Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota
Abstract
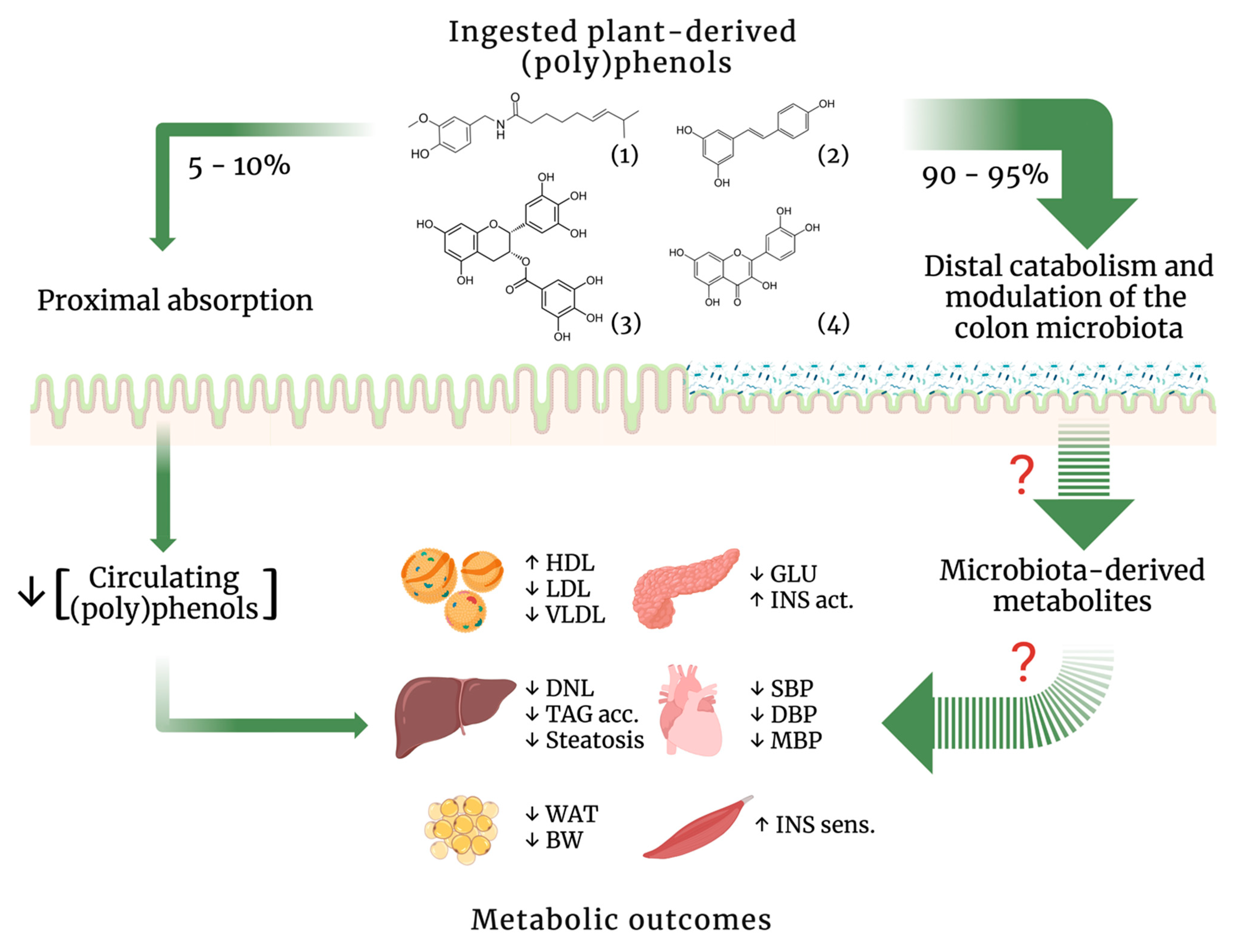
:1. Introduction
2. Gut Microbiota Development from Early-Life Colonization to Dysbiosis-Related Metabolic Disorders
3. Modulatory Effects of Plant-Derived (Poly)phenols on the Gut Microbiota and Their Metabolic Outcomes
4. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Margulis, L.; Fester, R. Bellagio conference and book. Symbiosis as Source of Evolutionary Innovation: Speciation and Morphogenesis. Conference—25–30 June 1989, Bellagio Conference Center, Italy. Symbiosis 1991, 11, 93–101. [Google Scholar] [PubMed]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.; Backhed, F.; Levin, B.R.; McFall-Ngai, M.J.; McLean, A.R. Evolution, human-microbe interactions, and life history plasticity. Lancet 2017, 390, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.C.; Marchesi, J.R.; Mougel, C.; Selosse, M.A. Host-microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. Gut microflora as a target for energy and metabolic homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 729–734. [Google Scholar] [CrossRef]
- Wolf, G. Gut microbiota: A factor in energy regulation. Nutr. Rev. 2006, 64, 47–50. [Google Scholar] [CrossRef]
- Hsiao, W.W.; Metz, C.; Singh, D.P.; Roth, J. The microbes of the intestine: An introduction to their metabolic and signaling capabilities. Endocrinol. Metab. Clin. N. Am. 2008, 37, 857–871. [Google Scholar] [CrossRef]
- Mitreva, M.; Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, K.M.; Gerlach, M.J.; Adam, T.; Heimesaat, M.M.; Rossi, L.; Surette, M.G.; Sloboda, D.M.; Braun, T. Fetal meconium does not have a detectable microbiota before birth. Nat. Microbiol. 2021, 6, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv. Nutr. 2016, 7, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, K.; Kimura, I. Gut Microbiota Dysbiosis Drives and Implies Novel Therapeutic Strategies for Diabetes Mellitus and Related Metabolic Diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, S.; Zhang, X. Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef]
- Martin, M.A.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, M.; Knauf, C.; Cani, P.D. Gut Microbes and Health: A Focus on the Mechanisms Linking Microbes, Obesity, and Related Disorders. Obesity 2018, 26, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Farhat, G.; Drummond, S.; Al-Dujaili, E.A.S. Polyphenols and Their Role in Obesity Management: A Systematic Review of Randomized Clinical Trials. Phytother. Res. PTR 2017, 31, 1005–1018. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Stinson, L.F.; Payne, M.S.; Keelan, J.A. Planting the seed: Origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit. Rev. Microbiol. 2017, 43, 352–369. [Google Scholar] [CrossRef]
