Is It Possible to Improve the Bioavailability of Resveratrol and Polydatin Derived from Polygoni cuspidati Radix as a Result of Preparing Electrospun Nanofibers Based on Polyvinylpyrrolidone/Cyclodextrin?
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Obtaining and Charakteristion of Biological Activity of Polygoni Cuspidati Extract
2.3.1. Plant Extraction Using Design of Experiment (DoE)
2.3.2. Determination of Selected Active Components Content and Total Phenolic Content (TPC)
2.3.3. Determination of Biological Activity
Antioxidant Activity
Anti-Hyaluronidase Activity
2.4. Obtaining of Electrospun Nanofibers Containing Polygoni Cuspidati Extract
Electrospun Nanofibers’ Preparation Using Design of Experiment (DoE)
2.5. The Identification of Optimized Electrospun Nanofibers
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. XRPD
2.5.3. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.6. Characterisation of Electrospun Nanofibers
2.6.1. Determination of Active Components Content
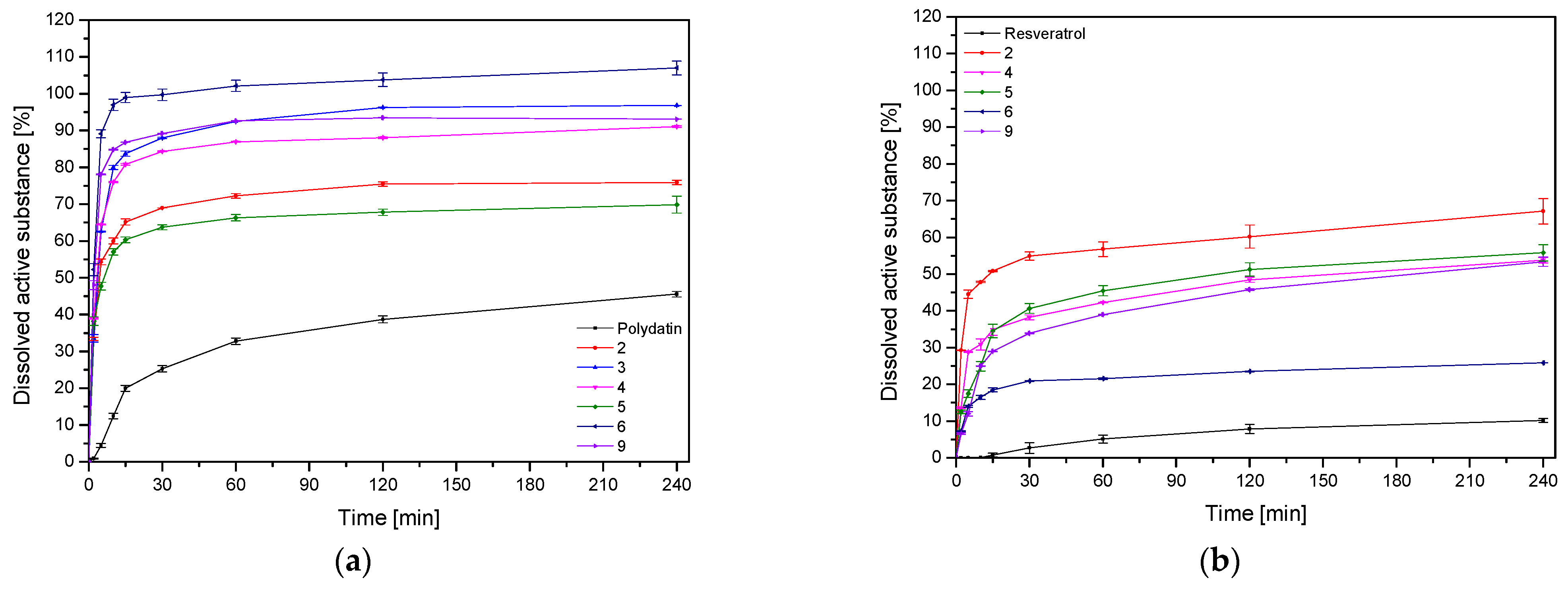
2.6.2. Dissolution Studies
2.6.3. Permeability Studies
2.6.4. In Vitro Assessment of Mucin–Biopolymer Bioadhesive Bond Strength
2.6.5. Antioxidant Activity
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garg, S.K.; Maurer, H.; Reed, K.; Selagamsetty, R. Diabetes and Cancer: Two Diseases with Obesity as a Common Risk Factor. Diabetes Obes. Metab. 2014, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Park, M.-Y.; Kwon, H.-J.; Sung, M.-K. Evaluation of Aloin and Aloe-Emodin as Anti-Inflammatory Agents in Aloe by Using Murine Macrophages. Biosci. Biotechnol. Biochem. 2009, 73, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.; Kwok, S.-T.; Zhang, Q.-W.; Chan, S.-W. A Review of the Pharmacological Effects of the Dried Root of Polygonum Cuspidatum (Hu Zhang) and Its Constituents. Evid.-Based Complement. Altern. Med. 2013, 2013, e208349. [Google Scholar] [CrossRef]
