Black Ginger (Kaempferia parviflora) Extract Enhances Endurance Capacity by Improving Energy Metabolism and Substrate Utilization in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Kaempferia parviflora Preparation
2.3. Experimental Protocol
2.4. Measurement of Plasma Biochemical Parameters, Liver Glycogen, and Biomarker of Oxidative Stress
2.5. Real-Time Quantitative Polymerase Chain Reaction (PCR)
2.6. Statistical Analysis
3. Results
3.1. Effect of KPE Administration on Mice Endurance Capacity
3.2. Effect of Exhaustive Exercise and KPE Administration on Metabolism Regulation in Plasma
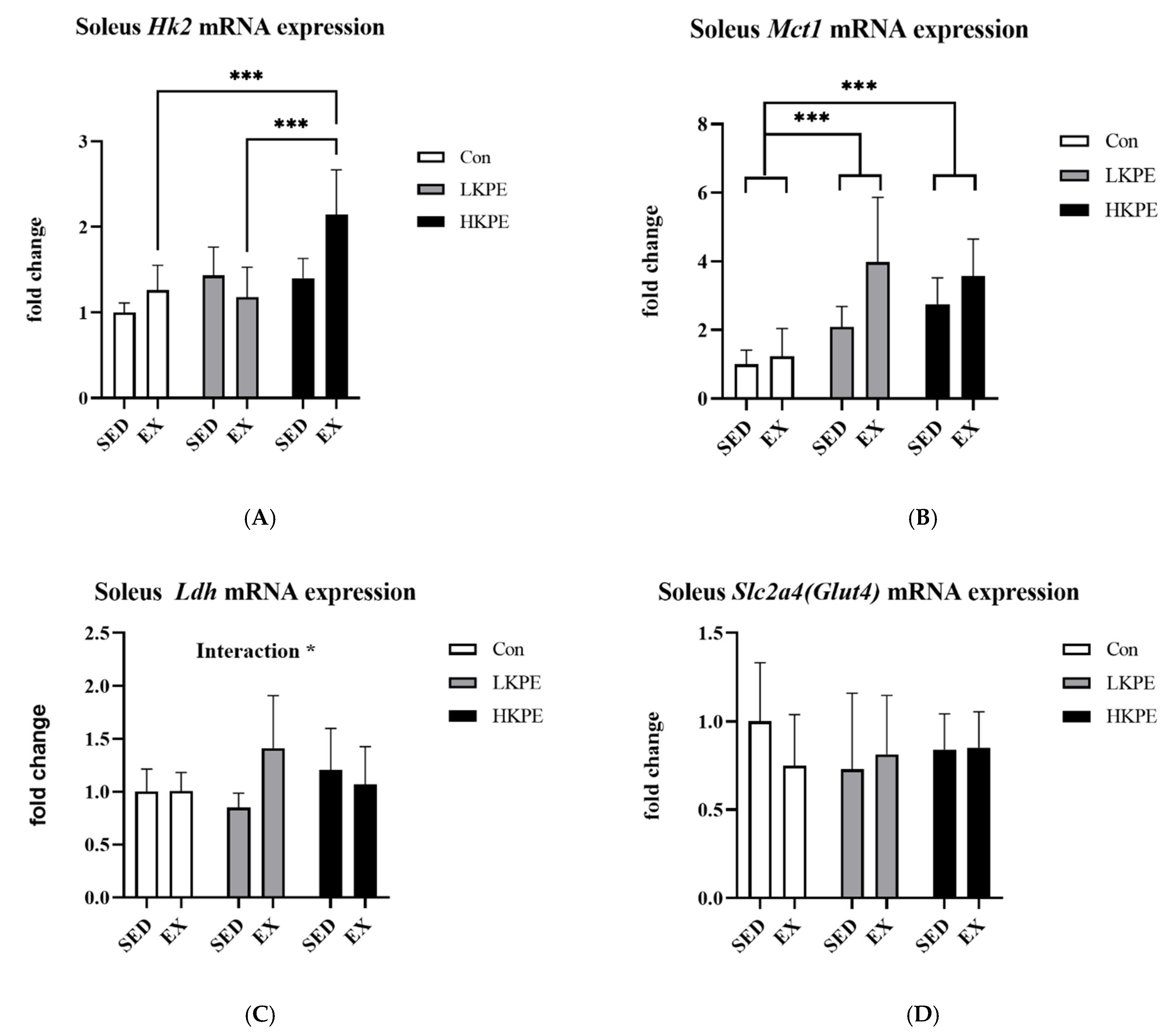
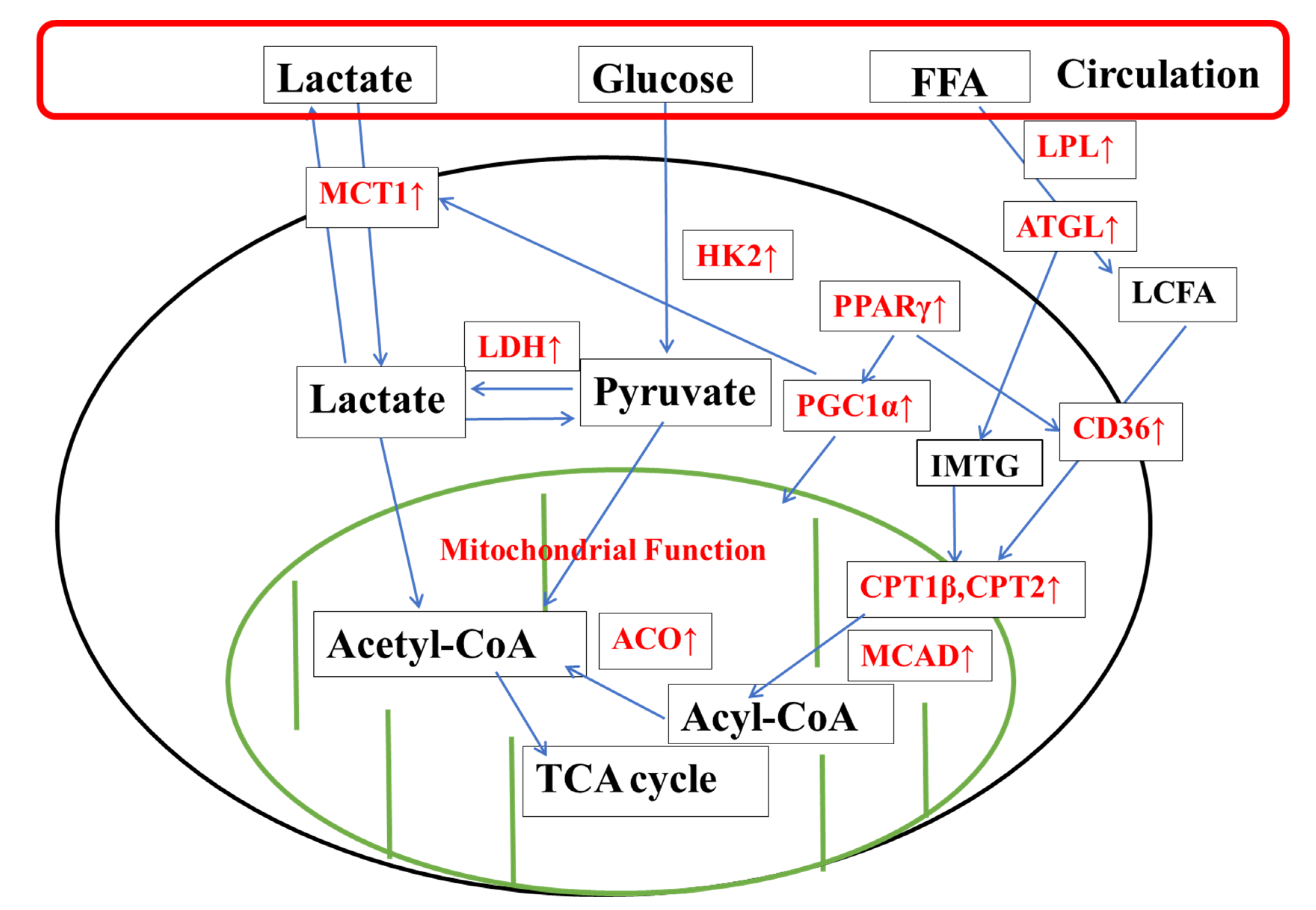
3.3. Effect of Exhaustive Exercise and KPE Administration on Fatty-Acid-Metabolism-Related Gene Expression in Soleus Muscle
3.4. Effect of Exhaustive Exercise and KPE Administration on Fatty Acid Transmembrane Transport Related Gene Expression in Soleus Muscle
3.5. Effect of Exhaustive Exercise and KPE Administration on Glucose-Metabolism-Related Gene Expression in Soleus Muscle
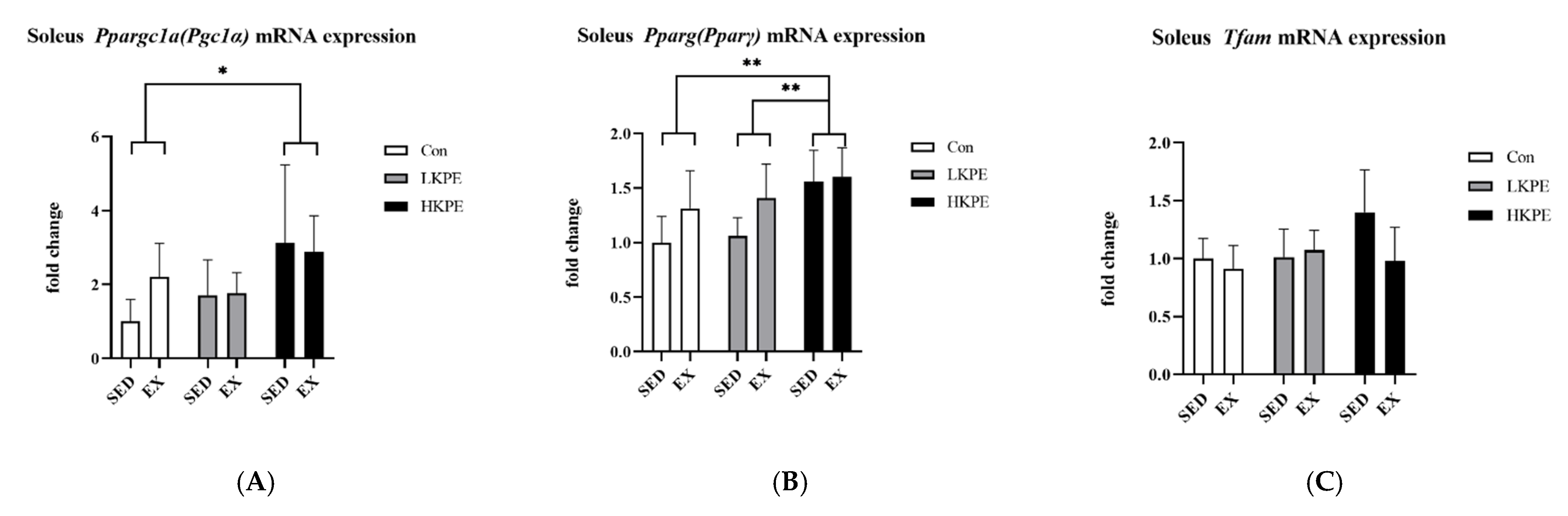
3.6. Effect of Exhaustive Exercise and KPE Administration on Mitochondrial-Function-Related Gene Expression in Soleus Muscle
3.7. Effect of Exhaustive Exercise and KPE Administration on Glucose-Metabolism-Related Gene Expression in Gastrocnemius Muscle
3.8. Effect of Exhaustive Exercise and KPE Administration on Glycogen-Metabolism-Related Gene Expression in Liver
4. Discussion
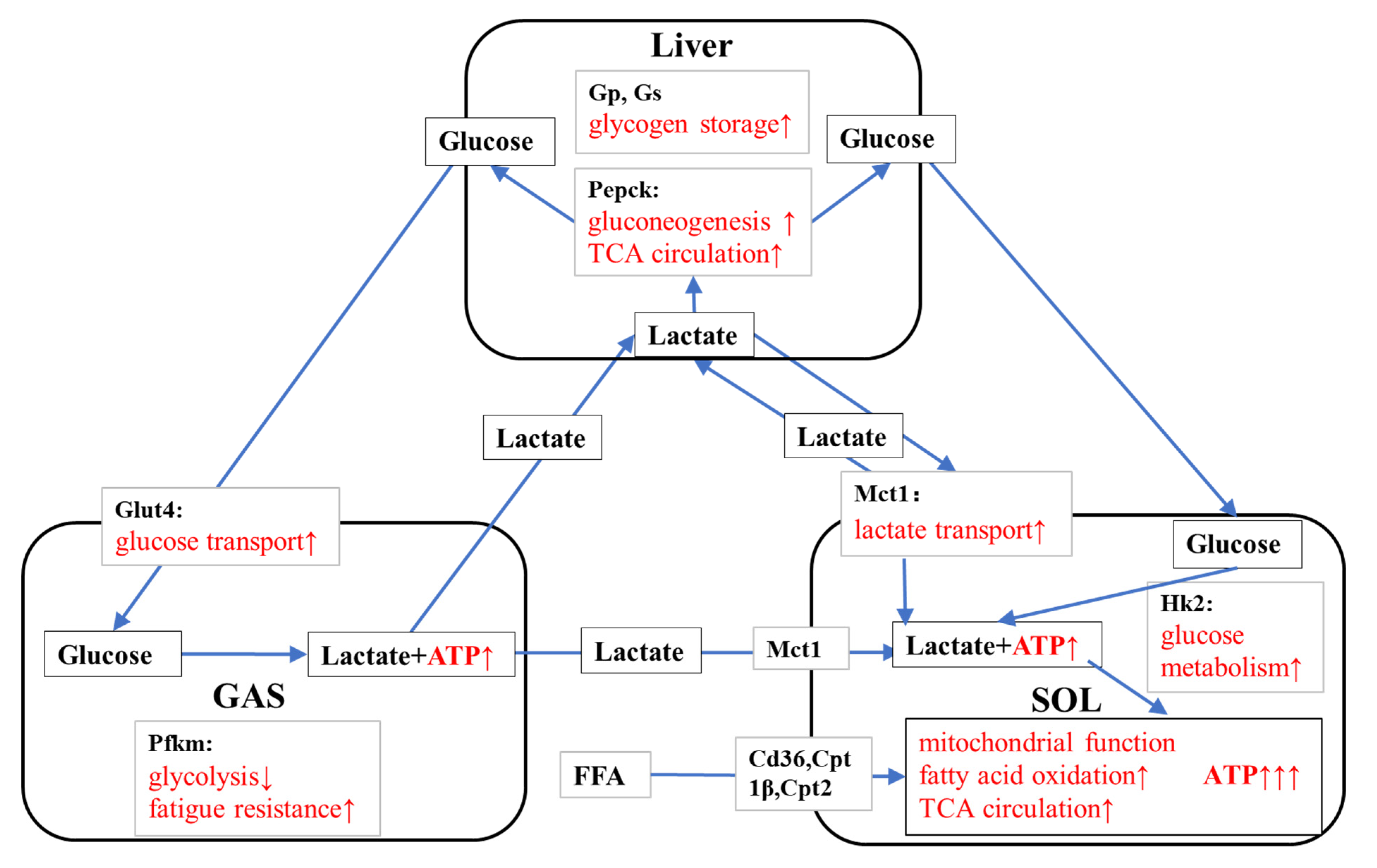
4.1. KPE Has the Potential to Improve Lipid and Lactate Transportation, Oxidation Capacity, Glycolysis and Mitochondrial Function in Soleus Muscle
4.2. KPE Has the Potential to Regulate Glucose Transport in Gastrocnemius Muscle and Liver
4.3. KPE Has the Potential to Improve Energy Metabolism and Substrate Utilization between Skeletal Muscle and Liver
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saokaew, S.; Wilairat, P.; Raktanyakan, P.; Dilokthornsakul, P.; Dhippayom, T.; Kongkaew, C.; Sruamsiri, R.; Chuthaputti, A.; Chaiyakunapruk, N. Clinical Effects of Krachaidum (Kaempferia parviflora): A Systematic Review. J. Evid.-Based Integr. Med. 2017, 22, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, H.; Li, W.; Feng, S.; Deng, D. Kaempferia parviflora and Its Methoxyflavones: Chemistry and Biological Activities. Evid.-Based Complement. Altern. Med. 2018, 2018, 4057456. [Google Scholar] [CrossRef]
