The Roles of Probiotics in the Gut Microbiota Composition and Metabolic Outcomes in Asymptomatic Post-Gestational Diabetes Women: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Recruitment
- (1)
- Impaired fasting glucose (IGT), FBG level ≥ 6.1 mmol/L and 2-h post-prandial (2HPP) level < 7.8 mmol/L.
- (2)
- Impaired glucose tolerance (IGT), FBG level < 6.1 mmol/L and 2HPP level ≥ 7.8 mmol/L.
- (3)
- Combination of IFG and IGT, FBG level between 6.1–6.9 mmol/L and 2HPP level between 7.8–11.0 mmol/L.
2.3. Intervention and Compliance
2.4. Dietary Intake and Physical Activity Assessments
2.5. Outcomes Measurements
2.5.1. Anthropometric and Blood Pressure Measurements
2.5.2. Biochemical Analysis
2.5.3. Gut Microbial Analysis
2.5.4. Functional Prediction Based on 16S rRNA Gene Data
2.6. Sample Size
2.7. Randomization and Blinding
2.8. Statistical Analysis
3. Results
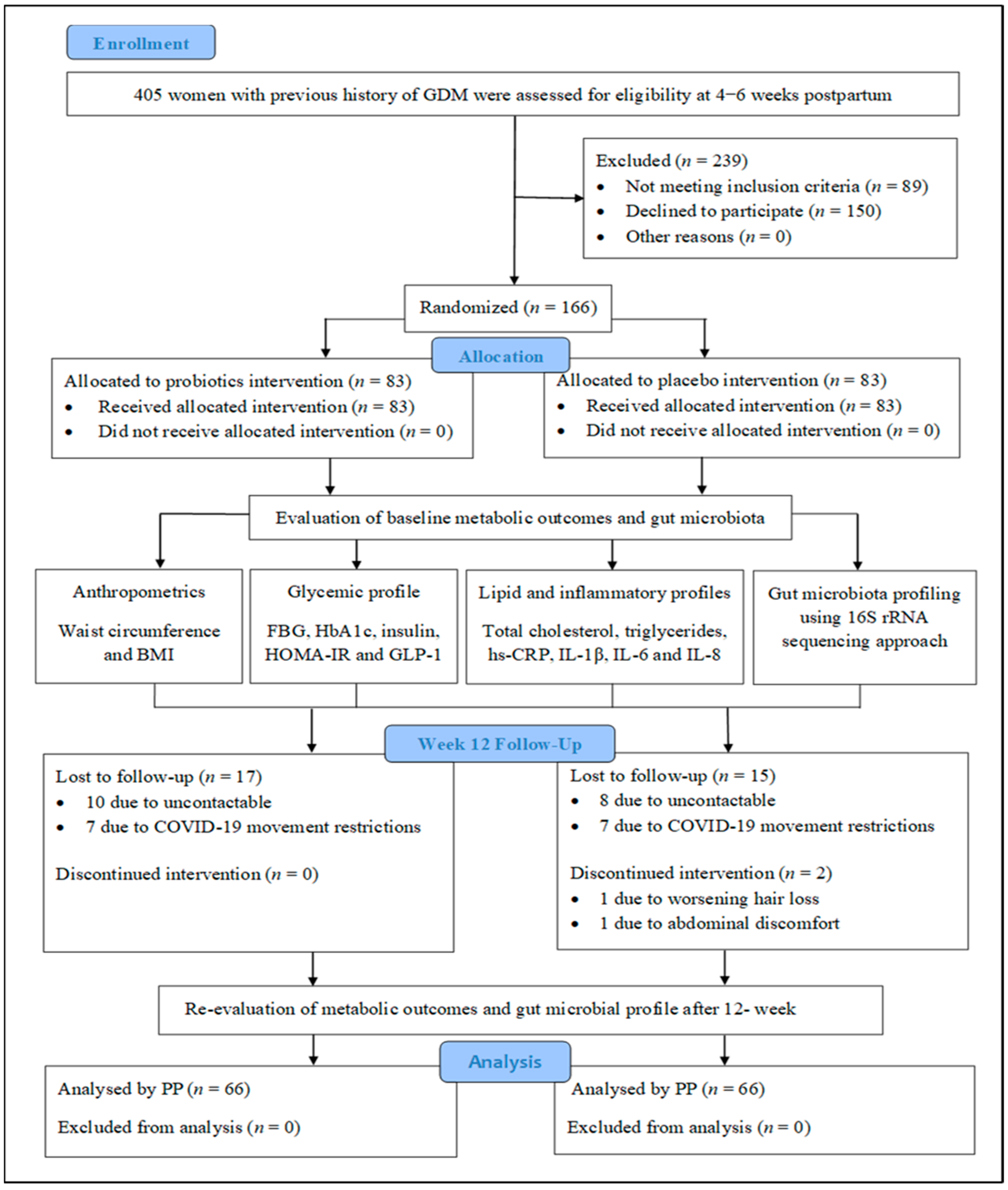
3.1. Participant Recruitment and Flow
3.2. Baseline Characteristics of Participants
3.3. Changes in Dietary Habits and Physical Activity Levels after the 12-Week Intervention
3.4. Outcomes and Estimation
3.4.1. Changes in Primary Outcomes
3.4.2. Changes in Anthropometric Measurements Outcomes
3.4.3. Changes in Lipid Outcomes
3.4.4. Changes in Relative Abundance of Gut Microbial Compositions
3.4.5. Changes in Gut Microbial α Diversity
3.4.6. Changes in Gut Microbial β Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; de Block, C.; et al. Prediction of Glucose Intolerance in Early Postpartum in Women with Gestational Diabetes Mellitus Based on the 2013 WHO Criteria. J. Clin. Med. 2019, 8, 383. [Google Scholar] [CrossRef]
- Rayanagoudar, G.; Hashi, A.A.; Zamora, J.; Khan, K.S.; Hitman, G.A.; Thangaratinam, S. Quantification of the type 2 diabetes risk in women with gestational diabetes: A systematic review and meta-analysis of 95,750 women. Diabetologia 2016, 59, 1403–1411. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas 2019. International Diabetes Federation. 2019. Available online: https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf (accessed on 3 July 2021).
- American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S200–S210. [Google Scholar] [CrossRef]
- Aroda, V.R.; Christophi, C.A.; Edelstein, S.L.; Zhang, P.; Herman, W.H.; Barrett-Connor, E.; Delahanty, L.M.; Montez, M.G.; Ackermann, R.T.; Zhuo, X.; et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: The diabetes prevention program outcomes study 10-year follow-up. J. Clin. Endocrinol. Metab. 2015, 100, 1646–1653. [Google Scholar] [CrossRef]