- Jimenez, E.; Marin, M.L.; Martin, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernandez, L.; Rodriguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Lee, S.E.; Jung, H.; Kim, G.; Romero, R.; Yoon, B.H. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J. Perinat. Med. 2010, 38, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Brussow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2020, 13, 423–434. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Van der Graaf, Y.; Cramer, M.J.; Kapelle, L.J.; de Borst, G.J.; Visseren, F.L.J.; Westerink, J. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Leon-Pedroza, J.I.; Gonzalez-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodriguez, D.; Escobedo, G.; Gonzalez-Chavez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cir. Cir. 2015, 83, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Francois, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Castagliuolo, I.; Di Leo, V.; Buda, A.; Pinzani, M.; Palu, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef]
- Hawkesworth, S.; Moore, S.E.; Fulford, A.J.; Barclay, G.R.; Darboe, A.A.; Mark, H.; Nyan, O.A.; Prentice, A.M. Evidence for metabolic endotoxemia in obese and diabetic Gambian women. Nutr. Diabetes 2013, 3, e83. [Google Scholar] [CrossRef]
- Anhe, F.F.; Barra, N.G.; Cavallari, J.F.; Henriksbo, B.D.; Schertzer, J.D. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. 2021, 36, 109691. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Bolognini, D.; Tobin, A.B.; Milligan, G.; Moss, C.E. The Pharmacology and Function of Receptors for Short-Chain Fatty Acids. Mol. Pharmacol. 2015, 89, 388–398. [Google Scholar] [CrossRef]
- Kibbie, J.J.; Dillon, S.M.; Thompson, T.A.; Purba, C.M.; McCarter, M.D.; Wilson, C.C. Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology 2021, 226, 152126. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S.; Wollam, A.; Shah, N.; Wang, C.; Magrini, V.; Wilson, R.K.; et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Yanez-Gascon, M.J.; Selma, M.V.; Gonzalez-Sarrias, A.; Toti, S.; Ceron, J.J.; Tomas-Barberan, F.; Dolara, P.; Espin, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.M.; Waget, A.; Klopp, P.; Serino, M.; Vachoux, C.; Pechere, L.; Drucker, D.J.; Champion, S.; Barthélemy, S.; Barra, Y.; et al. Resveratrol increases glucose induced GLP-1 secretion in mice: A mechanism which contributes to the glycemic control. PLoS ONE 2011, 6, e20700. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Jung, M.J.; Lee, J.; Shin, N.R.; Kim, M.S.; Hyun, D.W.; Yun, J.H.; Kim, P.S.; Whon, T.W.; Bae, J.W. Chronic Repression of mTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-induced Obese Mice. Sci. Rep. 2016, 6, 30887. [Google Scholar] [CrossRef]