- Strawa, J.W.; Jakimiuk, K.; Tomczyk, M. Zapotin, a Polymethoxyflavone, with Potential Therapeutic Attributes. Int. J. Mol. Sci. 2021, 22, 13227. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Murata, K.; Hayashi, H.; Matsumura, S. Suppression of benign prostate hyperplasia by Kaempferia parviflora rhizome. Pharmacogn. Res. 2013, 5, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Potikanond, S.; Sookkhee, S.; Na Takuathung, M.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia parviflora Extract Exhibits Anti-cancer Activity against HeLa Cervical Cancer Cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Kim, M.; Dibwe, D.; Omar, A.; Athikomkulchai, S.; Phrutivorapongkul, A.; Okada, T.; Tsuge, K.; Toyooka, N.; Awale, S. Anti-Austerity Activity of Thai Medicinal Plants: Chemical Constituents and Anti-Pancreatic Cancer Activities of Kaempferia parviflora. Plants 2021, 10, 229. [Google Scholar] [CrossRef]
- Chaipech, S.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O. Structures of Two New Phenolic Glycosides, Kaempferiaosides A and B, and Hepatoprotective Constituents from the Rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012, 60, 62–69. [Google Scholar] [CrossRef]
- Akase, T.; Shimada, T.; Terabayashi, S.; Ikeya, Y.; Sanada, H.; Aburada, M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Nat. Med. 2010, 65, 73–80. [Google Scholar] [CrossRef]
- Yoshino, S.; Kim, M.; Awa, R.; Kuwahara, H.; Kano, Y.; Kawada, T. Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci. Nutr. 2014, 2, 634–637. [Google Scholar] [CrossRef]
- Kim, M.-B.; Kim, T.; Kim, C.; Hwang, J.-K. Standardized Kaempferia parviflora Extract Enhances Exercise Performance Through Activation of Mitochondrial Biogenesis. J. Med. Food 2018, 21, 30–38. [Google Scholar] [CrossRef]