- Guo, J.; Chen, J.L.; Whittemore, R.; Whitaker, E. Postpartum Lifestyle Interventions to Prevent Type 2 Diabetes among Women with History of Gestational Diabetes: A Systematic Review of Randomized Clinical Trials. J. Women’s Health 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Buelo, A.K.; Kirk, A.; Lindsay, R.S.; Jepson, R.G. Exploring the effectiveness of physical activity interventions in women with previous gestational diabetes: A systematic review of quantitative and qualitative studies. Prev. Med. Rep. 2019, 14, 100877. [Google Scholar] [CrossRef]
- Razee, H.; van der Ploeg, H.P.; Blignault, I.; Smith, B.J.; Bauman, A.E.; McLean, M.; Wah Cheung, N. Beliefs, barriers, social support, and environmental influences related to diabetes risk behaviours among women with a history of gestational diabetes. Health Promot. J. Aust. 2010, 21, 130–137. [Google Scholar] [CrossRef]
- Davidson, M.B. Metformin Should Not Be Used to Treat Prediabetes. Diabetes Care 2020, 43, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Ozanne, S.E.; Aiken, C.E. Impact of metformin treatment during pregnancy on maternal outcomes: A systematic review/meta-analysis. Sci. Rep. 2021, 11, 9240. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.A.; Lamos, E.M.; Davis, S.N. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin. Drug Saf. 2013, 12, 153–175. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Kuang, Y.-S.; Lu, J.-H.; Li, S.-H.; Li, J.-H.; Yuan, M.-Y.; He, J.-R.; Chen, N.-N.; Xiao, W.-Q.; Shen, S.-Y.; Qiu, L.; et al. Connections between the human gut microbiome and gestational diabetes mellitus. GigaScience 2017, 6, gix058. [Google Scholar] [CrossRef]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef]
- Fugmann, M.; Breier, M.; Rottenkolber, M.; Banning, F.; Ferrari, U.; Sacco, V.; Grallert, H.; Parhofer, K.G.; Seissler, J.; Clavel, T.; et al. The stool microbiota of insulin resistant women with recent gestational diabetes, a high risk group for type 2 diabetes. Sci. Rep. 2015, 5, 13212. [Google Scholar] [CrossRef]
- Hasain, Z.; Raja Ali, R.A.; Abdul Razak, S.; Azizan, K.A.; El-Omar, E.; Razalli, N.H.; Mokhtar, N.M. Gut Microbiota Signature Among Asian Post-gestational Diabetes Women Linked to Macronutrient Intakes and Metabolic Phenotypes. Front. Microbiol. 2021, 12, 680622. [Google Scholar] [CrossRef]
