Unravelling the Therapeutic Potential of Nano-Delivered Functional Foods in Chronic Respiratory Diseases
Abstract
1. Introduction
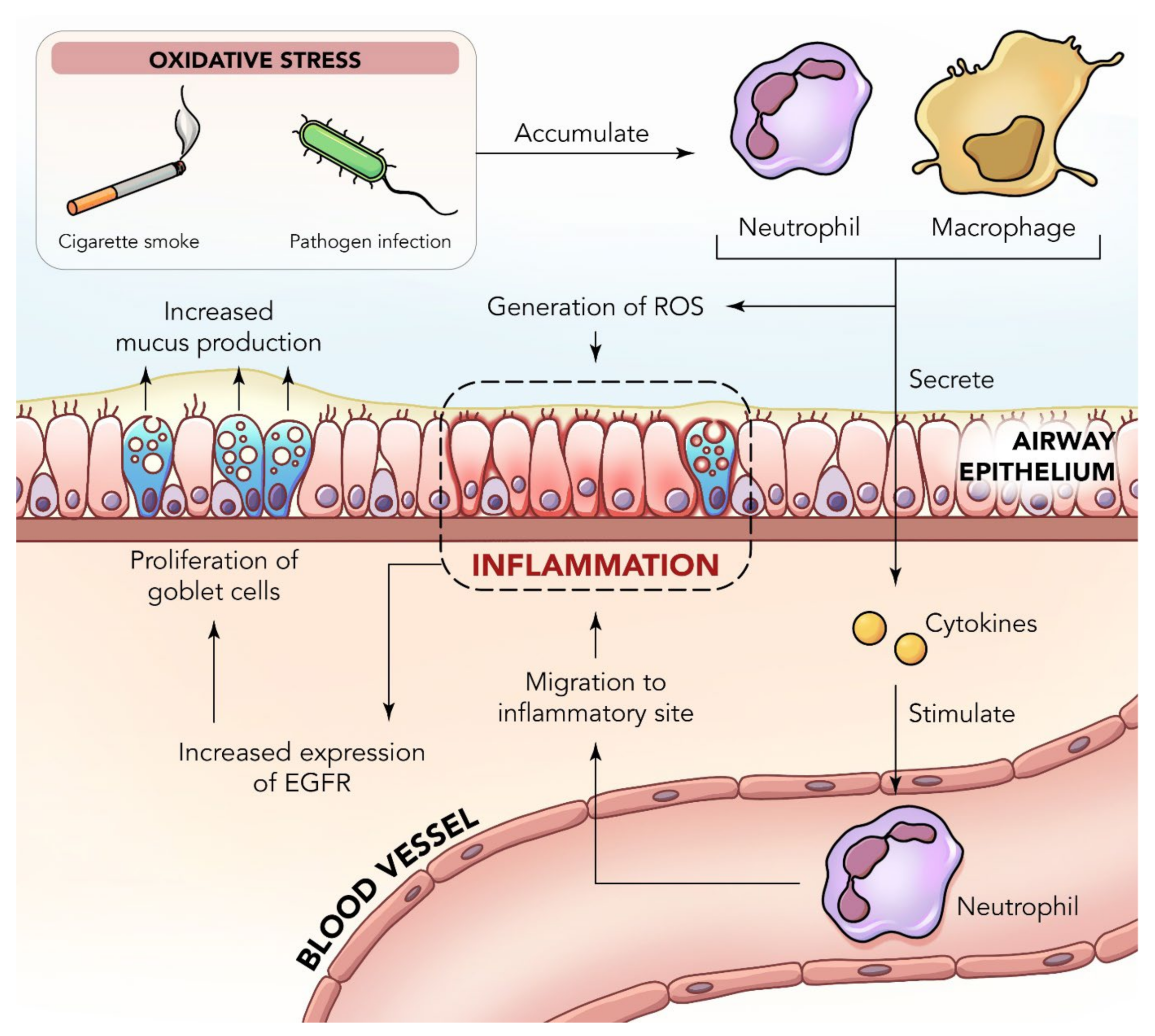
2. Inflammation in the Lungs
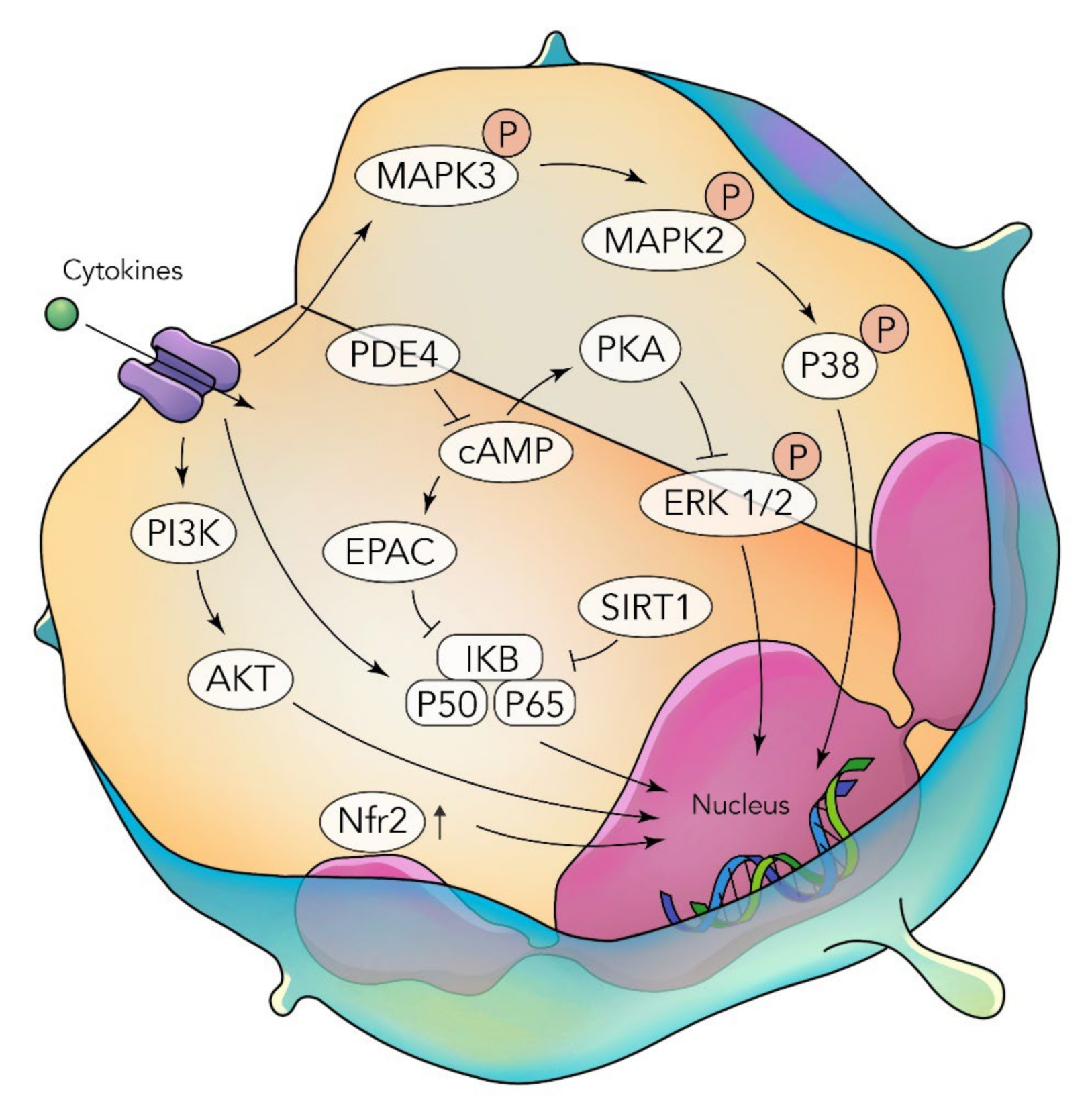
3. Functional Foods in the Management of Inflammatory Lung Disease
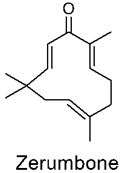
3.1. Zerumbone
Zerumbone Delivery Using Nanoparticles
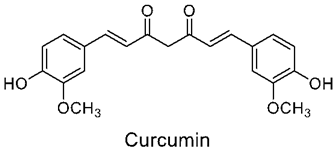
3.2. Curcumin
Curcumin Delivery Using Nanoparticles
3.3. Cinnamaldehyde
Cinnamaldehyde Delivery Using Nanoparticles
3.4. Eugenol
Eugenol Delivery Using Nanoparticles
3.5. Naringenin
Naringenin Delivery Using Nanoparticles
3.6. Capsaicin
Capsaicin Delivery Using Nanoparticles
3.7. Boswellic Acids
Boswellic Acid Delivery Using Nanoparticles
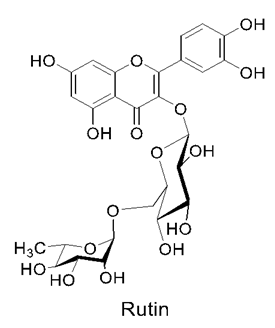
3.8. Rutin
Rutin Delivery Using Nanoparticles
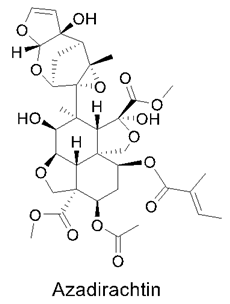
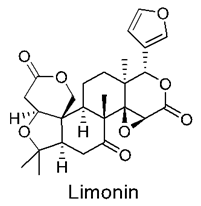
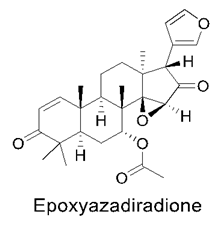
3.9. Neem Compounds
Neem Compound Delivery Using Nanoparticles
4. Clinical Trials on Nanotechnology-Based Drug Delivery Containing Functional Foods
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, J.W.E.; Williams, J.O.; Ramji, D.P. Nutraceuticals as therapeutic agents for atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt A, 1562–1572. [Google Scholar] [CrossRef]
- Hwang, Y.Y.; Ho, Y.S. Nutraceutical support for respiratory diseases. Food Sci. Hum. Wellness 2018, 7, 205–208. [Google Scholar] [CrossRef]
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kesharwani, S.S.; Mathur, H.; Tyagi, M.; Bhat, G.J.; Tummala, H. Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. Eur. J. Pharm. Sci. 2016, 82, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Dachineni, R.; Bhat, G.J.; Tummala, H. Hydrophobically modified inulin-based micelles: Transport mechanisms and drug delivery applications for breast cancer. J. Drug Deliv. Sci. Technol. 2019, 54, 101254. [Google Scholar] [CrossRef]
- Júlio, A.; Costa Lima, S.A.; Reis, S.; Santos de Almeida, T.; Fonte, P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J. Drug Deliv. Sci. Technol. 2020, 56, 100915. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050. [Google Scholar] [CrossRef]
- British Standards Institution. Available online: https://www.safenano.org/knowledgebase/standards/british-standards-institution/ (accessed on 26 June 2022).
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nanomicro Lett. 2020, 12, 45. [Google Scholar] [CrossRef]
