indirect Calorimetry: Methodological and Interpretative Problems. Am. J. Physiol. Metab. 1990, 258, E399–E412. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, D.R.; Leddy, J.J.; Venkatraman, J.T. A Perspective on Fat Intake in Athletes. J. Am. Coll. Nutr. 2000, 19, 345–350. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Mensink, M.; Saris, W.H.M.; Wagenmakers, A.J.M. Exogenous Glucose Oxidation during Exercise in Endurance-Trained and Untrained Subjects. J. Appl. Physiol. 1997, 82, 835–840. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brooks, G.A. Respiratory Gas-Exchange Ratios during Graded Exercise in Fed and Fasted Trained and Untrained Men. J. Appl. Physiol. 1999, 86, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Jiménez, A.; Hernández-Torres, R.P.; Torres-Durán, P.V.; Romero-Gonzalez, J.; Mascher, D.; Posadas-Romero, C.; Juárez-Oropeza, M.A. The Respiratory Exchange Ratio Is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clin. Med. Circ. Respir. Pulm. Med. 2008, 2, CCRPM.S449. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Shara, M. A Review of the Human Clinical Studies Involving Citrus aurantium (Bitter Orange) Extract and Its Primary Protoalkaloid p-Synephrine. Int. J. Med. Sci. 2012, 9, 527–538. [Google Scholar] [CrossRef]
- Hatton, R.C.; Winterstein, A.G.; McKelvey, R.P.; Shuster, J.; Hendeles, L. Efficacy and Safety of Oral Phenylephrine: Systematic Review and Meta-Analysis. Ann. Pharmacother. 2007, 41, 381–390. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koncz, D.; Tóth, B.; Bahar, M.A.; Roza, O.; Csupor, D. The Safety and Efficacy of Citrus aurantium (Bitter Orange) Extracts and p-Synephrine: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4019. https://doi.org/10.3390/nu14194019
Koncz D, Tóth B, Bahar MA, Roza O, Csupor D. The Safety and Efficacy of Citrus aurantium (Bitter Orange) Extracts and p-Synephrine: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(19):4019. https://doi.org/10.3390/nu14194019
Chicago/Turabian StyleKoncz, Dorottya, Barbara Tóth, Muh. Akbar Bahar, Orsolya Roza, and Dezső Csupor. 2022. "The Safety and Efficacy of Citrus aurantium (Bitter Orange) Extracts and p-Synephrine: A Systematic Review and Meta-Analysis" Nutrients 14, no. 19: 4019. https://doi.org/10.3390/nu14194019
APA StyleKoncz, D., Tóth, B., Bahar, M. A., Roza, O., & Csupor, D. (2022). The Safety and Efficacy of Citrus aurantium (Bitter Orange) Extracts and p-Synephrine: A Systematic Review and Meta-Analysis. Nutrients, 14(19), 4019. https://doi.org/10.3390/nu14194019