- Lin, Y.W.; Yang, F.J.; Chen, C.L.; Lee, W.T.; Chen, R.S. Free radical scavenging activity and antiproliferative potential of Polygonum cuspidatum root extracts. J. Nat. Med. 2010, 64, 146–152. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Szymańska, E.; Winnicka, K.; Szwajgier, D.; Baranowska-Wójcik, E.; Ruchała, M.A.; Simon, M.; Cielecka-Piontek, J. Cyclodextrin as Functional Carrier in Development of Mucoadhesive Tablets Containing Polygoni Cuspidati Extract with Potential for Dental Applications. Pharmaceutics 2021, 13, 1916. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability Is the Problem, What Is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef]
- Sinha, S.; Ali, M.; Baboota, S.; Ahuja, A.; Kumar, A.; Ali, J. Solid Dispersion as an Approach for Bioavailability Enhancement of Poorly Water-Soluble Drug Ritonavir. AAPS PharmSciTech 2010, 11, 518–527. [Google Scholar] [CrossRef]
- Zupančič, Š.; Baumgartner, S.; Lavrič, Z.; Petelin, M.; Kristl, J. Local Delivery of Resveratrol Using Polycaprolactone Nanofibers for Treatment of Periodontal Disease. J. Drug Deliv. Sci. Technol. 2015, 30, 408–416. [Google Scholar] [CrossRef]
- Xiang, S.; Tang, H.W.; Zhou, J.; Li1, X.Z. Electrospinning of Hydroxypropyl-Beta-Cyclodextrin/Polyvinylpyrrolidone Resveratrol-Loaded Nanofibers: Preparation and Characterization. Indian J. Pharm. Sci. 2019, 81, 618–625. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hu, S.C.-S.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Karakucuk, A.; Tort, S. Preparation, Characterization and Antimicrobial Activity Evaluation of Electrospun PCL Nanofiber Composites of Resveratrol Nanocrystals. Pharm. Dev. Technol. 2020, 25, 1216–1225. [Google Scholar] [CrossRef]