- Chan, Y.; Prasher, P.; Löbenberg, R.; Gupta, G.; Singh, S.K.; Oliver, B.G.; Chellappan, D.K.; Dua, K. Applications and practice of advanced drug delivery systems for targeting Toll-like receptors in pulmonary diseases. Nanomedicine 2021, 16, 783–786. [Google Scholar] [CrossRef]
- McKeever, T.M.; Lewis, S.A.; Smit, H.A.; Burney, P.; Cassano, P.A.; Britton, J. A multivariate analysis of serum nutrient levels and lung function. Respir. Res. 2008, 9, 67. [Google Scholar] [CrossRef]
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death/ (accessed on 2 July 2022).
- Eapen, M.S.; Myers, S.; Walters, E.H.; Sohal, S.S. Airway inflammation in chronic obstructive pulmonary disease (COPD): A true paradox. Expert Rev. Respir. Med. 2017, 11, 827–839. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, E.; Xiao, M.; Chen, C.; Xu, W. Study of anti-inflammatory activities of α-D-glucosylated eugenol. Arch. Pharm. Res. 2013, 36, 109–115. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Paudel, K.R.; Satija, S.; Chellappan, D.K.; Dureja, H.; Gupta, G.; Tambuwala, M.M.; Negi, P.; et al. Plants derived therapeutic strategies targeting chronic respiratory diseases: Chemical and immunological perspective. Chem. Biol. Interact. 2020, 325, 109125. [Google Scholar] [CrossRef]
- Shukla, S.D.; Vanka, K.S.; Chavelier, A.; Shastri, M.D.; Tambuwala, M.M.; Bakshi, H.A.; Pabreja, K.; Mahmood, M.Q.; O’toole, R.F. Chronic respiratory diseases: An introduction and need for novel drug delivery approaches. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 1–31. [Google Scholar]
- Chronic Respiratory Diseases. Available online: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1 (accessed on 30 June 2022).
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11 (Suppl. S17), S2129–S2140. [Google Scholar] [CrossRef]
- Lugg, S.T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D.R. Cigarette smoke exposure and alveolar macrophages: Mechanisms for lung disease. Thorax 2021, 77, 94–101. [Google Scholar] [CrossRef]
- Duncan, D. Chronic obstructive pulmonary disease: An overview. Br. J. Nurs. 2016, 25, 360–366. [Google Scholar] [CrossRef]
- Hatipoglu, U. Chronic obstructive pulmonary disease: More than meets the eye. Ann. Thorac. Med. 2018, 13, 1–6. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Raja, A.; Brown, B.D. Chronic Obstructive Pulmonary Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559281/ (accessed on 30 July 2022).