- Sung, M.M.; Kim, T.T.; Denou, E.; Soltys, C.M.; Hamza, S.M.; Byrne, N.J.; Masson, G.; Park, H.; Wishart, D.S.; Madsen, K.L.; et al. Improved Glucose Homeostasis in Obese Mice Treated with Resveratrol Is Associated with Alterations in the Gut Microbiome. Diabetes 2017, 66, 418–425. [Google Scholar] [CrossRef]
- Liao, W.; Yin, X.; Li, Q.; Zhang, H.; Liu, Z.; Zheng, X.; Zheng, L.; Feng, X. Resveratrol-Induced White Adipose Tissue Browning in Obese Mice by Remodeling Fecal Microbiota. Molecules 2018, 23, 3356. [Google Scholar] [CrossRef]
- Brandt, N.; Kotowska, D.; Kristensen, C.M.; Olesen, J.; Lutzhoft, D.O.; Halling, J.F.; Hansen, M.; Al-Soud, W.A.; Hansen, L.; Kiilerich, P.; et al. The impact of exercise training and resveratrol supplementation on gut microbiota composition in high-fat diet fed mice. Physiol. Rep. 2018, 6, e13881. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, e1800066. [Google Scholar] [CrossRef] [PubMed]
- Sreng, N.; Champion, S.; Martin, J.C.; Khelaifia, S.; Christensen, J.E.; Padmanabhan, R.; Azalbert, V.; Blasco-Baque, V.; Loubieres, P.; Pechere, L.; et al. Resveratrol-mediated glycemic regulation is blunted by curcumin and is associated to modulation of gut microbiota. J. Nutr. Biochem. 2019, 72, 108218. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Milton-Laskibar, I.; Marcos-Zambrano, L.J.; Gomez-Zorita, S.; Fernandez-Quintela, A.; Carrillo de Santa Pau, E.; Martinez, J.A.; Portillo, M.P. Gut Microbiota Induced by Pterostilbene and Resveratrol in High-Fat-High-Fructose Fed Rats: Putative Role in Steatohepatitis Onset. Nutrients 2021, 13, 1738. [Google Scholar] [CrossRef]
- Etxeberria, U.; Hijona, E.; Aguirre, L.; Milagro, F.I.; Bujanda, L.; Rimando, A.M.; Martinez, J.A.; Portillo, M.P. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 2017, 61, 1500906. [Google Scholar] [CrossRef]
- Unno, T.; Sakuma, M.; Mitsuhashi, S. Effect of dietary supplementation of (-)-epigallocatechin gallate on gut microbiota and biomarkers of colonic fermentation in rats. J. Nutr. Sci. Vitaminol. 2014, 60, 213–219. [Google Scholar] [CrossRef]
- Ikarashi, N.; Ogawa, S.; Hirobe, R.; Kon, R.; Kusunoki, Y.; Yamashita, M.; Mizukami, N.; Kaneko, M.; Wakui, N.; Machida, Y.; et al. Epigallocatechin gallate induces a hepatospecific decrease in the CYP3A expression level by altering intestinal flora. Eur. J. Pharm. Sci. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Unno, T.; Hisada, T.; Takahashi, S. Hesperetin Modifies the Composition of Fecal Microbiota and Increases Cecal Levels of Short-Chain Fatty Acids in Rats. J. Agric. Food Chem. 2015, 63, 7952–7957. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, A.B.; Sun, S.; Ajami, N.J.; Ross, M.C.; Wang, H.; Zhang, L.; Reuhl, K.; Kobayashi, K.; Onishi, J.C.; et al. Green Tea Polyphenols Modify the Gut Microbiome in db/db Mice as Co-Abundance Groups Correlating with the Blood Glucose Lowering Effect. Mol. Nutr. Food Res. 2019, 63, e1801064. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Su, Q.; Liu, Y. Sinapine reduces non-alcoholic fatty liver disease in mice by modulating the composition of the gut microbiota. Food Funct. 2019, 10, 3637–3649. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Liu, Z.; Liu, L.; Yang, K.; Wei, Y.; Liu, Y.; Chen, X.; Sun, X.; Wen, D. Hydroxytyrosol prevents PM2.5-induced adiposity and insulin resistance by restraining oxidative stress related NF-kappaB pathway and modulation of gut microbiota in a murine model. Free Radic. Biol. Med. 2019, 141, 393–407. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. mBio 2017, 8, e00470-17. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Shen, M.; Zhao, X.; Zhu, H.; Yang, Y.; Lu, S.; Tan, Y.; Li, G.; Li, M.; Wang, J.; et al. Anti-obesity Effect of Capsaicin in Mice Fed with High-Fat Diet Is Associated with an Increase in Population of the Gut Bacterium Akkermansia muciniphila. Front. Microbiol. 2017, 8, 272. [Google Scholar] [CrossRef]
- Song, J.X.; Ren, H.; Gao, Y.F.; Lee, C.Y.; Li, S.F.; Zhang, F.; Li, L.; Chen, H. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Front. Physiol. 2017, 8, 602. [Google Scholar] [CrossRef]
- Hui, S.; Liu, Y.; Chen, M.; Wang, X.; Lang, H.; Zhou, M.; Yi, L.; Mi, M. Capsaicin Improves Glucose Tolerance and Insulin Sensitivity through Modulation of the Gut Microbiota-Bile Acid-FXR Axis in Type 2 Diabetic db/db Mice. Mol. Nutr. Food Res. 2019, 63, e1900608. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Tang, Y.; Yin, H.; Liu, X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef] [Green Version]
- Baboota, R.K.; Khare, P.; Mangal, P.; Singh, D.P.; Bhutani, K.K.; Kondepudi, K.K.; Kaur, J.; Bishnoi, M. Dihydrocapsiate supplementation prevented high-fat diet-induced adiposity, hepatic steatosis, glucose intolerance, and gut morphological alterations in mice. Nutr. Res. 2018, 51, 40–56. [Google Scholar] [CrossRef]
- Ding, Y.; Song, Z.; Li, H.; Chang, L.; Pan, T.; Gu, X.; He, X.; Fan, Z. Honokiol Ameliorates High-Fat-Diet-Induced Obesity of Different Sexes of Mice by Modulating the Composition of the Gut Microbiota. Front. Immunol. 2019, 10, 2800. [Google Scholar] [CrossRef]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; da Rosa, J.C.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.; Breslow, J.L.; Holt, P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. J. Clin. Transl. Res. 2019, 4, 122–135. [Google Scholar]
- Most, J.; Penders, J.; Lucchesi, M.; Goossens, G.H.; Blaak, E.E. Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur. J. Clin. Nutr. 2017, 71, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R. Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef]