- Maria Leena, M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Edible Coating with Resveratrol Loaded Electrospun Zein Nanofibers with Enhanced Bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Wu, Z.; Liang, H.; Yan, P.; Song, J.; Ma, N.; Zhao, Q. Enhanced Bioavailability and Anticancer Effect of Curcumin-Loaded Electrospun Nanofiber: In Vitro and In Vivo Study. Nanoscale Res. Lett. 2015, 10, 439. [Google Scholar] [CrossRef]
- Torres-Martínez, E.J.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- Noriega, P.; Mafud, D. de F.; Souza, B. de; Soares-Scott, M.; Rivelli, D.P.; Barros, S.B. de M.; Bacchi, E.M. Applying Design of Experiments (DOE) to Flavonoid Extraction from Passiflora Alata and P. Edulis. Rev. Bras. De Farmacogn. 2012, 22, 1119–1129. [Google Scholar] [CrossRef]
- Ćućuz, V.; Cvejić, J.; Torović, L.; Gojković-Bukarica, L.; Acevska, J.; Dimitrovska, A.; Aldawoud, T.M.S.; Galanakis, C.M. Design of Experiments (DoE) to Model Phenolic Compounds Recovery from Grape Pomace Using Ultrasounds. J. Food Sci. Technol. 2022, 59, 2913–2924. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant Activity and Mechanism of Resveratrol and Polydatin Isolated from Mulberry (Morus alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef]
- Zykova, T.A.; Zhu, F.; Zhai, X.; Ma, W.-Y.; Ermakova, S.P.; Lee, K.W.; Bode, A.M.; Dong, Z. Resveratrol Directly Targets COX-2 to Inhibit Carcinogenesis. Mol. Carcinog. 2008, 47, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the Way towards Effective Plant-Based Inhibitors of Hyaluronidase and Tyrosinase: A Critical Review on a Structure–Activity Relationship. J. Enzyme Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef]