- Tamimi, A.; Serdarevic, D.; Hanania, N.A. The effects of cigarette smoke on airway inflammation in asthma and COPD: Therapeutic implications. Respir. Med. 2012, 106, 319–328. [Google Scholar] [CrossRef]
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 26. [Google Scholar] [CrossRef]
- Zhou, H.X.; Ou, X.M.; Tang, Y.J.; Wang, L.; Feng, Y.L. Advanced Chronic Obstructive Pulmonary Disease: Innovative and Integrated Management Approaches. Chin. Med. J. 2015, 128, 2952. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.J. Regulation of Chemokine–Receptor Interactions and Functions. Int. J. Mol. Sci. 2017, 18, 2415. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.P.R.; Pinheiro, N.M.; Mernak, M.I.B.; Righetti, R.F.; Martins, M.A.; Lago, J.H.; Lopes, F.D.; Tibério, I.F.; Prado, C.M. Evidences of Herbal Medicine-Derived Natural Products Effects in Inflammatory Lung Diseases. Mediat. Inflamm. 2016, 2016, 2348968. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Da Silveira ESá, R.; Andrade, L.N.; De Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Dusio, G.F.; Gasheva, O.Y.; Skoog, H.; Tobin, R.; Peddaboina, C.; Meininger, C.J.; Zawieja, D.C.; Newell-Rogers, M.K.; Gashev, A.A. Mast cells and histamine are triggering the NF-κB-mediated reactions of adult and aged perilymphatic mesenteric tissues to acute inflammation. Aging 2016, 8, 3065. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Pavlisko, E.; Voynow, J.A. Pathogenic triad in COPD: Oxidative stress, protease-antiprotease imbalance, and inflammation. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 413–421. [Google Scholar] [CrossRef]
- Bodas, M.; Patel, N.; Silverberg, D.; Walworth, K.; Vij, N. Master Autophagy Regulator Transcription Factor EB Regulates Cigarette Smoke-Induced Autophagy Impairment and Chronic Obstructive Pulmonary Disease-Emphysema Pathogenesis. Antioxid. Redox Signal. 2017, 27, 150–167. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Huang, L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node. Mol. Ther. 2017, 25, 1665–1675. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Mubarak, R.A.; Roberts, N.; Mason, R.J.; Alper, S.; Chu, H.W. Comparison of pro- and anti-inflammatory responses in paired human primary airway epithelial cells and alveolar macrophages. Respir. Res. 2018, 19, 126. [Google Scholar] [CrossRef]
- Rovina, N.; Koutsoukou, A.; Koulouris, N.G. Inflammation and immune response in COPD: Where do we stand? Mediat. Inflamm. 2013, 2013, 413735. [Google Scholar] [CrossRef]
- Bulugonda, R.K.; Gangappa, D.; Beeda, H.; Philip, G.H.; Muralidhara Rao, D.; Faisal, S.M. Mangiferin from Pueraria tuberosa reduces inflammation via inactivation of NLRP3 inflammasome. Sci. Rep. 2017, 7, 42683. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.; Korashy, H.M.; Sayed-Ahmed, M.M.; Attia, S.M.; Shabanah, O.A.; Ahmad, S.F. Dexamethasone Attenuates LPS-induced Acute Lung Injury through Inhibition of NF-κB, COX-2, and Pro-inflammatory Mediators. Immunol. Investig. 2016, 45, 349–369. [Google Scholar] [CrossRef]
- Cheng, J.; Dackor, R.T.; Bradbury, J.A.; Li, H.; DeGraff, L.M.; Hong, L.K.; King, D.; Lih, F.B.; Gruzdev, A.; Edin, M.L.; et al. Contribution of alveolar type II cell-derived cyclooxygenase-2 to basal airway function, lung inflammation, and lung fibrosis. FASEB J. 2016, 30, 160–173. [Google Scholar] [CrossRef]
- Su, B.; Liu, T.; Fan, H.; Chen, F.; Ding, H.; Wu, Z.; Wang, H.; Hou, S. Inflammatory Markers and the Risk of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150586. [Google Scholar] [CrossRef]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory Action of Curcumin and Its Use in the Treatment of Lifestyle-related Diseases. Eur. Cardiol. Rev. 2019, 14, 117. [Google Scholar] [CrossRef]
- Shastri, M.D.; Allam, V.S.R.R.; Shukla, S.D.; Jha, N.K.; Paudel, K.R.; Peterson, G.M.; Patel, R.P.; Hansbro, P.M.; Chellappan, D.K.; Dua, K. Interleukin-13: A pivotal target against influenza-induced exacerbation of chronic lung diseases. Life Sci. 2021, 283, 119871. [Google Scholar] [CrossRef]
- Lin, B.F.; Chiang, B.L.; Ma, Y.; Lin, J.Y.; Chen, M.L. Traditional herbal medicine and allergic asthma. Evid.-Based Complement. Altern. Med. 2015, 2015, 510989. [Google Scholar] [CrossRef]
- Liu, F.; Xuan, N.X.; Ying, S.M.; Li, W.; Chen, Z.H.; Shen, H.H. Herbal Medicines for Asthmatic Inflammation: From Basic Researches to Clinical Applications. Mediat. Inflamm. 2016, 2016, 6943135. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Bansal, P.; Jialal, I. Chronic Inflammation. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; StatPearls Publishing: Treasure Island, FL, USA, 2020; pp. 300–314. [Google Scholar]
- Durham, A.L.; Caramori, G.; Chung, K.F.; Adcock, I.M. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl. Res. 2016, 167, 192–203. [Google Scholar] [CrossRef]
- Heffler, E.; Madeira, L.N.G.; Ferrando, M.; Puggioni, F.; Racca, F.; Malvezzi, L.; Passalacqua, G.; Canonica, G.W. Inhaled Corticosteroids Safety and Adverse Effects in Patients with Asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 776–781. [Google Scholar] [CrossRef]
- Clarke, R.; Lundy, F.T.; McGarvey, L. Herbal treatment in asthma and COPD—Current evidence. Clin. Phytoscience 2015, 1, 4. [Google Scholar] [CrossRef]
- Ram, A.; Balachandar, S.; Vijayananth, P.; Singh, V.P. Medicinal plants useful for treating chronic obstructive pulmonary disease (COPD): Current status and future perspectives. Fitoterapia 2011, 82, 141–151. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef]
- Chanda, S.; Tiwari, R.K.; Kumar, A.; Singh, K. Nutraceuticals inspiring the current therapy for lifestyle diseases. Adv. Pharmacol. Sci. 2019, 2019, 6908716. [Google Scholar] [CrossRef]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210. [Google Scholar] [CrossRef]
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013, 4, 4–8. [Google Scholar]
- Varzakas, T.; Kandylis, P.; Dimitrellou, D.; Salamoura, C.; Zakynthinos, G.; Proestos, C. Innovative and fortified food: Probiotics, prebiotics, GMOs, and superfood. In Preparation and Processing of Religious and Cultural Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–129. [Google Scholar]