- Tremblay, A.; Arguin, H.; Panahi, S. Capsaicinoids: A spicy solution to the management of obesity? Int. J. Obes. 2016, 40, 1198–1204. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Gonlachanvit, S. Chili Peppers, Curcumins, and Prebiotics in Gastrointestinal Health and Disease. Curr. Gastroenterol. Rep. 2016, 18, 19. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Xie, H.; Wen, Z.; Zhang, Y.; Wu, C.; Huang, L.; Wu, J.; Xie, C.; Wang, T.; et al. Gut Microbiota Composition and Structure of the Ob/Ob and Db/Db Mice. Int. J. Endocrinol. 2019, 2019, 1394097. [Google Scholar] [CrossRef]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, E.S.; Liang, L.; Smillie, S.J.; Kaiser, F.; Purcell, R.; Rivett, D.W.; Alam, S.; Howat, S.; Collins, H.; Thompson, S.J.; et al. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 2012, 188, 5741–5751. [Google Scholar] [CrossRef] [PubMed]
- Polia, F.; Pastor-Belda, M.; Martínez-Blázquez, A.; Horcajada, M.-N.; Tomás-Barberán, F.A.; García-Villalba, R. Technological and Biotechnological Processes To Enhance the Bioavailability of Dietary (Poly)phenols in Humans. J. Agric. Food Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A survey of modulation of gut microbiota by dietary polyphenols. BioMed Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Freitas, P.L.; Barros, M.V.C.; Froes, R.B.L.; Franca, L.M.; Paes, A.M.A. Prebiotic effects of plant-derived (poly)phenols on host metabolism: Is there a role for short-chain fatty acids? Crit. Rev. Food Sci. Nutr. 2022, 1–9. [Google Scholar] [CrossRef]
| (Poly)phenol | Model | Regimen | Gut Microbiota Modulation | Metabolic Outcomes | Ref. |
|---|---|---|---|---|---|
| Pre-clinical Studies | |||||
| Resveratrol | DSS-induced colitic rats | 1 mg/kg/day supplemented to the diet for 25 days. | ↑ Bifidobacterium , ↓ Enterococcus faecalis, ↑ Lactobacillus. | ↑ food intake, ↓ body weight loss associated to the animal model. | [64] |
| HFD-fed mice | 60 mg/kg/day supplemented to the diet for 5 weeks. | ↓ Bacteroides vulgatus, ↓ Alistipes putredinis, ↓ Parabacteroides johnsonii. | ↑ glucose tolerance, ↑ GLP-1 and insulin levels, ↑ GLP-1 intestinal content. | [65] | |
| HFD-fed mice | 200 mg/kg/day supplemented to the diet for 12 weeks. | ↑ Bacteroidetes, ↑ Bifidobacterium, ↑ Lactobacillus, ↓ Enterococcus faecalis. | ↓ body weight, ↓ abdominal adipose tissue, ↓ liver weight, ↓ glycemia, ↓ dyslipidemia. | [66] | |
| HFD-fed mice | 200 mg/kg/day by oral gavage for 8 weeks. | ↓ Lactococcus, ↓ Oscillibacter, ↓ Clostridium XI, ↓ Flavonifractor, ↓ Hydrogenoanaerobacterium. | ↓ body weight, ↓ epididymal adipose tissue, ↑ glucose tolerance, ↑ insulin sensitivity. | [67] | |
| HFHS-fed mice | 400 mg/kg/day supplemented to the diet for 8 weeks. | ↑ Bacteroides, ↓ Turicibacteraceae, ↓ Lachnospiraceae, ↑ Parabacteroides, ↓ Akkermansia. | ↓ fat mass, ↑ glucose tolerance. | [68] | |