- Coles, S.R.; Jacobs, D.K.; Meredith, J.O.; Barker, G.; Clark, A.J.; Kirwan, K.; Stanger, J.; Tucker, N. A Design of Experiments (DoE) Approach to Material Properties Optimization of Electrospun Nanofibers. J. Appl. Polym. Sci. 2010, 117, 2251–2257. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Dvořák, J.; Rosiak, N.; Tykarska, E.; Szymańska, E.; Winnicka, K.; Ruchała, M.A.; Cielecka-Piontek, J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics 2021, 13, 417. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, D.; Zhang, Z.; Pan, J.; Cui, Z.; Yu, D.-G.; Annie Bligh, S.-W. Testing of Fast Dissolution of Ibuprofen from Its Electrospun Hydrophilic Polymer Nanocomposites. Polym. Test. 2021, 93, 106872. [Google Scholar] [CrossRef]
- Torres-Martínez, E.J.; Vera-Graziano, R.; Cervantes-Uc, J.M.; Bogdanchikova, N.; Olivas-Sarabia, A.; Valdez-Castro, R.; Serrano-Medina, A.; Iglesias, A.L.; Pérez-González, G.L.; Cornejo-Bravo, J.M.; et al. Preparation and Characterization of Electrospun Fibrous Scaffolds of Either PVA or PVP for Fast Release of Sildenafil Citrate. e-Polymers 2020, 20, 746–758. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; White, K.; Li, X.-L.; Zhu, L.-M. Dissolution Improvement of Electrospun Nanofiber-Based Solid Dispersions for Acetaminophen. AAPS PharmSciTech 2010, 11, 809–817. [Google Scholar] [CrossRef]
- Sizílio, R.H.; Galvão, J.G.; Trindade, G.G.G.; Pina, L.T.S.; Andrade, L.N.; Gonsalves, J.K.M.C.; Lira, A.A.M.; Chaud, M.V.; Alves, T.F.R.; Arguelho, M.L.P.M.; et al. Chitosan/Pvp-Based Mucoadhesive Membranes as a Promising Delivery System of Betamethasone-17-Valerate for Aphthous Stomatitis. Carbohydr. Polym. 2018, 190, 339–345. [Google Scholar] [CrossRef]
- Vecchi, C.F.; Cesar, G.B.; de Souza, P.R.; Caetano, W.; Bruschi, M.L. Mucoadhesive Polymeric Films Comprising Polyvinyl Alcohol, Polyvinylpyrrolidone, and Poloxamer 407 for Pharmaceutical Applications. Pharm. Dev. Technol. 2021, 26, 138–149. [Google Scholar] [CrossRef]