- Berthon, B.S.; Wood, L.G. Nutrition and Respiratory Health—Feature Review. Nutrients 2015, 7, 1618–1643. [Google Scholar] [CrossRef]
- Nurmatov, U.; Devereux, G.; Sheikh, A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011, 127, 724–733.e30. [Google Scholar] [CrossRef]
- Madl, A.K.; Plummer, L.E.; Carosino, C.; Pinkerton, K.E. Nanoparticles, Lung Injury, and the Role of Oxidant Stress. Annu. Rev. Physiol. 2014, 76, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mendoza, M.E.; Castillo-Henkel, C.; Navarrete, A. Relaxant action mechanism of berberine identified as the active principle of Argemone ochroleuca Sweet in guinea-pig tracheal smooth muscle. J. Pharm. Pharmacol. 2008, 60, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Shang, J.H.; Pu, S.B.; Wang, H.S.; Wang, B.; Liu, L.; Liu, Y.P.; Hong-Mei, S.; Luo, X.D. of total alkaloids from Alstonia scholaris on airway inflammation in rats. J. Ethnopharmacol. 2016, 178, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Henry, K.C.; Abrahamsson, T.R.; Wu, R.Y.; Sherman, P.M. Probiotics, Prebiotics, and Synbiotics for the Prevention of Necrotizing Enterocolitis. Adv. Nutr. 2016, 7, 928–937. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Y.; Zhang, W.; Chen, X. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem. Cell Biol. 2018, 96, 742–751. [Google Scholar] [CrossRef]
- Veith, C.; Drent, M.; Bast, A.; van Schooten, F.J.; Boots, A.W. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol. Appl. Pharmacol. 2017, 336, 40–48. [Google Scholar] [CrossRef]
- Md, S.; Kit, B.C.M.; Jagdish, S.; David, D.J.P.; Pandey, M.; Chatterjee, L.A. Development and in vitro evaluation of a zerumbone loaded nanosuspension drug delivery system. Crystals 2018, 8, 286. [Google Scholar] [CrossRef]
- Albaayit, S.F.A.; Rasedee, A.; Abdullah, N. Zerumbone-Loaded Nanostructured Lipid Carrier Gel Facilitates Wound Healing in Rats. Rev. Bras. Farmacogn. 2020, 30, 272–278. [Google Scholar] [CrossRef]
- Haque, M.A.; Jantan, I.; Harikrishnan, H. Zerumbone suppresses the activation of inflammatory mediators in LPS-stimulated U937 macrophages through MyD88-dependent NF-κB/MAPK/PI3K-Akt signaling pathways. Int. Immunopharmacol. 2018, 55, 312–322. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Galván, G.C.; Johnson, C.B.; Price, R.S.; Liss, M.A.; Jolly, C.A.; deGraffenried, L.A. Effects of Obesity on the Regulation of Macrophage Population in the Prostate Tumor Microenvironment. Nutr. Cancer 2017, 69, 996–1002. [Google Scholar] [CrossRef]
- Ho, Y.C.; Lee, S.S.; Yang, M.L.; Huang-Liu, R.; Lee, C.Y.; Li, Y.C.; Kuan, Y.H. Zerumbone reduced the inflammatory response of acute lung injury in endotoxin-treated mice via Akt-NFκB pathway. Chem. Biol. Interact. 2017, 271, 9–14. [Google Scholar] [CrossRef]
- Foong, J.N.; Selvarajah, G.T.; Rasedee, A.; Rahman, H.S.; How, C.W.; Beh, C.Y.; Teo, G.Y.; Ku, C.L. Zerumbone-loaded nanostructured lipid carrier induces apoptosis of canine mammary adenocarcinoma cells. BioMed Res. Int. 2018, 2018, 8691569. [Google Scholar] [CrossRef]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am. J. Physiol.-Ren. Physiol. 2005, 288, 605–613. [Google Scholar] [CrossRef]
- Fang, Y.; Lin, Y.; Su, Y.; Fang, J. Tryptanthrin-loaded nanoparticles for delivery into cultured human breast cancer cells, MCF7: The effects of solid lipid/liquid lipid ratios in the inner core. Chem. Pharm. Bull. 2011, 59, 266–271. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Saeed, M.I.; Yeap, S.K. Zerumbone-loaded nanostructured lipid carriers: Preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013, 8, 2769–2781. [Google Scholar] [CrossRef]
- Nathaniel, C.; Elaine-Lee, Y.L.; Yee, B.C.; How, C.W.; Yim, H.S.; Rasadee, A.; Ng, H.S. Zerumbone-loaded nanostructured lipid carrier induces apoptosis in human colorectal adenocarcinoma (caco-2) cell line. Nanosci. Nanotechnol. Lett. 2016, 8, 294–302. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, S.-W.; Quan, H.; Jeong, C.-W.; Choi, J.-I.; Bae, H.-B. Effect of curcumin (Curcuma longa extract) on LPS-induced acute lung injury is mediated by the activation of AMPK. J. Anesth. 2016, 30, 100–108. [Google Scholar] [CrossRef]
- Xu, J.M.; Ding, H.R.; Li, Z.X.; Wang, H.J. Relationship between Autophagy and Curcumin-induced Anticancer Effect. Zhongguo Yi Xue Ke Xue Yuan Xue Bao Acta Acad. Med. Sin. 2018, 40, 568–572. [Google Scholar]
- Wong, R.; Li, J.; Zhao, Y.; Li, Y.; Tin, L. Investigating the therapeutic potential and mechanism of curcumin in breast cancer based on RNA sequencing and bioinformatics analysis. Breast Cancer 2018, 25, 206–212. [Google Scholar] [CrossRef]
- Bakır, B.; Yetkin Ay, Z.; Büyükbayram, H.İ.; Kumbul Doğuç, D.; Bayram, D.; Candan, İ.A.; Uskun, E. Effect of Curcumin on Systemic T Helper 17 Cell Response; Gingival Expressions of Interleukin-17 and Retinoic Acid Receptor-Related Orphan Receptor γt; and Alveolar Bone Loss in Experimental Periodontitis. J. Periodontol. 2016, 87, e183–e191. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Simental-Mendía, L.E.; Bo, S.; Sahebkar, A. Effect of curcumin on circulating interleukin-6 concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 111, 394–404. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Chen, G.; Wang, D.; Tang, S.; Deng, H.; Wang, J.; Li, S.; Lan, J.; Tong, J.; et al. Curcumin Attenuates Asthmatic Airway Inflammation and Mucus Hypersecretion Involving a PPARγ-Dependent NF-κB Signaling Pathway In Vivo and In Vitro. Mediat. Inflamm. 2019, 2019, 4927430. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2017, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Karimian, M.S.; Pirro, M.; Johnston, T.P.; Majeed, M.; Sahebkar, A. Curcumin and Endothelial Function: Evidence and Mechanisms of Protective Effects. Curr. Pharm. Des. 2017, 23, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xie, Y.; Yan, R.; Shan, H.; Tang, J.; Cai, Y.; Yin, J.; Chen, M.; Zhang, J.; Yang, X.; et al. Curcumin ameliorates alveolar epithelial injury in a rat model of chronic obstructive pulmonary disease. Life Sci. 2016, 164, 1–8. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang J woong Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Papaioannou, A.I.; Papaporfyriou, A.; Baker, J.R.; Vuppusetty, C.; Loukides, S.; Barnes, P.J.; Ito, K. Decreased Serum Sirtuin-1 in COPD. Chest 2017, 152, 343–352. [Google Scholar] [CrossRef]