- Hasan, S.; Aho, V.; Pereira, P.; Paulin, L.; Koivusalo, S.B.; Auvinen, P.; Eriksson, J.G. Gut microbiome in gestational diabetes: A cross-sectional study of mothers and offspring 5 years postpartum. Acta Obstet. Gynecol. Scand. 2018, 97, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Bueno, A.A.; De Souza, R.G.M.H.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Probiotics and Prebiotics|World Gastroenterology Organisation. Available online: https://www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics (accessed on 1 July 2021).
- Sato, J.; Kanazawa, A.; Azuma, K.; Ikeda, F.; Goto, H.; Komiya, K.; Kanno, R.; Tamura, Y.; Asahara, T.; Takahashi, T.; et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci. Rep. 2017, 7, 12115. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef]
- Nor, M.H.M.; Ayob, N.; Mokhtar, N.M.; Ali, R.A.R.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The Effect of Probiotics (MCP® BCMC® Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Brennan, L.; McAuliffe, F.M. Acceptability of and compliance with a probiotic capsule intervention in pregnancy. Int. J. Gynecol. Obstet. 2014, 125, 279–280. [Google Scholar] [CrossRef]
- Hasain, Z.; Che Roos, N.A.; Rahmat, F.; Mustapa, M.; Raja Ali, R.A.; Mokhtar, N.M. Diet and Pre-Intervention Washout Modifies the Effects of Probiotics on Gestational Diabetes Mellitus: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 3045. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Wu, S.; Guo, C.; Long, S.; Tan, H. Effects of Probiotic Supplement in Pregnant Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2019, 2019, 5364730. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yue, R.; Zhang, B.; Li, Z.; Shui, J.; Huang, X. Effects of probiotics on blood glucose, biomarkers of inflammation and oxidative stress in pregnant women with gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Med. Clin. 2020, 154, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, T.; Molnár, B.; Németh, D.; Hegyi, P.; Szakács, Z.; Bálint, A.; Garami, A.; Soós, A.; Márta, K.; Solymár, M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 11787. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Jafari, P.; Ebrahimi, M.T.; Amini, M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: A double-blind randomized clinical trial. Acta Diabetol. 2018, 55, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Amini, M. Probiotic and synbiotic supplementation could improve metabolic syndrome in prediabetic adults: A randomized controlled trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2991–2996. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Ministry of Health Malaysia. CPG Management of Type 2 Diabetes Mellitus (5th Edition). 2015. Available online: https://www.moh.gov.my/moh/resources/Penerbitan/CPG/Endocrine/3a.pdf (accessed on 3 July 2021).