| HFD-fed mice | 400 mg/kg/day supplemented to the diet for 4 weeks *. | ↑ Lactobacillus, ↑ Bifidobacterium, ↓ Proteobacteria. | ↓ body weight gain, ↑ glucose homeostasis, ↓ perigonadal and inguinal adipose tissue, ↑ white adipose tissue browning. | [69] | |
| HFD-fed mice | 400 mg/kg/day supplemented to the diet for 16 weeks. | ↑ Erysipelotrichaceae family, ↑ Allobaculum spp. | ↓ body weight, ↓ subcutaneous and visceral adipose tissue, ↑ lean mass, ↓ food intake. | [70] | |
| Fecal transplantation from HFD-fed RSV-treated to HFD-fed untreated mice | 300 mg/kg/day by oral gavage for 16 weeks. | ↑ Bacteroides, ↑ Lachnospiraceae, ↑ Lachnoclostridium, ↑ Parabacteroides, ↑ Ruminiclostridium, ↑ Blautia. | ↓ body weight, ↓ white adipose tissue, ↑ white adipose tissue browning, ↓ blood glucose, ↑ insulin sensitivity, ↓ hepatic steatosis, ↓ serum LPS levels. | [71] | |
| Perinatal and post-weaning HFr-fed rats | 50 mg/L in drinking water to mothers and offspring up to 12 weeks old. | ↓ Bacteroidetes, ↑ Lactobacillus, ↑ Bifidobacterium, ↑ Akkermansia. | ↑ body weight, ↓ blood pressure, ↓ renal oxidative stress, ↑ nutrient-sensing signals. | [72]. | |
| HFD-fed mice | 60 mg/kg/day supplemented to the diet for 5 weeks. | ↓ Rikenellaceae,↑ Ruminococcaceae, ↓ Peptostreptococcaceae, ↓ Proteobacteria. | ↑ glucose tolerance. | [73] | |
| HFD-fed rats | 10 mg/kg/day supplemented to the diet for 8 weeks. | ↓ Bacteroides, ↑ Lachnospiraceae, ↓ Desulfovibrionaceae. | ↓ blood glucose, ↑ insulin sensitivity. | [74]. | |
| HFHFr-fed rats | 30 mg/kg/day supplemented to the diet for 8 weeks. | ↑ Blautia, ↑ Moryella, ↑ Lactococcus. | ↓ liver weight, ↓ hepatic transaminases levels, ↓ steatohepatitis. | [75] | |
| Pterostilbene | Obese Zucker (fa/fa) rats | 15 mg/kg/day by oral gavage for 6 weeks. | ↑ Mollicutes, ↓ Negativicutes, ↓ Lachnospiraceae, ↓ Defluviitaleaceae, ↑ Verrucomicrobia. | ↓ body weight gain, ↓ white adipose tissue, ↓ insulin levels, ↑ insulin sensitivity. | [76] |
| HFHF-fed rats | 15 and 30 mg/kg/day supplemented to the diet for 8 weeks. | ↓ Clostridium sensu stricto 1, ↑ Erysipelatoclostridium, ↑ Fourrnierella, ↑ Akkermansia. | ↓ hepatic transaminases levels, ↓ steatohepatitis. | [75] | |
| EGCG | Wistar rats | 300 and 600 mg/kg/day supplemented to the diet for 4 weeks *. | ↑ Bacteroides, ↓ Prevotella, ↓ Clostridium, ↓ Bifidobacterium. | ↓ liver weight, ↓ abdominal adipose tissue (higher dose). | [77] |
| ICR mice | 50, 750, or 1500 mg/kg/day supplemented to the diet for 2–10 days *. | ↓ Clostridium cluster IV, ↓ Clostridium cluster XIVa. | ↓ CYP3A gene and protein expression in the liver, ↓ pregnane X receptor (PXR) protein expression in the liver (higher dose). | [78] | |