- Toda, K.; Takeda, S.; Hitoe, S.; Nakamura, S.; Matsuda, H.; Shimoda, H. Enhancement of energy production by black ginger extract containing polymethoxy flavonoids in myocytes through improving glucose, lactic acid and lipid metabolism. J. Nat. Med. 2016, 70, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Hitoe, S.; Takeda, S.; Shimoda, H. Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon 2016, 2, e00115. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-E.; Woo, S.W.; Kim, M.-B.; Kim, C.; Hwang, J.-K. Standardized Kaempferia parviflora Extract Inhibits Intrinsic Aging Process in Human Dermal Fibroblasts and Hairless Mice by Inhibiting Cellular Senescence and Mitochondrial Dysfunction. Evid.-Based Complement. Altern. Med. 2017, 2017, 6861085. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Takeuchi, T.; Nozaki, T.; Ishihara, K.; Matsuo, T. Kaempferia parviflora Ethanol Extract, a Peroxisome Proliferator-Activated Receptor γ Ligand-binding Agonist, Improves Glucose Tolerance and Suppresses Fat Accumulation in Diabetic NSY Mice. J. Food Sci. 2019, 84, 339–348. [Google Scholar] [CrossRef]
- Okabe, Y.; Shimada, T.; Horikawa, T.; Kinoshita, K.; Koyama, K.; Ichinose, K.; Aburada, M.; Takahashi, K. Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine 2014, 21, 800–806. [Google Scholar] [CrossRef]
- Kobayashi, H.; Horiguchi-Babamoto, E.; Suzuki, M.; Makihara, H.; Tomozawa, H.; Tsubata, M.; Shimada, T.; Sugiyama, K.; Aburada, M. Effects of ethyl acetate extract of Kaempferia parviflora on brown adipose tissue. J. Nat. Med. 2016, 70, 54–61. [Google Scholar] [CrossRef]
- Chatchawan, U.; Promthep, K.; Eungpinichpong, W.; Sripanidkulchai, B. Effect of Kaempferia parviflora Extract on Physical Fitness of Soccer Players: A Randomized Double-Blind Placebo-Controlled Trial. Med Sci. Monit. Basic Res. 2015, 21, 100–108. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Kwon, D.; Kim, M.-B.; Hwang, J.-K. Standardized Kaempferia parviflora Wall. ex Baker (Zingiberaceae) Extract Inhibits Fat Accumulation and Muscle Atrophy in ob/ob Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, 8161042. [Google Scholar] [CrossRef]
- Haskell, W.L.; Kiernan, M. Methodologic issues in measuring physical activity and physical fitness when evaluating the role of dietary supplements for physically active people. Am. J. Clin. Nutr. 2000, 72, 541S–550S. [Google Scholar] [CrossRef]