- Gavini, E.; Rassu, G.; Haukvik, T.; Lanni, C.; Racchi, M.; Giunchedi, P. Mucoadhesive Microspheres for Nasal Administration of Cyclodextrins. J. Drug Target 2009, 17, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal Absorption and First-Pass Metabolism of Polyphenol Compounds in Rat and Their Transport Dynamics in Caco-2 Cells. PLoS ONE 2012, 7, e29647. [Google Scholar] [CrossRef]
- Fenyvesi, F.; Nguyen, T.L.P.; Haimhoffer, Á.; Rusznyák, Á.; Vasvári, G.; Bácskay, I.; Vecsernyés, M.; Ignat, S.-R.; Dinescu, S.; Costache, M.; et al. Cyclodextrin Complexation Improves the Solubility and Caco-2 Permeability of Chrysin. Materials 2020, 13, 3618. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M. The Solubility–Permeability Interplay and Its Implications in Formulation Design and Development for Poorly Soluble Drugs. AAPS J. 2012, 14, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Blaskovich, M.A.T.; Falconer, J.R. Understanding the Relationship between Solubility and Permeability of γ-Cyclodextrin-Based Systems Embedded with Poorly Aqueous Soluble Benznidazole. Int. J. Pharm. 2022, 616, 121487. [Google Scholar] [CrossRef] [PubMed]
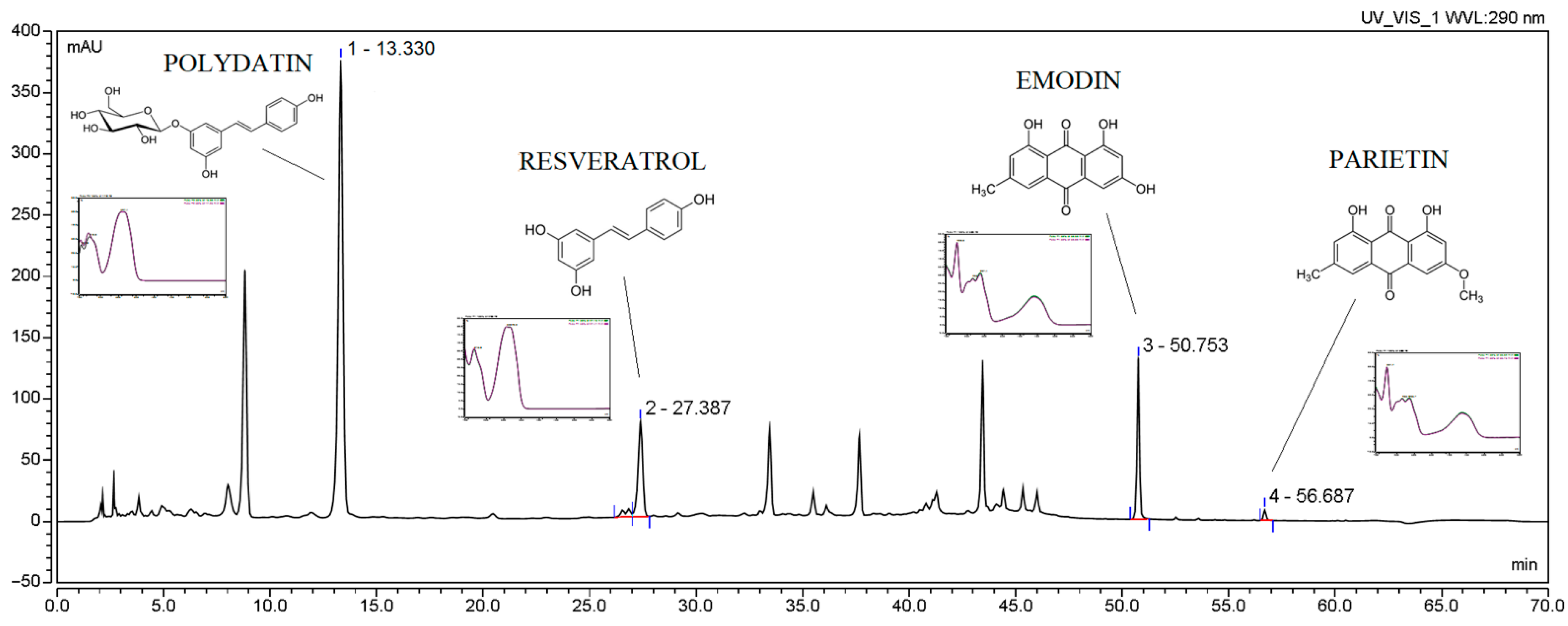
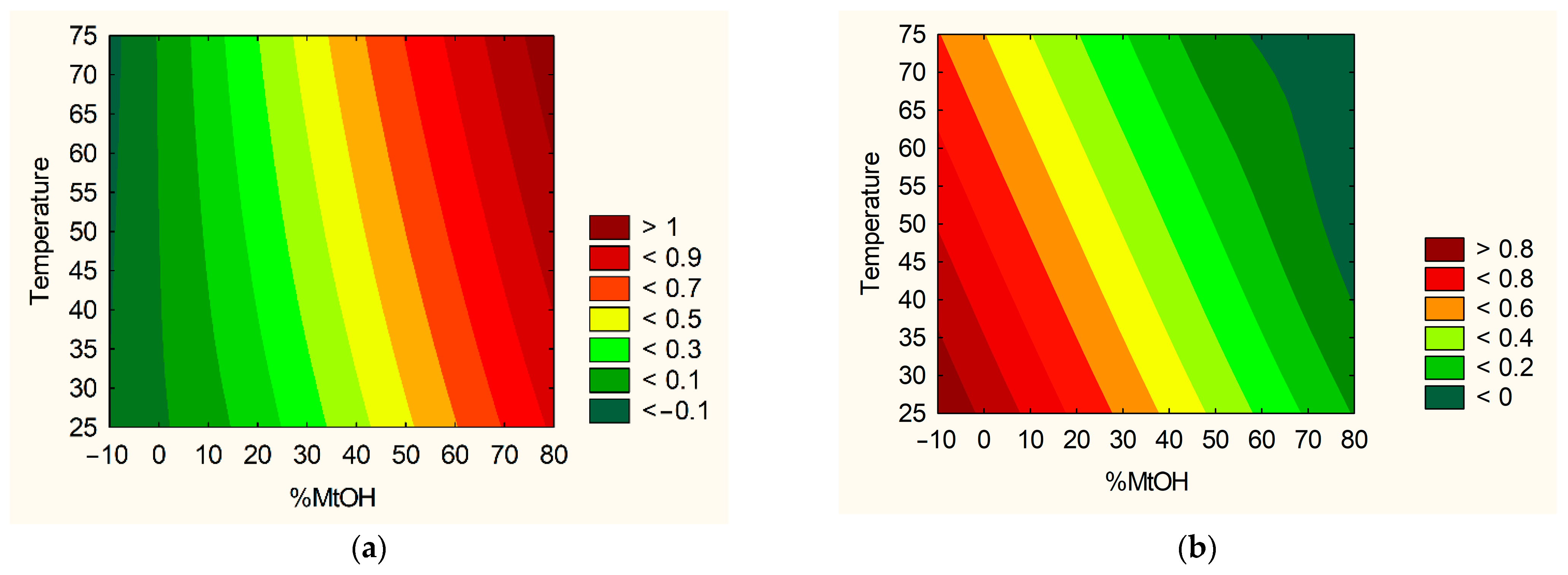

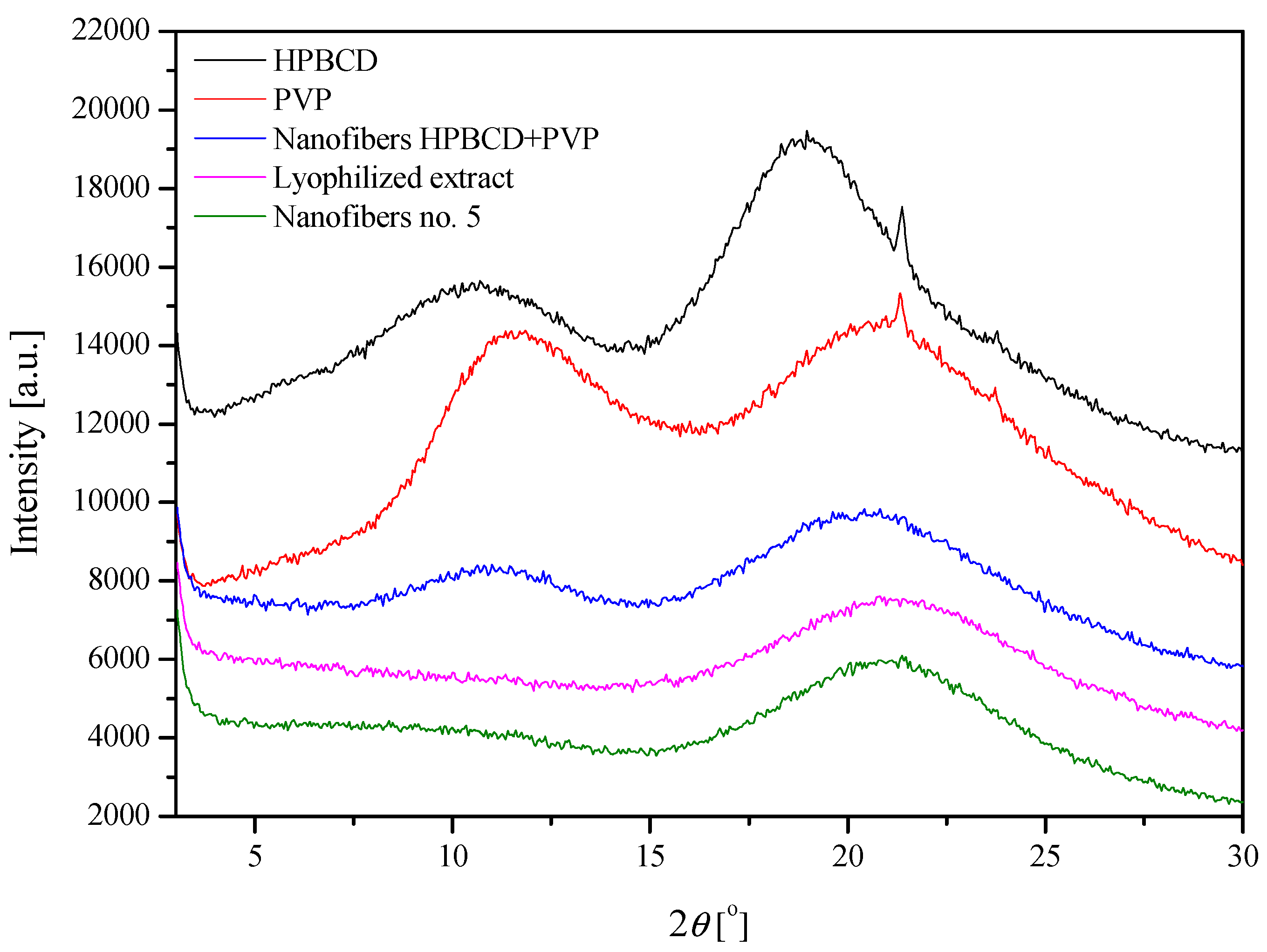
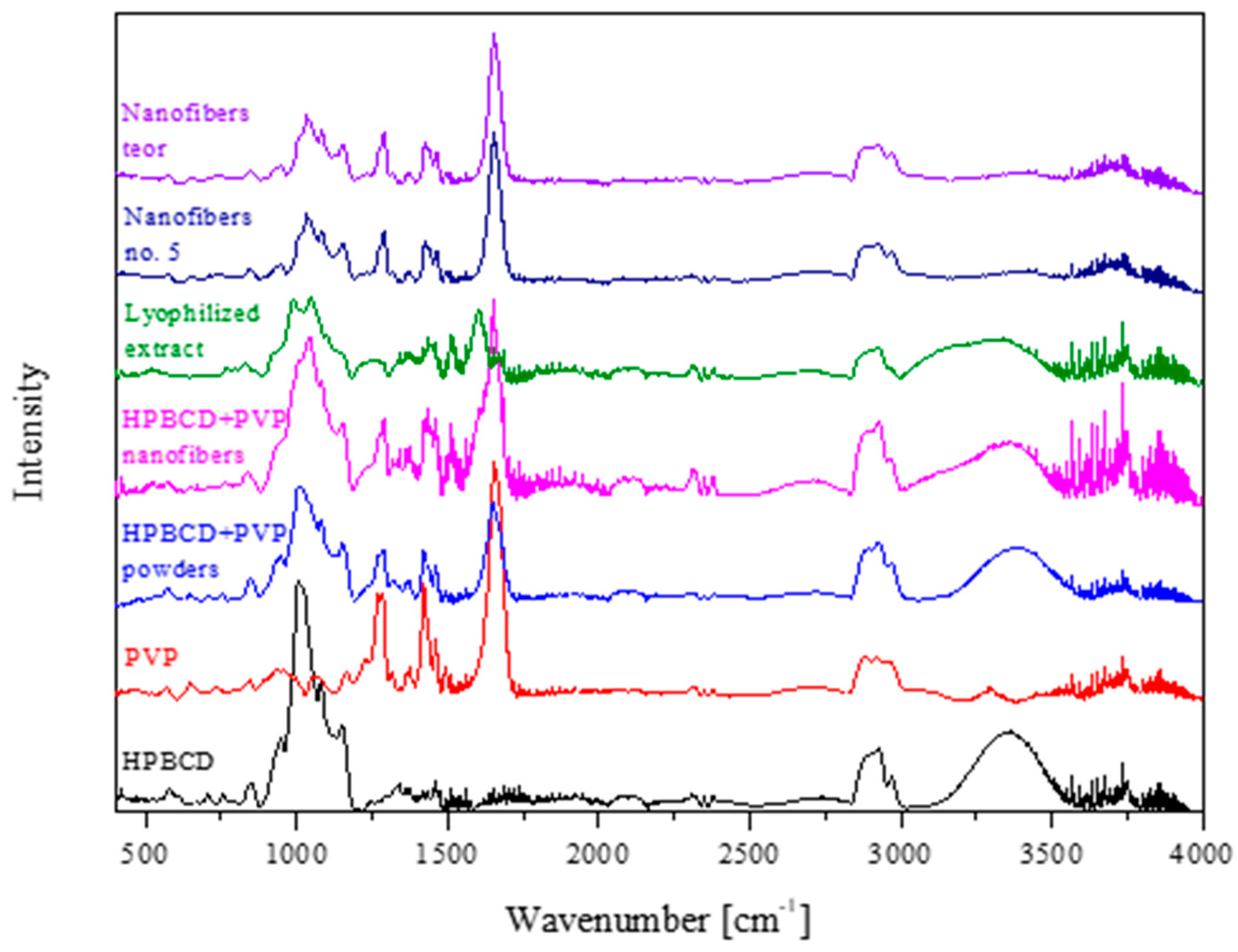
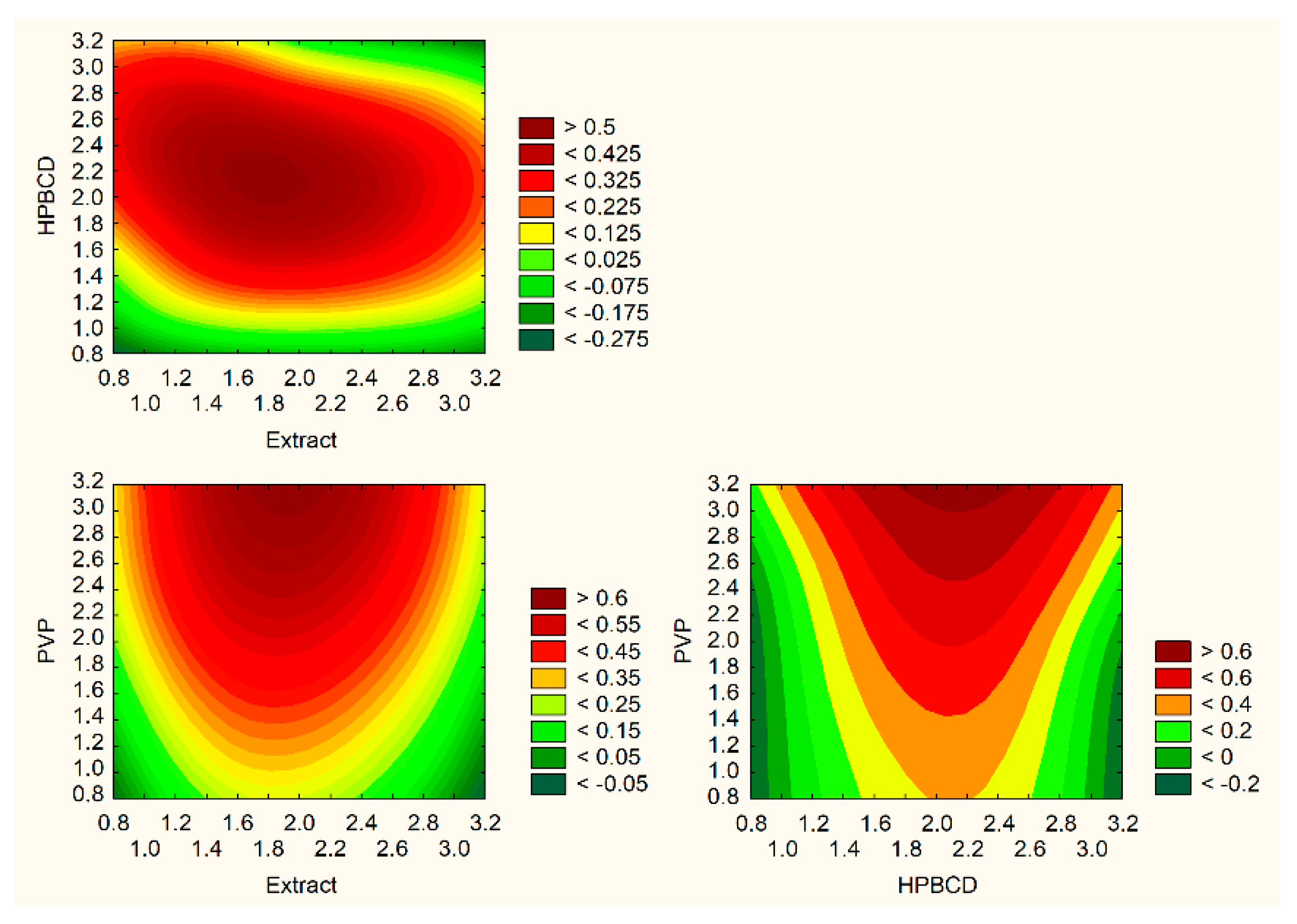

| No. | % of Methanol in the Extraction Mixture | Temperature | Number of Cycles |
|---|---|---|---|
| W1 | 0 | 30 | 3 |
| W2 | 0 | 50 | 5 |
| W3 | 0 | 70 | 4 |
| W4 | 35 | 30 | 5 |
| W5 | 35 | 50 | 4 |
| W6 | 35 | 70 | 3 |
| W7 | 70 | 30 | 4 |
| W8 | 70 | 50 | 3 |
| W9 | 70 | 70 | 5 |
| No. | Content | ||
|---|---|---|---|
| W10 Extract (g) | HPβCD (g) | PVP (g) | |
| F1 | 1 | 1 | 1 |
| F2 | 1 | 2 | 3 |
| F3 | 1 | 3 | 2 |
| F4 | 2 | 1 | 3 |
| F5 | 2 | 2 | 2 |
| F6 | 2 | 3 | 1 |
| F7 | 3 | 1 | 2 |
| F8 | 3 | 2 | 1 |
| F9 | 3 | 3 | 3 |
| No. | Content (µg/1 g Plant Material) Mean ± SD | Sum of Active Compounds (µg/1 g Plant Material) Mean | TPC (mg GAE/1 g Plant Material) Mean ± SD | |||