- Di Vincenzo, S.; Heijink, I.H.; Noordhoek, J.A.; Cipollina, C.; Siena, L.; Bruno, A.; Ferraro, M.; Postma, D.S.; Gjomarkaj, M.; Pace, E. SIRT1/FoxO3 axis alteration leads to aberrant immune responses in bronchial epithelial cells. J. Cell. Mol. Med. 2018, 22, 2272–2282. [Google Scholar] [CrossRef]
- Guo, R.; Liu, W.; Liu, B.; Zhang, B.; Li, W.; Xu, Y. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: An insight into endoplasmic reticulum stress response mechanism. Int. J. Cardiol. 2015, 191, 36–45. [Google Scholar] [CrossRef]
- Tang, F.; Ling, C. Curcumin ameliorates chronic obstructive pulmonary disease by modulating autophagy and endoplasmic reticulum stress through regulation of SIRT1 in a rat model. J. Int. Med. Res. 2019, 47, 4764–4774. [Google Scholar] [CrossRef]
- Le, K.M.; Trinh, N.T.; Nguyen, V.D.X.; Van Nguyen, T.D.; Thi Nguyen, T.H.; Van Vo, T.; Tran, T.Q.; Ngo, D.N.; Vong, L.B. Investigating the Anti-Inflammatory Activity of Curcumin-Loaded Silica-Containing Redox Nanoparticles. J. Nanomater. 2021, 2021, 6655375. [Google Scholar] [CrossRef]
- Hanci, D.; Altun, H.; Çetinkaya, E.A.; Muluk, N.B.; Cengiz, B.P.; Cingi, C. Cinnamaldehyde is an effective anti-inflammatory agent for treatment of allergic rhinitis in a rat model. Int. J. Pediatric Otorhinolaryngol. 2016, 84, 81–87. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.M. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011, 51, 547–562. [Google Scholar] [CrossRef]
- Lee, S.C.; Wang, S.Y.; Li, C.C.; Liu, C.T. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal. 2018, 26, 211–220. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, L.; Fan, S.; Wang, J.; Luo, T.; Tang, Y.; Chen, Z.; Yu, L. Cinnamomum cassia Presl: A review of its traditional uses, phytochemistry, pharmacology and toxicology. Molecules 2019, 24, 3473. [Google Scholar] [CrossRef]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 429320. [Google Scholar] [CrossRef]
- Wang, F.; Pu, C.; Zhou, P.; Wang, P.; Liang, D.; Wang, Q.; Hu, Y.; Li, B.; Hao, X. Cinnamaldehyde prevents endothelial dysfunction induced by high glucose by activating Nrf2. Cell. Physiol. Biochem. 2015, 36, 315–324. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, P.; Zhao, Y.; Zhang, L.; Zhang, Z.; Dong, X.; Wu, Z.; Xu, Y.; Chen, Y. Trans-Cinnamaldehyde Inhibits Microglial Activation and Improves Neuronal Survival against Neuroinflammation in BV2 Microglial Cells with Lipopolysaccharide Stimulation. Evid.-Based Complement. Altern. Med. 2017, 2017, 4730878. [Google Scholar] [CrossRef]
- Chung, J.; Kim, S.; Lee, H.A.; Park, M.H.; Kim, S.; Song, Y.R.; Na, H.S. Trans-cinnamic aldehyde inhibits Aggregatibacter actinomycetemcomitans-induced inflammation in THP-1-derived macrophages via autophagy activation. J. Periodontol. 2018, 89, 1262–1271. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Fu, Y.; Yang, P.; Qin, Z.; Chen, Y.; Xu, Y. Trans-cinnamaldehyde improves memory impairment by blocking microglial activation through the destabilization of iNOS mRNA in mice challenged with lipopolysaccharide. Neuropharmacology 2016, 110 Pt A, 503–518. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Dong, L.; Wen, Y.; Zheng, X.; Zhang, C.; Chen, R.; Zhang, Y.; Li, Y.; He, T.; et al. Cinnamaldehyde inhibits inflammation and brain damage in a mouse model of permanent cerebral ischaemia. Br. J. Pharmacol. 2015, 172, 5009–5023. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Schink, A.; Naumoska, K.; Kitanovski, Z.; Kampf, C.J.; Fröhlich-Nowoisky, J.; Thines, E.; Pöschl, U.; Schuppan, D.; Lucas, K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018, 9, 5950–5964. [Google Scholar] [CrossRef]
- Kot, B.; Wicha, J.; Piechota, M.; Wolska, K.; Grużewska, A. Antibiofilm activity of trans-cinnamaldehyde, p-coumaric, and ferulic acids on uropathogenic Escherichia coli. Turk. J. Med. Sci. 2015, 45, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.R.; Narayanan, A.; Baskaran, S.A.; Venkitanarayanan, K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J. Urol. 2010, 184, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Camacho, S.; Michlig, S.; de Senarclens-Bezençon, C.; Meylan, J.; Meystre, J.; Pezzoli, M.; Markram, H.; Le Coutre, J. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci. Rep. 2015, 5, 7919. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Xiong, J.J.; Jiang, H.; Zhang, C.; Wen, Y.E. The antimicrobial activities of the cinnamaldehyde adducts with amino acids. Int. J. Food Microbiol. 2011, 150, 164–170. [Google Scholar] [CrossRef]
- Gunawardena, D.; Karunaweera, N.; Lee, S.; van Der Kooy, F.; Harman, D.G.; Raju, R.; Bennett, L.; Gyengesi, E.; Sucher, N.J.; Münch, G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts—Identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015, 6, 910–919. [Google Scholar] [CrossRef]
- Xia, T.; Gao, R.; Zhou, G.; Liu, J.; Li, J.; Shen, J. Trans-cinnamaldehyde inhibits IL-1β-stimulated inflammation in chondrocytes by suppressing NF-κB and p38-JNK pathways and exerts chondrocyte protective effects in a rat model of osteoarthritis. BioMed Res. Int. 2019, 2019, 4039472. [Google Scholar] [CrossRef]
- Hosni, A.A.; Abdel-Moneim, A.A.; Abdel-Raheim, E.S.; Mohamed, S.M.; Helmy, H. Cinnamaldehyde potentially attenuates gestational hyperglycemia in rats through modulation of PPARγ, proinflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2017, 88, 52–60. [Google Scholar] [CrossRef]
- Kim, M.E.; Na, J.Y.; Lee, J.S. Anti-inflammatory effects of trans-cinnamaldehyde on lipopolysaccharide-stimulated macrophage activation via MAPKs pathway regulation. Immunopharmacol. Immunotoxicol. 2018, 40, 219–224. [Google Scholar] [CrossRef]