- Imamura, H.; Nhat, K.P.H.; Togawa, H.; Saito, K.; Iino, R.; Kato-Yamada, Y.; Nagai, T.; Noji, H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl. Acad. Sci. USA 2009, 106, 15651–15656. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Exercise Metabolism: Fuels for the Fire. Cold Spring Harb. Perspect. Med. 2017, 8, a029744. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.C.; Greenhaff, P.; Constantin-Teodosiu, D.; Saris, W.H.M.; Wagenmakers, A. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Alghannam, A.; Ghaith, M.; Alhussain, M. Regulation of Energy Substrate Metabolism in Endurance Exercise. Int. J. Environ. Res. Public Health 2021, 7, 4963. [Google Scholar] [CrossRef] [PubMed]
- Emhoff, C.-A.W.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Direct and indirect lactate oxidation in trained and untrained men. J. Appl. Physiol. 2013, 115, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Casazza, G.A.; Tovar, A.P.; Richardson, C.E.; Cortez, A.N.; Davis, B.A. Energy Availability, Macronutrient Intake, and Nutritional Supplementation for Improving Exercise Performance in Endurance Athletes. Curr. Sports Med. Rep. 2018, 17, 215–223. [Google Scholar] [CrossRef]
- Narkhede, A.N.; Jagtap, S.; Nirmal, P.S.; Giramkar, S.A.; Nagarkar, B.E.; Kulkarni, O.; Harsulkar, A.M. Anti-fatigue effect of Amarkand on endurance exercise capacity in rats. BMC Complement. Altern. Med. 2016, 16, 23. [Google Scholar] [CrossRef]
- Xia, F.; Zhong, Y.; Li, M.; Chang, Q.; Liao, Y.; Liu, X.; Pan, R. Antioxidant and Anti-Fatigue Constituents of Okra. Nutrients 2015, 7, 8846–8858. [Google Scholar] [CrossRef]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single Dose Administration of Taheebo Polyphenol Enhances Endurance Capacity in Mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- D’unienville, N.M.A.; Blake, H.T.; Coates, A.M.; Hill, A.M.; Nelson, M.J.; Buckley, J.D. Effect of food sources of nitrate, polyphenols, L-arginine and L-citrulline on endurance exercise performance: A systematic review and meta-analysis of randomised controlled trials. J. Int. Soc. Sports Nutr. 2021, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Sripanidkulchai, B.; Promthep, K.; Tuntiyasawasdikul, S.; Tabboon, P.; Areemit, R. Supplementation of Kaempferia parviflora Extract Enhances Physical Fitness and Modulates Parameters of Heart Rate Variability in Adolescent Student-Athletes: A Randomized, Double-Blind, Placebo-Controlled Clinical Study. J. Diet. Suppl. 