| HFD-fed mice | 320 mg/kg/day (roughly) supplemented to the diet for 8 weeks *. | ↑ Allobaculum, ↑ Clostridium, ↑ Parabacteroides, ↓ Lachnospiraceae, ↓ Ruminococcous, ↑ Adlercreutzia, ↓ Desulfovibrionaceae, ↑ Akkermansia. | ↓ body weight, ↓ hepatic steatosis, ↓ hepatic TG, ↓ serum non-esterified fatty acids. | [79] | |
| Quercetin | HFD-fed mice | 50 mg/kg/day aglycone quercetin supplemented to the diet for 16 weeks *. | ↑ Bacteroidia,↑ Erysipelotrichi, ↓ Bacilli, ↓ Clostridia, ↓ Helicobacter, ↑ Betaproteobacteria, ↓ Desulfovibrio, ↓ Deltaproteobacteria, ↑ Akkermansia. | ↓ body weight gain, ↓ epididymal fat pads, ↓ glycemia, ↓ insulinemia, ↑ insulin sensitivity, ↓ plasma TG, ↓ plasma alanine aminotransferase activity, ↓ hepatic steatohepatitis. | [80] |
| Hesperetin | Wistar rats | 500 mg/kg/day supplemented to the diet for 3 weeks *. | ↓ Clostridium subcluster XIVa, ↑ Clostridium clusters IV, XVIII. | ↓ abdominal adipose tissue. | [81] |
| Theaflavins | db/db mice | 100 mg/kg/day supplemented to the diet for 7 weeks *. | ↓ Barnesiella, ↓ Odoribacter, ↓ Lachnospiraceae, ↓ Desulfovibrio. | ↓ insulinemia. | [82] |
| Sinapine | HFD-fed mice | 500 mg/kg/day supplemented to the diet for 12 weeks *. | ↑ Prevotellaceae,↑ Lactobacillaceae, ↓ Lachnospiraceae, ↓ Erysipelotrichaceae, ↓ Peptostreptococcaceae, ↑ Blautia, ↑ Bifidobacterium, ↑ Eggerthellaceae, ↓ Desulfovibrio, ↑ Akkermansiaceae. | ↓ body weight, ↓ food efficiency, ↓ white adipose tissue, ↓ blood glucose, ↓ plasma TG, ↓ plasma LDL-C, ↓ insulinemia, ↑ insulin sensitivity, ↓ hepatic steatosis. | [83] |
| Hydroxytyrosol | Fine particulate matter-exposed mice | 50 mg/kg/day by oral gavage for 4 weeks. | ↑ Bacteroidetes, ↑ Akkermansia. | ↓ visceral adipose tissue, ↑ glucose tolerance, ↑ insulin sensitivity, ↓ hepatic oxidative stress, ↓ hepatic inflammation. | [84] |
| HFD-fed mice | 50 mg/kg/day by oral gavage for 8 weeks. | Unchanged Bacteroidetes/Firmicutes. | ↓ white adipose tissue, ↓ liver weight, ↓ blood glucose, ↑ insulin sensitivity, ↓ hepatic steatosis, ↓ plasma LPS. | [85] | |
| Capsaicin | HFD-fed mice | 2 mg/kg/day supplemented to the diet for 12 weeks *. | ↓ LPS-producing S24-7 family, ↑ Ruminococcaceae, ↑ Lachnospiraceae. | ↓ body weight gain, ↓ white adipose tissue, ↑ glucose tolerance, ↓ serum LPS, ↓ serum proinflammatory cytokines. | [86] |
| HFD-fed mice | 10 mg/kg/day supplemented to the diet for 9 weeks *. | ↑ Bacteroides, ↑ Prevotella, ↑ Coprococcus, ↑ Akkermansia, ↓ Proteobacteria, ↑ Acidobacteria. | ↓ body weight gain, ↓ food intake, ↑ glucose tolerance. | [87] | |
| ob/ob mice | 6 and 12 mg/kg/day supplemented to the diet for 6 weeks *. | ↓ Bacteroides, ↑ Roseburia, ↑ Parabacteroides. | ↑ glucose tolerance, ↑ insulin sensitivity. | [88] | |
| db/db mice | 10 mg/kg/day added to the diet for 4 or 8 weeks *. | ↓ Lactobacillus. | ↓ blood glucose, ↓ insulinemia, ↑ glucose tolerance, ↑ insulin sensitivity. | [89] | |