|---|---|---|---|---|---|---|
| Polydatin | Resveratrol | Emodin | Parietin | |||
| W1 | 174.29 ± 0.68 | 185.85 ± 10.75 | 7.85 ± 0.41 | 0.08 ± 0.02 | 452.61 | 7.85 ± 0.41 |
| W2 | 288.76 ± 22.64 | 206.69 ± 3.99 | 11.97 ± 0.62 | 0.23 ± 0.02 | 729.16 | 11.97 ± 0.62 |
| W3 | 1384.68 ± 6.51 | 307.38 ± 1.57 | 12.95 ± 1.95 | 0.18 ± 0.01 | 1892.28 | 12.95 ± 1.95 |
| W4 | 1060.43 ± 8.74 | 324.95 ± 15.49 | 20.35 ± 0.72 | 0.19 ± 0.08 | 1631.68 | 20.35 ± 0.72 |
| W5 | 1388.60 ± 66.33 | 353.33 ± 12.53 | 21.96 ± 0.88 | 0.20 ± 0.01 | 2001.47 | 21.96 ± 0.88 |
| W6 | 2891.96 ± 26.31 | 369.26 ± 3.88 | 23.11 ± 1.06 | 0.15 ± 0.02 | 3738.75 | 23.11 ± 1.06 |
| W7 | 2730.72 ± 30.61 | 414.12 ± 5.30 | 23.09 ± 2.22 | 0.29 ± 0.02 | 3931.77 | 23.09 ± 2.22 |
| W8 | 4179.04 ± 29.44 | 449.50 ± 1.24 | 30.75 ± 2.14 | 0.13 ± 0.08 | 4925.62 | 30.75 ± 2.14 |
| W9 | 4199.43 ± 68.10 | 652.67 ± 17.44 | 35.01 ± 1.77 | 0.14 ± 0.02 | 5241.30 | 35.01 ± 1.77 |
| No. | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | CUPRAC IC50 (mg/mL) | FRAP IC50 (mg/mL) | Hyaluronidase Inhibition IC50 (mg/mL) |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| W1 | 0.80 ± 0.02 | 0.66 ± 0.08 | 2.16 ± 0.02 | 0.49 ± 0.03 | 73.52 ± 2.37 |
| W2 | 0.65 ± 0.01 | 0.49 ± 0.04 | 1.63 ± 0.03 | 0.30 ± 0.01 | 44.02 ± 1.48 |
| W3 | 0.50 ± 0.01 | 0.42 ± 0.04 | 1.33 ± 0.30 | 0.29 ± 0.01 | 33.21 ± 2.40 |
| W4 | 0.45 ± 0.01 | 0.44 ± 0.04 | 0.45 ± 0.02 | 0.23 ± 0.01 | 25.45 ± 0.87 |
| W5 | 0.31 ± 0.02 | 0.38 ± 0.03 | 0.30 ± 0.02 | 0.18 ± 0.01 | 19.98 ± 1.67 |
| W6 | 0.25 ± 0.01 | 0.31 ± 0.02 | 0.27 ± 0.03 | 0.13 ± 0.01 | 11.34 ± 2.45 |
| W7 | 0.22 ± 0.03 | 0.21 ± 0.04 | 0.19 ± 0.01 | 0.14 ± 0.01 | 10.43 ± 1.33 |
| W8 | 0.16 ± 0.02 | 0.18 ± 0.02 | 0.13 ± 0.01 | 0.11 ± 0.01 | 4.69 ± 0.33 |