2020, 19, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hwang, J.-K. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients 2020, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, Bioavailability, Tissue Distribution, Excretion, and Metabolite Identification of Methoxyflavones in Kaempferia parviflora Extract in Rats. Drug Metab. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef]
- Rang, Y.; Ma, S.; Yang, J.; Liu, H.; Suzuki, K.; Liu, C. A Low-Protein High-Fat Diet Leads to Loss of Body Weight and White Adipose Tissue Weight via Enhancing Energy Expenditure in Mice. Metabolites 2021, 11, 301. [Google Scholar] [CrossRef]
- Ma, S.; Yang, J.; Tominaga, T.; Liu, C.; Suzuki, K. A Low-Carbohydrate Ketogenic Diet and Treadmill Training Enhanced Fatty Acid Oxidation Capacity but Did Not Enhance Maximal Exercise Capacity in Mice. Nutrients 2021, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Miyake, J.U.N.; Nishida, Y. Understanding the Characteristics of the Triceps Surae Muscle Contraction and its Effect of Metabolic Control. Rigakuryoho Kagaku 2011, 26, 315–321. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, W.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M.; Zhu, J. Pterostilbene Enhances Endurance Capacity via Promoting Skeletal Muscle Adaptations to Exercise Training in Rats. Molecules 2020, 25, 186. [Google Scholar] [CrossRef]
- Shaw, C.S.; Clark, J.A.; Shepherd, S.O. HSL and ATGL: The movers and shakers of muscle lipolysis. J. Physiol. 2013, 591, 6137–6138. [Google Scholar] [CrossRef]
- Watt, M.J.; Heigenhauser, G.J.F.; Dyck, D.J.; Spriet, L.L. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J. Physiol. 2002, 541, 969–978. [Google Scholar] [CrossRef]
- Wu, S.A.; Kersten, S.; Qi, L. Lipoprotein Lipase and Its Regulators: An Unfolding Story. Trends Endocrinol. Metab. 2021, 32, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, L.; Laviolette, M.; Rodrigue-Way, A.; Sow, B.; Brochu, M.; Caron, V.; Tremblay, A. The CD36-PPARγ Pathway in Metabolic Disorders. Int. J. Mol. Sci. 2018, 1, 1529. [Google Scholar] [CrossRef] [PubMed]