- Mohd Yusoff, Z.; Amat, A.; Naim, D.; Othman, S. Postnatal Care Practices among the Malays, Chinese and Indians: A Comparison. SHS Web Conf. 2018, 45, 05002. [Google Scholar] [CrossRef]
- Krueger, K.P.; Felkey, B.G.; Berger, B.A. Improving adherence and persistence: A review and assessment of interventions and description of steps toward a national adherence initiative. J. Am. Pharm. Assoc. 2003, 43, 668–679. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Kamath, D.Y.; Xavier, D.; Sigamani, A.; Pais, P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: An Indian perspective. Indian J. Med. Res. 2015, 142, 261–268. [Google Scholar] [PubMed]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef]
- Iglesias Molli, A.E.; Bergonzi, M.F.; Spalvieri, M.P.; Linari, M.A.; Frechtel, G.D.; Cerrone, G.E. Relationship between the IL-1β serum concentration, mRNA levels and rs16944 genotype in the hyperglycemic normalization of T2D patients. Sci. Rep. 2020, 10, 9985. [Google Scholar] [CrossRef]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef]
- Lee, C.H.; Shih, A.Z.L.; Woo, Y.C.; Fong, C.H.Y.; Leung, O.Y.; Janus, E.; Cheung, B.M.Y.; Lam, K.S.L. Optimal cut-offs of homeostasis model assessment of insulin resistance (HOMA-IR) to identify dysglycemia and type 2 diabetes mellitus: A15-year prospective study in Chinese. PLoS ONE 2016, 11, e0163424. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B.; Baradar Jalili, R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. DARU J. Pharm. Sci. 2019, 27, 827–837. [Google Scholar] [CrossRef]
- Laitinen, K.; Poussa, T.; Isolauri, E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: A randomised controlled trial. Br. J. Nutr. 2009, 101, 1679–1687. [Google Scholar] [CrossRef] [Green Version]
- Kim, C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet. Med. 2014, 31, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern.-Fetal Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 496.e1–496.e11. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Olbrich dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, C.; Zhang, C.; Zhao, J.; Yu, L.; Zhang, H.; Narbad, A.; Chen, W.; Zhai, Q. Effects of Probiotic Supplementation on Cardiovascular Risk Factors in Hypercholesterolemia: A Systematic Review and Meta-Analysis of Randomized Clinical Trial. J. Funct. Foods 2020, 74, 104177. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef] [Green Version]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591. [Google Scholar] [PubMed]
- Morisset, A.S.; Dubé, M.C.; Côté, J.A.; Robitaille, J.; Weisnagel, S.J.; Tchernof, A. Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2011, 90, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A.; di Benedetto, M.G.; Giacobbe, J.; Pariante, C.M. Pro- and anti-inflammatory properties of interleukin (IL6) in vitro: Relevance for major depression and for human hippocampal neurogenesis. Int. J. Neuropsychopharmacol. 2020, 23, 738–750. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Oral administration of Lactobacillus rhamnosus CCFM0528 improves glucose tolerance and cytokine secretion in high-fat-fed, streptozotocin-induced type 2 diabetic mice. J. Funct. Foods 2014, 10, 318–326. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Zheng, Q.X.; Jiang, X.M.; Wang, H.W.; Ge, L.; Lai, Y.T.; Jiang, X.Y.; Chen, F.; Huang, P.P. Probiotic supplements alleviate gestational diabetes mellitus by restoring the diversity of gut microbiota: A study based on 16S rRNA sequencing. J. Microbiol. 2021, 59, 827–839. [Google Scholar] [CrossRef]