| W9 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.11 ± 0.01 | 0.09 ± 0.01 | 4.35 ± 0.28 |
| IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | ||
| Resveratrol | 22.32 ± 0.20 | 10.84 ± 0.23 | 20.82 ± 1.29 | 9.17 ± 0.68 | |
| Polydatin | 35.06 ± 1.11 | 25.21 ± 2.32 | 68.73 ± 0.48 | 12.90 ± 0.29 | |
| Sample | PVP | HPβCD | Lyophilized Extract | Nanofiber HPBCD-PVP | Nanofiber no. 5 |
|---|---|---|---|---|---|
| (1) Peak position [2θ] | 11.45 | 10.26 | - | 11.11 | 9.48 |
| (2) Peak position [2θ] | 21.28 | 18.72 | 21.37 | 20.61 | 21.16 |
| Matrix peak position displacement [2θ] | - | - | - | (1) −0.34 (2) −0.67 | (1) −1.97 (2) −0.12 |
| Matrix peak position displacement [Å] | - | (1) 0.24 (2) 0.13 | (1) 1.76 (2) 0.02 |
| Nanofibers 2 | Nanofibers 3 | Nanofibers 4 | Nanofibers 5 | Nanofibers 6 | Nanofibers 9 | |
|---|---|---|---|---|---|---|
| Content (µg/100 mg Nanofibers) | ||||||
| Solvent: methanol | ||||||
| Polydatin | 5.16 ± 0.11 | 8.35 ± 0.11 | 13.66 ± 0.87 | 33.32 ± 1.60 | 15.04 ± 3.48 | 9.45 ± 0.33 |
| Resveratrol | 3.70 ± 0.01 | 1.28 ± 0.01 | 5.93 ± 0.59 | 8.60 ± 0.39 | 1.68 ± 0.33 | 1.05 ± 0.04 |
| Solvent: artificial saliva solution at pH 6.8 | ||||||
| Polydatin | 8.20 ± 0.01 | 6.96 ± 0.06 | 15.46 ± 0.01 | 27.46 ± 1.08 | 18.62 ± 0.27 | 19.24 ± 0.08 |
| Resveratrol | 0.81 ± 0.03 | 0.60 ± 0.03 | 1.02 ± 0.01 | 11.00 ± 0.52 | 1.79 ± 0.02 | 2.26 ± 0.05 |
| Nanofibers 2 | Nanofibers 3 | Nanofibers 4 | Nanofibers 5 | Nanofibers 6 | Nanofibers 9 | |
|---|---|---|---|---|---|---|
| Total Amount of Released Drug from 100 mg of Nanofibers (µg) at 15 min | ||||||
| Polydatin | 5.75 ± 0.07 | 5.83 ± 0.04 | 12.50 ± 0.03 | 16.56 ± 0.22 | 18.80 ± 0.29 | 17.47 ± 0.02 |
| Resveratrol | 0.31 ± 0.01 | 0 | 0.31 ± 0.02 | 3.80 ± 0.20 | 0.26 ± 0.01 | 0.56 ± 0.01 |
| Standards | W10 | Nanofibers 2 | Nanofibers 3 | Nanofibers 4 | Nanofibers 5 | Nanofibers 6 | Nanofibers 9 | |