- Bezaire, V.; Bruce, C.R.; Heigenhauser, G.J.F.; Tandon, N.N.; Glatz, J.F.C.; Luiken, J.J.J.F.; Bonen, A.; Spriet, L.L. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: Essential role in fatty acid oxidation. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E509–E515. [Google Scholar] [CrossRef]
- Jeong, J.; Kwon, E.G.; Im, S.K.; Seo, K.S.; Baik, M. Expression of Fat Deposition and Fat Removal Genes Is As-sociated with Intramuscular Fat Content in Longissimus Dorsi Muscle of Korean Cattle Steers. J. Anim. Sci. 2012, 90, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, J.-J.; Zhang, Y.-N.; Limbu, S.M.; Du, Z.-Y.; Qin, J.-G.; Chen, L.-Q. Forskolin reduces fat accumulation in Nile tilapia (Oreochromis niloticus) through stimulating lipolysis and beta-oxidation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 230, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, V.A. Fatty Acid Beta-Oxidation Disorders: A Brief Review. Ann. Neurosci. 2016, 23, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Benton, C.R.; Yoshida, Y.; Lally, J.; Han, X.-X.; Hatta, H.; Bonen, A. PGC-1α increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol. Genom. 2008, 35, 45–54. [Google Scholar] [CrossRef]
- Bonen, A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur. J. Appl. Physiol. 2001, 86, 6–11. [Google Scholar] [CrossRef]
- Wyatt, E.; Wu, R.; Rabeh, W.; Park, H.-W.; Ghanefar, M.; Ardehali, H. Regulation and Cytoprotective Role of Hexokinase III. PLoS ONE 2010, 5, e13823. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Summermatter, S.; Santos, G.; Perez-Schindler, J.; Handschin, C. Skeletal muscle PGC-1alpha controls whole-body lactate homeostasis through estrogen-related receptor alpha-dependent activation of LDH B and repression of LDH A. Proc. Natl. Acad. Sci. USA 2013, 110, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Hayasaki, H.; Shimada, M.; Kanbara, K.; Watanabe, M. Regional Difference in Muscle Fiber Type and Glu-cose Uptake of Mouse Gastrocnemius at Rest. Cell. Mol. Biol. 2001, 47, 135–140. [Google Scholar]
- Grassi, B.; Rossiter, H.B.; Zoladz, J.A.; Rossiter, H.; Zoladz, J. Skeletal Muscle Fatigue and Decreased Efficiency. Two Sides of the Same Coin? Exerc. Sport Sci. Rev. 2015, 43, 75–83. [Google Scholar] [CrossRef]