- Halkjær, S.I.; de Knegt, V.E.; Lo, B.; Nilas, L.; Cortes, D.; Pedersen, A.E.; Mirsepasi-Lauridsen, H.C.; Andersen, L.O.B.; Nielsen, H.V.; Stensvold, C.R.; et al. Multistrain Probiotic Increases the Gut Microbiota Diversity in Obese Pregnant Women: Results from a Randomized, Double-Blind Placebo-Controlled Study. Curr. Dev. Nutr. 2020, 4, nzaa095. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Pither, M.D.; Martufi, M.; Scarinci, I.; Guzmán-Caldentey, J.; Łakomiec, E.; Jachymek, W.; Bruijns, S.C.M.; Santamaría, S.M.; Frick, J.S.; et al. Pairing Bacteroides Vulgatus LPS Structure with Its Immunomodulatory Effects on Human Cellular Models. ACS Cent. Sci. 2020, 6, 1602–1616. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Kaji, A.; Sakai, R.; Osaka, T.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Uchiyama, K.; Takagi, T.; et al. Intake of sucrose affects gut dysbiosis in patients with type 2 diabetes. J. Diabetes Investig. 2020, 11, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Morino, K.; Sekine, O.; Ohashi, N.; Kume, S.; Chano, T.; Iwasaki, K.; Harada, N.; Inagaki, N.; Ugi, S.; et al. Diverse metabolic effects of O-GlcNAcylation in the pancreas but limited effects in insulin-sensitive organs in mice. Diabetologia 2017, 60, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Maity, C.; Gupta, A.K.; Saroj, D.B.; Biyani, A.; Bagkar, P.; Kulkarni, J.; Dixit, Y. Impact of a Gastrointestinal Stable Probiotic Supplement Bacillus coagulans LBSC on Human Gut Microbiome Modulation. J. Diet. Suppl. 2021, 18, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Juge, N. Mucosal glycan degradation of the host by the gut microbiota. Glycobiology 2021, 31, 691–696. [Google Scholar] [CrossRef]
- Briliūtė, J.; Urbanowicz, P.A.; Luis, A.S.; Baslé, A.; Paterson, N.; Rebello, O.; Hendel, J.; Ndeh, D.A.; Lowe, E.C.; Martens, E.C.; et al. Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nat. Microbiol. 2019, 4, 1571–1581. [Google Scholar] [CrossRef]
- Panwar, H.; Rashmi, H.M.; Batish, V.K.; Grover, S. Probiotics as potential biotherapeutics in the management of type 2 diabetes—prospects and perspectives. Diabetes/Metab. Res. Rev. 2013, 29, 103–112. [Google Scholar] [CrossRef]
- Larsen, M.P.; Torekov, S.S. Glucagon-Like Peptide 1: A Predictor of Type 2 Diabetes? J. Diabetes Res. 2017, 2017, 7583506. [Google Scholar] [CrossRef] [Green Version]








| Characteristics | Probiotics (n = 66) | Placebo (n = 66) | p-Value |
|---|---|---|---|
| Age (years) | 34.85 ± 4.25 | 34.27 ± 4.79 | 0.487 |
| Pre-pregnancy BMI (kg/m2) | 28.56 ± 5.10 | 27.12 ± 5.52 | 0.122 |
| Ethnicity, n (%) | 1.000 # | ||
| Malay | 60 (90.9) | 60 (90.9) | |
| Non-Malay | 6 (9.1) | 6 (9.1) | |
| Education level, n (%) | 0.059 # | ||
| Secondary | 15 (22.7) | 26 (39.4) | |
| Tertiary | 51 (77.3) | 40 (60.6) | |
| Number of children | 3 ± 1 | 3 ± 1 | 0.813 |
| Family history of T2DM, n (%) | 52 (78.8) | 40 (60.6) | 0.037 #* |
| Received hypoglycemic agents in pregnancy, n (%) | 29 (43.9) | 26 (39.4) | 0.724 # |
| Exclusive breastfeeding, n (%) | 30 (45.5) | 25 (37.9) | 0.480 # |
| Postpartum OGTT assessment, n (%) | |||
| Frequency of postpartum GI, n (%) | 30 (45.5) | 31 (47.0) | 1.000 # |
| Pre-intervention assessment | |||