- Yuan, J.H.; Dieter, M.P.; Bucher, J.R.; Jameson, C.W. Toxicokinetics of cinnamaldehyde in F344 rats. Food Chem. Toxicol. 1992, 30, 997–1004. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Muhoza, B.; Duhoranimana, E.; Xia, S.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X. Fabrication of low environment-sensitive nanoparticles for cinnamaldehyde encapsulation by heat-induced gelation method. Food Hydrocoll. 2020, 105, 105789. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Suwalka, S.; Yogi, M.R.; Ali, W.; Das, A.; Alagirusamy, R. Green synthesis of chitosan-cinnamaldehyde cross-linked nanoparticles: Characterization and antibacterial activity. Carbohydr. Polym. 2019, 226, 115298. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Shibamoto, T. Antioxidant Properties of Aroma Compounds Isolated from Soybeans and Mung Beans. J. Agric. Food Chem. 2000, 48, 4290–4293. [Google Scholar] [CrossRef] [PubMed]
- García-Ramón, J.A.; Carmona-García, R.; Valera-Zaragoza, M.; Aparicio-Saguilán, A.; Bello-Pérez, L.A.; Aguirre-Cruz, A.; Alvarez-Ramirez, J. Morphological, barrier, and mechanical properties of banana starch films reinforced with cellulose nanoparticles from plantain rachis. Int. J. Biol. Macromol. 2021, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Chami, N.; Bennis, S.; Chami, F.; Aboussekhra, A.; Remmal, A. Study of anticandidal activity of carvacrol and eugenol in vitro and in vivo. Oral. Microbiol. Immunol. 2005, 20, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Nurzyñska-Wierdak, R. Morphological Variability and Essential Oil Composition of four Ocimum basilicum L. cultivars. J. Essent. Oil Bear. Plants 2014, 17, 112–119. [Google Scholar] [CrossRef]
- Hartanti, L.; Yonas, S.M.K.; Mustamu, J.J.; Wijaya, S.; Setiawan, H.K.; Soegianto, L. Influence of extraction methods of bay leaves (Syzygium polyanthum) on antioxidant and HMG-CoA Reductase inhibitory activity. Heliyon 2019, 5, e01485. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Bhattacharjee, P. Use of eugenol-lean clove extract as a flavoring agent and natural antioxidant in mayonnaise: Product characterization and storage study. J. Food Sci. Technol. 2015, 52, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Omidi, M.; Bushehri, A.A.S.; Golshani, A.; Smith, M.L. The Antifungal Eugenol Perturbs Dual Aromatic and Branched-Chain Amino Acid Permeases in the Cytoplasmic Membrane of Yeast. PLoS ONE 2013, 8, e76028. [Google Scholar] [CrossRef]
- Dai, J.P.; Zhao, X.F.; Zeng, J.; Wan, Q.Y.; Yang, J.C.; Li, W.Z.; Chen, X.X.; Wang, G.F.; Li, K.S. Drug screening for autophagy inhibitors based on the dissociation of Beclin1-Bcl2 complex using BiFC technique and mechanism of eugenol on anti-influenza A virus activity. PLoS ONE 2013, 8, e61026. [Google Scholar] [CrossRef]
- Taher, Y.A.; Samud, A.M.; El-Taher, F.E.; Ben-Hussin, G.; Elmezogi, J.S.; Al-Mehdawi, B.F.; Salem, H.A. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J. Med. 2015, 10, 28685. [Google Scholar] [CrossRef]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, P.; Cañigueral, S. Biological and Nonbiological Antioxidant Activity of Some Essential Oils. J. Agric. Food Chem. 2016, 64, 4716–4724. [Google Scholar] [CrossRef]
- Hamed, S.F.; Sadek, Z.; Edris, A. Antioxidant and Antimicrobial Activities of Clove Bud Essential Oil and Eugenol Nanoparticles in Alcohol-Free Microemulsion. J. Oleo Sci. 2012, 61, 641–648. [Google Scholar] [CrossRef]
- Kim, S.S.; Oh, O.J.; Min, H.Y.; Park, E.J.; Kim, Y.; Park, H.J.; Han, Y.N.; Lee, S.K. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003, 73, 337–348. [Google Scholar] [CrossRef]
- Dervis, E.; Yurt Kilcar, A.; Medine, E.I.; Tekin, V.; Cetkin, B.; Uygur, E.; Muftuler, F.Z.B. In Vitro Incorporation of Radioiodinated Eugenol on Adenocarcinoma Cell Lines (Caco2, MCF7, and PC3). Radiopharmaceuticals 2017, 32, 75–81. Available online: https://home.liebertpub.com/cbr (accessed on 22 April 2022).
- Baldisserotto, B.; Parodi, T.V.; Stevens, E.D. Lack of postexposure analgesic efficacy of low concentrations of eugenol in zebrafish. Vet. Anaesth. Analg. 2018, 45, 48–56. [Google Scholar] [CrossRef]
- Li, Y.; She, Q.; Han, Z.; Sun, N.; Liu, X.; Li, X. Anaesthetic Effects of Eugenol on Grass Shrimp (Palaemonetes sinensis) of Different Sizes at Different Concentrations and Temperatures. Sci. Rep. 2018, 8, 11007. [Google Scholar] [CrossRef]
- De Araújo Lopes, A.; da Fonseca, F.N.; Rocha, T.M.; de Freitas, L.B.; Araújo, E.V.O.; Wong, D.V.T.; Lima Júnior, R.C.P.; Leal, L.K.A.M. Eugenol as a Promising Molecule for the Treatment of Dermatitis: Antioxidant and Anti-inflammatory Activities and Its Nanoformulation. Oxidative Med. Cell. Longev. 2018, 2018, 8194849. [Google Scholar] [CrossRef]
- Piper, K.; Garelnabi, M. Eicosanoids: Atherosclerosis and cardiometabolic health. J. Clin. Transl. Endocrinol. 2020, 19, 100216. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.C.; Li, X.; Abbas, M.; Wu, S.Y.; Ren, S.Z.; Liu, Q.X.; Liu, Y.; Chen, P.W.; Duan, Y.T.; et al. Design, synthesis and evaluation of novel diaryl-1,5-diazoles derivatives bearing morpholine as potent dual COX-2/5-LOX inhibitors and antitumor agents. Eur. J. Med. Chem. 2019, 169, 168–184. [Google Scholar] [CrossRef]
- Shaaban, M.A.; Kamal, A.M.; Faggal, S.I.; Farag, N.A.; Aborehab, N.M.; Elsahar, A.E.; Mohamed, K.O. Design, synthesis, and biological evaluation of new pyrazoloquinazoline derivatives as dual COX-2/5-LOX inhibitors. Arch. Pharm. 2020, 353, 2000027. [Google Scholar] [CrossRef]
- Das Chagas Pereira de Andrade, F.; Mendes, A.N. Computational analysis of eugenol inhibitory activity in lipoxygenase and cyclooxygenase pathways. Sci. Rep. 2020, 10, 16204. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Murakami, Y. Eugenol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Rajabnejhad, S.; Partoazar, A.R.; Mehr, S.E.; Faridi-Majidi, R.; Sahebgharani, M.; Syedmoradi, L.; Rajabnejhad, M.R.; Amani, A. Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm. Dev. Technol. 2016, 21, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Zobeiri, M.; Belwal, T.; Parvizi, F.; Naseri, R.; Farzaei, M.H.; Nabavi, S.F.; Sureda, A.; Nabavi, S.M. Naringenin and its Nano-formulations for Fatty Liver: Cellular Modes of Action and Clinical Perspective. Curr. Pharm. Biotechnol. 2018, 19, 196–205. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Bido, G.D.S.; Ferrarese, M.D.L.L.; Marchiosi, R.; Ferrarese-Filho, O. Naringenin inhibits the growth and stimulates the lignification of soybean root. Braz. Arch. Biol. Technol. 2010, 53, 533–542. [Google Scholar] [CrossRef]