- Ausina, P.; Da Silva, D.; Majerowicz, D.; Zancan, P.; Sola-Penna, M. Insulin specifically regulates expression of liver and muscle phosphofructokinase isoforms. Biomed. Pharmacother. 2018, 103, 228–233. [Google Scholar] [CrossRef]
- Ranallo, R.F.; Rhodes, E.C.; Buchanan, J.M. Lipid Metabolism During Exercise. Sports Med. 1998, 26, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- Hearris, M.A.; Hammond, K.M.; Fell, J.M.; Morton, J.P. Regulation of Muscle Glycogen Metabolism during Exercise: Implications for Endurance Performance and Training Adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef]
- Katz, A.; Westerblad, H. Regulation of glycogen breakdown and its consequences for skeletal muscle function after training. Mamm. Genome 2014, 25, 464–472. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Betts, J.A. Dietary sugars, exercise and hepatic carbohydrate metabolism. Proc. Nutr. Soc. 2019, 78, 246–256. [Google Scholar] [CrossRef]
- Burgess, S.C.; He, T.; Yan, Z.; Lindner, J.; Sherry, A.D.; Malloy, C.R.; Browning, J.D.; Magnuson, M.A. Cytosolic Phosphoenolpyruvate Carboxykinase Does Not Solely Control the Rate of Hepatic Gluconeogenesis in the Intact Mouse Liver. Cell Metab. 2007, 5, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.G.; Biensø, R.S.; Hassing, H.A.; Jakobsen, A.H.; Pilegaard, H. Exercise-induced regulation of key factors in substrate choice and gluconeogenesis in mouse liver. Mol. Cell. Biochem. 2015, 403, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hoene, M.; Plomgaard, P.; Hansen, J.S.; Zhao, X.; Li, J.; Wang, X.; Clemmesen, J.O.; Secher, N.H.; Häring, H.U.; et al. Muscle-Liver Substrate Fluxes in Exercising Humans and Potential Effects on Hepatic Metabolism. J. Clin. Endocrinol. Metab. 2019, 105, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate Link Between Glycolytic and Oxidative Metabolism. Sports Med. 2007, 37, 341–343. [Google Scholar] [CrossRef]








| Gene Symbol for Primer | Forward | Reverse |
|---|---|---|
| Hk2 | CTGTCTACAAGAAACATCCCCATTT | CACCGCCGTCACCATAGC |
| Slc2a4 (Glut4) | CCGCGGCCTCCTATGAGATACT | AGGCACCCCGAAGATGAGT |
| Mct1 | GTGACCATTGTGGAATGCTG | CTCCGCTTTCTGTTCTTTGG |
| Ldh | ACAGTTGTTGGGTTGGTG | CCGGCTCTGCCCTCTTG |
| Cd36 | TGGCCTTACTTGGGATTGG | CCAGTGTATATGTAGGCTCATCCA |