| Months after delivery | 4.08 ± 1.09 | 4.12 ± 1.07 | 0.809 |
| Macronutrient intake | |||
| Total energy intakes (kcal/day) | 1600.82 ± 341.31 | 1606.88 ± 319.96 | 0.916 |
| Carbohydrate (% total kcal) | 58.2 ± 7.9 | 56.0 ± 6.9 | 0.095 |
| Fat (% total kcal) | 33.9 ± 4.7 | 32.3 ± 13.1 | 0.056 |
| Proteins (% total kcal) | 16.8 ± 2.4 | 16.1 ± 2.5 | 0.116 |
| Cholesterol intakes (mg/day) | 224.05 ± 106.15 | 215.46 ± 125.40 | 0.672 |
| Total dietary fiber (g/day) | 6.45 ± 4.29 | 4.65 ± 2.27 | 0.003 ** |
| Physical activities (minutes/week) | |||
| Commuting | 56.62 ± 49.87 | 62.05 ± 55.30 | 0.555 |
| Leisure-time activities | 40.89 ± 48.61 | 32.50 ± 39.78 | 0.286 |
| Household activities | 1491.71 ± 559.01 | 1520.99 ± 672.38 | 0.786 |
| Activities at work | 1470.98 ± 823.99 | 1230.68 ± 935.24 | 0.120 |
| Total average 1-week physical activities | 3060.21 ± 640.84 | 2846.21 ± 709.51 | 0.071 |
| Anthropometric measurements | |||
| BMI (kg/m2) | 29.96 ± 4.67 | 27.87 ± 5.79 | 0.024 * |
| Waist circumference (cm) | 93.99 ± 10.39 | 89.01 ± 9.78 | 0.005 * |
| Overweight/Obese, n (%) | 64 (97.0) | 55 (83.3) | 0.016 #* |
| Systolic blood pressure (mmHg) | 115.74 ± 11.77 | 116.41 ± 12.77 | 0.756 |
| Diastolic blood pressure (mmHg) | 79.47 ± 10.97 | 78.50 ± 10.48 | 0.605 |
| Biochemical outcomes | |||
| FBG (mmol/L) | 5.36 ± 1.14 | 5.00 ± 0.95 | 0.056 |
| HbAIc (%) | 5.66 ± 0.66 | 5.66 ± 0.56 | 0.944 |
| Total cholesterol (mmol/L) | 4.91 ± 0.70 | 5.12 ± 0.85 | 0.103 |
| Triglycerides (mmol/L) | 1.07 ± 0.77 | 1.26 ± 0.75 | 0.082 |
| hs-CRP (mg/L) | 5.05 ± 3.50 | 3.76 ± 3.03 | 0.026 * |
| Biomarkers | Probiotics (n = 15) | Placebo (n = 15) | p-Value |
|---|---|---|---|
| Fasting serum insulin (μIU/mL) | 13.99 (54.43) | 13.16 (51.45) | 0.852 |
| HOMA-IR | 3.05 (18.37) | 3.74 (14.45) | 0.852 |
| Fasting active GLP-1 (pmol/L) | 0.00 (1.25) | 0.00 (0.93) | 0.909 |
| Interleukin-1β (pg/mL) | 0.13 (0.01) | 0.13 (0.04) | 0.868 |
| Interleukin-6 (pg/mL) | 0.26 (0.53) | 0.29 (0.43) | 0.820 |
| Interleukin-8 (pg/mL) | 0.65 (1.54) | 0.80 (0.77) | 0.852 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasain, Z.; Raja Ali, R.A.; Ahmad, H.F.; Abdul Rauf, U.F.; Oon, S.F.; Mokhtar, N.M. The Roles of Probiotics in the Gut Microbiota Composition and Metabolic Outcomes in Asymptomatic Post-Gestational Diabetes Women: A Randomized Controlled Trial. Nutrients 2022, 14, 3878. https://doi.org/10.3390/nu14183878
Hasain Z, Raja Ali RA, Ahmad HF, Abdul Rauf UF, Oon SF, Mokhtar NM. The Roles of Probiotics in the Gut Microbiota Composition and Metabolic Outcomes in Asymptomatic Post-Gestational Diabetes Women: A Randomized Controlled Trial. Nutrients. 2022; 14(18):3878. https://doi.org/10.3390/nu14183878
Chicago/Turabian StyleHasain, Zubaidah, Raja Affendi Raja Ali, Hajar Fauzan Ahmad, Ummul Fahri Abdul Rauf, Seok Fang Oon, and Norfilza Mohd Mokhtar. 2022. "The Roles of Probiotics in the Gut Microbiota Composition and Metabolic Outcomes in Asymptomatic Post-Gestational Diabetes Women: A Randomized Controlled Trial" Nutrients 14, no. 18: 3878. https://doi.org/10.3390/nu14183878
APA StyleHasain, Z., Raja Ali, R. A., Ahmad, H. F., Abdul Rauf, U. F., Oon, S. F., & Mokhtar, N. M. (2022). The Roles of Probiotics in the Gut Microbiota Composition and Metabolic Outcomes in Asymptomatic Post-Gestational Diabetes Women: A Randomized Controlled Trial. Nutrients, 14(18), 3878. https://doi.org/10.3390/nu14183878