- Koopman, F.; Beekwilder, J.; Crimi, B.; van Houwelingen, A.; Hall, R.D.; Bosch, D.; van Maris, A.J.; Pronk, J.T.; Daran, J.M. De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 155. [Google Scholar] [CrossRef]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef]
- Karim, N.; Jia, Z.; Zheng, X.; Cui, S.; Chen, W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci. Technol. 2018, 79, 35–54. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A., Jr. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Pedata, F.; Dettori, I.; Coppi, E.; Melani, A.; Fusco, I.; Corradetti, R.; Pugliese, A.M. Purinergic signalling in brain ischemia. Neuropharmacology 2016, 104, 105–130. [Google Scholar] [CrossRef]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A., Jr. Naringenin Inhibits Superoxide Anion-Induced Inflammatory Pain: Role of Oxidative Stress, Cytokines, Nrf-2 and the NO-cGMP-PKG-KATP Channel Signaling Pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Mizokami, S.S.; Borghi, S.M.; Bordignon, J.; Silva, R.L.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; Casagrande, R.; et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J. Nutr. Biochem. 2016, 33, 8–14. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef]
- Manchope, M.F.; Casagrande, R.; Verri, W.A. Naringenin: An analgesic and anti-inflammatory citrus flavanone. Oncotarget 2017, 8, 3766. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Zhang, P.; Liu, T.; Zhou, L.; Yang, G.; Lin, R.; Zhang, J. Solubilities of Naringin and Naringenin in Different Solvents and Dissociation Constants of Naringenin. J. Chem. Eng. Data 2015, 60, 932–940. [Google Scholar] [CrossRef]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-inflammatory and anticancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef]
- Vaghasiya, H.; Kumar, A.; Sawant, K. Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride. Eur. J. Pharm. Sci. 2013, 49, 311–322. [Google Scholar] [CrossRef]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911. [Google Scholar]
- Fan, T.; Chen, C.; Guo, H.; Xu, J.; Zhang, J.; Zhu, X.; Yang, Y.; Zhou, Z.; Li, L.; Huang, Y. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur. J. Pharm. Biopharm. 2014, 88, 518–528. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef]
- Hayman, M.; Kam, P.C.A. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Jolayemi, A.T.; Ojewole, J.A.O. Comparative anti-inflammatory properties of Capsaicin and ethyl-aAcetate extract of Capsicum frutescens linn [Solanaceae] in rats. Afr. Health Sci. 2013, 13, 357–361. [Google Scholar]
- Zimmer, A.R.; Leonardi, B.; Miron, D.; Schapoval, E.; de Oliveira, J.R.; Gosmann, G. Antioxidant and anti-inflammatory properties of Capsicum baccatum: From traditional use to scientific approach. J. Ethnopharmacol. 2012, 139, 228–233. [Google Scholar] [CrossRef]
- Meghvansi, M.K.; Siddiqui, S.; Khan, M.H.; Gupta, V.K.; Vairale, M.G.; Gogoi, H.K.; Singh, L. Naga chilli: A potential source of capsaicinoids with broad-spectrum ethnopharmacological applications. J. Ethnopharmacol. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- 164. Zsiborás, C.; Mátics, R.; Hegyi, P.; Balaskó, M.; Pétervári, E.; Szabó, I.; Sarlós, P.; Mikó, A.; Tenk, J.; Rostás, I.; et al. Capsaicin and capsiate could be appropriate agents for treatment of obesity: A meta-analysis of human studies. Crit. Rev. Food Sci. Nutr. 2017, 58, 1419–1427. [Google Scholar] [CrossRef]
- Ramachandran, S.; Srivastava, S.K. Abstract 4422: Capsaicin suppresses pancreatic tumor growth by inhibiting tumor cell metabolism. Cancer Res. 2017, 77 (Suppl. S13), 4422. [Google Scholar] [CrossRef]
- Chung, M.K.; Campbell, J.N. Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations. Pharmaceuticals 2016, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nolan, N.A.; Brown, K.C.; Miles, S.L.; Akers, A.T.; Colclough, K.W.; Seidler, J.M.; Rimoldi, J.M.; Valentovic, M.A.; Dasgupta, P. Anticancer Activity of Natural and Synthetic Capsaicin Analogs. J. Pharmacol. Exp. Ther. 2018, 364, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.W. Capsaicin as an Anti-Obesity Drug. Prog. Drug Res. 2014, 68, 171–179. [Google Scholar] [PubMed]
- Shang, K.; Amna, T.; Amina, M.; Al-Musayeib, N.; Al-Deyab, S.; Hwang, I. Influence of Capsaicin on Inflammatory Cytokines Induced by Lipopolysaccharide in Myoblast Cells Under In vitro Environment. Pharmacogn. Mag. 2017, 13 (Suppl. S1), S26. [Google Scholar]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Cho, S.Y.; Kim, H.W.; Lee, M.K.; Kim, H.J.; Kim, J.B.; Choe, J.S.; Lee, Y.M.; Jang, H.H. Antioxidant and Anti-Inflammatory Activities in Relation to the Flavonoids Composition of Pepper (Capsicum annuum L.). Antioxidants 2020, 9, 986. [Google Scholar] [CrossRef]
- Kim, C.S.; Kawada, T.; Kim, B.S.; Han, I.S.; Choe, S.Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell. Signal. 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Abolmaali, S.S.; Tamaddon, A.M.; Farvadi, F.S.; Daneshamuz, S.; Moghimi, H. Pharmaceutical nanoemulsions and their potential topical and transdermal applications. Iran. J. Pharm. Sci. 2011, 7, 139–150. [Google Scholar]
- Lovelyn, C.; Attama, A.A.; Lovelyn, C.; Attama, A.A. Current State of Nanoemulsions in Drug Delivery. J. Biomater. Nanobiotechnology 2011, 2, 626–639. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ghosh, S.; Ray, S.; Kaurav, S.; Mazumder, B. In vitro & in vivo Studies on Lornoxicam Loaded Nanoemulsion Gels for Topical Application. Curr. Drug Deliv. 2014, 11, 132–138. [Google Scholar]
- Azizi, M.; Esmaeili, F.; Partoazar, A.; Ejtemaei Mehr, S.; Amani, A. Efficacy of nano- and microemulsion-based topical gels in delivery of ibuprofen: An in vivo study. J. Microencapsul. 2017, 34, 195–202. [Google Scholar] [CrossRef]