| Cpt1β | CCCATGTGCTCCTACCAGAT | CCTTGAAGAAGCGACCTTTG |
| Cpt2 | GAAGAAGCTGAGCCCTGATG | GCCATGGTATTTGGAGCACT |
| Lpl | GAGAAGCCATCCGTGTGATT | TATGCTTTGCTGGGGTTTTC |
| Pnpla2 (Atgl) | GAGCCCCGGGGTGGAACAAGAT | AAAAGGTGGTGGGCAGGAGTAAGG |
| Aco | TGTTAAGAAGAGTGCCACCA | ATCCATCTCTTCATAACCAAATTT |
| Acadm (Mcad) | GCTCGTGAGCACATTGAAAA | CATTGTCCAAAAGCCAAACC |
| Hadh | ACTACATCAAAATGGGCTCTCAG | AGCAGAAATGGAATGCGGACC |
| Acacb (Acc2) | GGGCTCCCTGGATGACAAC | TTCCGGGAGGAGTTCTGGA |
| Mlycd (Mcd) | ACTCCATCAGCCTGACCCAG | ACCCCTTGAGGCTCTCGTGA |
| Pparg | GATGGAAGACCACTCGCATT | AACCATTGGGTCAGCTCTTG |
| Ppargc1a(Pgc-1α) | GACTGGAGGAAGACTAAACGGCCA | GCCAGTCACAGGAGGCATCTTT |
| Tfam | CCAAAAAGACCTCGTTCAGC | CTTCAGCCATCTGCTCTTCC |
| Gp | TGGCAGAAGTGGTGAACAATGAC | CCGTGGAGATCTGCTCCGATA |
| Gs | ACTGCTTGGGCGTTATCTCTGTG | ATGCCCGCTCCATGCGTA |
| Pfkm | GGAGTGCGTGCAGGTGACCAAA | ATCACGGCCACTGTGTGCAACC |
| Pck1 (Pepck) | CACCATCACCTCCTGGAAGA | GGGTGCAGAATCTCGAGTTG |
| G6pc (G6pase) | GTGGCAGTGGTCGGAGACT | ACGGGCGTTGTCCAAAC |
| Cs | GCATGAAGGGACTTGTGTA | TCTGGCACTCAGGGATACT |
| Pfkl | CATGAATGCAGCTGTGCGCTCC | CCAGCCCACTTCTTGCACCTGA |
| 18s | CGGCTACCACATCCAAGGA | AGCTGGAATTACCGCGGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Tagawa, T.; Ma, S.; Suzuki, K. Black Ginger (Kaempferia parviflora) Extract Enhances Endurance Capacity by Improving Energy Metabolism and Substrate Utilization in Mice. Nutrients 2022, 14, 3845. https://doi.org/10.3390/nu14183845
Huang J, Tagawa T, Ma S, Suzuki K. Black Ginger (Kaempferia parviflora) Extract Enhances Endurance Capacity by Improving Energy Metabolism and Substrate Utilization in Mice. Nutrients. 2022; 14(18):3845. https://doi.org/10.3390/nu14183845
Chicago/Turabian StyleHuang, Jiapeng, Takashi Tagawa, Sihui Ma, and Katsuhiko Suzuki. 2022. "Black Ginger (Kaempferia parviflora) Extract Enhances Endurance Capacity by Improving Energy Metabolism and Substrate Utilization in Mice" Nutrients 14, no. 18: 3845. https://doi.org/10.3390/nu14183845
APA StyleHuang, J., Tagawa, T., Ma, S., & Suzuki, K. (2022). Black Ginger (Kaempferia parviflora) Extract Enhances Endurance Capacity by Improving Energy Metabolism and Substrate Utilization in Mice. Nutrients, 14(18), 3845. https://doi.org/10.3390/nu14183845