|---|---|---|---|---|---|---|---|---|
| Apparent Permeability Coefficient Papp × 10−6 (cm/s) | ||||||||
| Polydatin | 0.0036 ± 0.0001 | 0.0096 ± 0.0007 | 3.6213 ± 0.4921 | 0.7235 ± 0.0460 | 0.1740 ± 0.0043 | 0.2060 ± 0.0155 | 0.0455 ± 0.0039 | 0.0088 ± 0.0003 |
| Resveratrol | 1.0924 ± 0.0778 | 0.0281 ± 0.0017 | 47.5107 ± 6.1468 | 11.7056 ± 0.6146 | 11.3306 ± 0.2093 | 13.4603 ± 0.9008 | 0.1387 ± 0.0070 | 0.1102 ± 0.0042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczkowska-Walendowska, M.; Miklaszewski, A.; Cielecka-Piontek, J. Is It Possible to Improve the Bioavailability of Resveratrol and Polydatin Derived from Polygoni cuspidati Radix as a Result of Preparing Electrospun Nanofibers Based on Polyvinylpyrrolidone/Cyclodextrin? Nutrients 2022, 14, 3897. https://doi.org/10.3390/nu14193897
Paczkowska-Walendowska M, Miklaszewski A, Cielecka-Piontek J. Is It Possible to Improve the Bioavailability of Resveratrol and Polydatin Derived from Polygoni cuspidati Radix as a Result of Preparing Electrospun Nanofibers Based on Polyvinylpyrrolidone/Cyclodextrin? Nutrients. 2022; 14(19):3897. https://doi.org/10.3390/nu14193897
Chicago/Turabian StylePaczkowska-Walendowska, Magdalena, Andrzej Miklaszewski, and Judyta Cielecka-Piontek. 2022. "Is It Possible to Improve the Bioavailability of Resveratrol and Polydatin Derived from Polygoni cuspidati Radix as a Result of Preparing Electrospun Nanofibers Based on Polyvinylpyrrolidone/Cyclodextrin?" Nutrients 14, no. 19: 3897. https://doi.org/10.3390/nu14193897
APA StylePaczkowska-Walendowska, M., Miklaszewski, A., & Cielecka-Piontek, J. (2022). Is It Possible to Improve the Bioavailability of Resveratrol and Polydatin Derived from Polygoni cuspidati Radix as a Result of Preparing Electrospun Nanofibers Based on Polyvinylpyrrolidone/Cyclodextrin? Nutrients, 14(19), 3897. https://doi.org/10.3390/nu14193897