| HFD-fed TRPV1−/− mice | 2 mg/kg/day by oral gavage for 12 weeks. | ↑ Bacteroides, ↑ Prevotella, ↓ endotoxemic S24-7 family, ↑ Coprococcus, ↓ Actinobacteria, ↓ Desulfovibrio, ↓ Escherichia, ↓ Helicobacter, ↓ Sutterella, ↑ Akkermansia, ↓ Cyanobacteria, ↑ Tenericutes. | ↓ body weight gain, ↓ food intake, ↓ blood glucose, ↓ plasma TG, TC, and LDL-C, ↓ insulinemia. | [90] | |
| Dihydrocapsiate | HFD-fed mice | 2 and 10 mg/kg/day by oral gavage for 12 weeks. | No change in Lactobacillus, Bifidobacterium, and Akkermansia. | ↓ plasma TG, ↓ insulinemia, ↑ glucose tolerance, ↓ hepatic steatosis. | [91] |
| Honokiol | HFD-fed mice | 200, 400 and 800 mg/kg/day supplemented to the diet for 8 weeks. | ↑ Bacteroides, ↓ Muribaculaceae, ↓ Oscillospira, ↓ Ruminococcus, ↓ Lactococcus, ↓ Dehalobacterium, ↓ Unclassified_Clostridiales, ↓ Unclassified_Ruminococcaceae, ↑ Unclassified_Enterobacteriaceae, ↑ Bilophila, ↑ Akkermansia, ↑ Fusobacterium. | ↓ body weight, ↓ white adipose tissue, ↓ serum TG, and TC, ↓ serum free fatty acids, ↓ blood glucose. | [92] |
| Clinical Studies | |||||
| Trans-resveratrol | MetS humans | 2 g/day orally for 30 days. | ↓ Rikenellaceae, ↓ Butyricimonas, ↑ Gemellaceae, ↑ Turicibacter, ↓ Ruminococcus, ↓ Oscillospira, ↓ Clostridium, ↓ Odoribacter, ↓ Alistipes, ↑ Gammaproteobacteria, ↑ Akkermansia, ↑ Atopobium. | ↑ glucose tolerance in Caucasian subjects only. | [93] |
| Trans-resveratrol + EGCG | Overweight humans | 80 mg/day RVS and 282 mg/day EGCG orally for 12 weeks. | ↓ Faecalibacteriuim prausnitzii, ↓ Bacteroidetes (only in men). | ↑ skeletal muscle mitochondrial oxidative capacity, ↑ increased fat oxidation. | [94] |
| Capsaicin | Humans | 0.078 mg/kg/day for 2 weeks, 1 week washout and then 0.156 mg/kg/day for 2 weeks *. | ↑ Lachnospiraceae, ↑ Ruminococcaceae, ↑ Faecalibacterium. | ↑ plasma GLP-1, ↑ GIP and ghrelin. | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, P.L.d.; Miranda, J.P.N.; França, L.M.; Paes, A.M.d.A. Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota. Nutrients 2022, 14, 3510. https://doi.org/10.3390/nu14173510
Freitas PLd, Miranda JPN, França LM, Paes AMdA. Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota. Nutrients. 2022; 14(17):3510. https://doi.org/10.3390/nu14173510
Chicago/Turabian StyleFreitas, Perla Lopes de, João Paulo Nascimento Miranda, Lucas Martins França, and Antonio Marcus de Andrade Paes. 2022. "Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota" Nutrients 14, no. 17: 3510. https://doi.org/10.3390/nu14173510
APA StyleFreitas, P. L. d., Miranda, J. P. N., França, L. M., & Paes, A. M. d. A. (2022). Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota. Nutrients, 14(17), 3510. https://doi.org/10.3390/nu14173510