- Sandig, A.G.; Campmany, A.C.C.; Campos, F.F.; Villena, M.J.M.; Naveros, B.C. Transdermal delivery of imipramine and doxepin from newly oil-in-water nanoemulsions for an analgesic and anti-allodynic activity: Development, characterization and in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Schwarz, J.C.; Valenta, C. Nanoemulsions in Dermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Springer: Berlin/Heidelberg, Germany, 2015; pp. 255–266. [Google Scholar]
- Ghiasi, Z.; Esmaeli, F.; Aghajani, M.; Ghazi-Khansari, M.; Faramarzi, M.A.; Amani, A. Enhancing analgesic and anti-inflammatory effects of capsaicin when loaded into olive oil nanoemulsion: An in vivo study. Int. J. Pharm. 2019, 559, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Shafiq, S.; Haq, N.; Alanazi, F.K.; Alsarra, I.A. Nanoemulsions as potential vehicles for transdermal and dermal delivery of hydrophobic compounds: An overview. Expert Opin. Drug Deliv. 2012, 9, 953–974. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, J.A.; Kim, J.T.; Cha, D.S.; Cho, J.H.; Park, H.J.; Shin, G.H. Preparation of a Capsaicin-Loaded Nanoemulsion for Improving Skin Penetration. J. Agric. Food Chem. 2014, 62, 725–732. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia Serrata, A Potential Antiinflammatory Agent: An Overview. Indian J. Pharm. Sci. 2011, 73, 255. [Google Scholar]
- Büchele, B.; Simmet, T. Analysis of 12 different pentacyclic triterpenic acids from frankincense in human plasma by high-performance liquid chromatography and photodiode array detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 795, 355–362. [Google Scholar] [CrossRef]
- Eltahir, H.M.; Fawzy, M.A.; Mohamed, E.M.; Alrehany, M.A.; Shehata, A.M.; Abouzied, M.M. Antioxidant, anti inflammatory and anti fibrotic effects of Boswellia serrate gum resin in CCl4 induced hepatotoxicity. Exp. Ther. Med. 2020, 19, 1313–1321. [Google Scholar] [CrossRef]
- Efferth, T.; Oesch, F. Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin. Cancer Biol. 2022, 80, 39–57. [Google Scholar] [CrossRef]
- Soliman, A.M.; Majeed, R.R.; Himyari, H.F.A.; Dhanalekshmi, U.M.; Khan, S.A. In vitro Cytotoxic, Antioxidant and Antimicrobial Activities of Alcoholic and Chloroform Extracts of Dhofari Frankincense. Dhaka Univ. J. Pharm. Sci. 2020, 19, 105–110. [Google Scholar] [CrossRef]
- Alotaibi, B.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elseady, W.S.; Saleh, A.; Alotaibi, K.N.; El-Sherbeni, S.A. Antibacterial, immunomodulatory, and lung protective effects of Boswellia dalzielii oleoresin ethanol extract in pulmonary diseases: In vitro and in vivo studies. Antibiotics 2021, 10, 1444. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Frankincense (乳香 Rǔ Xiāng; Boswellia Species): From the Selection of Traditional Applications to the Novel Phytotherapy for the Prevention and Treatment of Serious Diseases. J. Tradit. Complement. Med. 2013, 3, 221–226. [Google Scholar] [CrossRef]
- Liu, J.J.; Toy, W.C.; Liu, S.; Cheng, A.; Lim, B.K.; Subramaniam, T.; Sum, C.F.; Lim, S.C. Acetyl-keto-β-boswellic acid induces lipolysis in mature adipocytes. Biochem. Biophys. Res. Commun. 2013, 431, 192–196. [Google Scholar] [CrossRef]
- Altmann, A.; Poeckel, D.; Fischer, L.; Schubert-Zsilavecz, M.; Steinhilber, D.; Werz, O. Coupling of boswellic acid-induced Ca2+ mobilisation and MAPK activation to lipid metabolism and peroxide formation in human leucocytes. Br. J. Pharmacol. 2004, 141, 223–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Ning, Z.; Lu, C.; Zhao, S.; Wang, J.; Liu, B.; Xu, X.; Liu, Y. Triterpenoid resinous metabolites from the genus Boswellia: Pharmacological activities and potential species-identifying properties. Chem. Cent. J. 2013, 7, 153. [Google Scholar] [CrossRef]
- Ali, E.N.; Mansour, S.Z. Boswellic acids extract attenuates pulmonary fibrosis induced by bleomycin and oxidative stress from gamma irradiation in rats. Chin. Med. 2011, 6, 36. [Google Scholar] [CrossRef]
- Cuaz-Pérolin, C.; Billiet, L.; Baugé, E.; Copin, C.; Scott-Algara, D.; Genze, F.; Büchele, B.; Syrovets, T.; Simmet, T.; Rouis, M. Antiinflammatory and antiatherogenic effects of the NF-κB inhibitor acetyl-11-Keto-β-boswellic acid in LPS-challenged ApoE−/− mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 272–277. [Google Scholar] [CrossRef]
- Bairwa, K.; Jachak, S.M. Nanoparticle formulation of 11-keto-β-boswellic acid (KBA): Anti-inflammatory activity and in vivo pharmacokinetics. Pharm. Biol. 2016, 54, 2909–2916. [Google Scholar] [CrossRef]
- Thakur, S.; Mohan, G.K. In Vivo Antiinflammatory Activity of Facile Boswellic Acid Silver Nanoparticles and In Vitro Drug Release Kinetics. BioNanoScience 2022, 12, 670–684. [Google Scholar] [CrossRef]
- Solanki, N.; Mehta, M.; Chellappan, D.K.; Gupta, G.; Hansbro, N.G.; Tambuwala, M.M.; AAAljabali, A.; Paudel, K.R.; Liu, G.; Satija, S.; et al. Antiproliferative effects of boswellic acid-loaded chitosan nanoparticles on human lung cancer cell line A549. Future Med. Chem. 2020, 12, 2019–2034. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Njoku, R.C.C.; John, I.G.; Amadi, B.A.; Mgbudom-Okah, C.J.; Onuah, C.L. Antioxidant and anti-inflammatory protective effects of rutin and kolaviron against busulfan-induced testicular injuries in rats. Syst. Biol. Reprod. Med. 2022, 68, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Huang, H.C.; Chang, C.Y.Y.; Lee, H.J.; Liu, C.T.; Lo, H.Y.; Ho, T.Y.; Lin, W.C.; Yen, H.R. A Potential Herbal Adjuvant Combined with a Peptide-Based Vaccine Acts Against HPV-Related Tumors Through Enhancing Effector and Memory T-Cell Immune Responses. Front. Immunol. 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 2012, 210, 340–352. [Google Scholar] [CrossRef]
- Soni, H.; Malik, J.; Singhai, A.K.; Sharma, S. Antimicrobial and antiinflammatory activity of the hydrogels containing rutin delivery. Asian J. Chem. 2013, 25, 8371–8373. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Rutin: A Potential Antiviral for Repurposing as a SARS-CoV-2 Main Protease (Mpro) Inhibitor. Nat. Prod. Commun. 2021, 16. [Google Scholar] [CrossRef]
- Carvalho, T.T.; Mizokami, S.S.; Ferraz, C.R.; Manchope, M.F.; Borghi, S.M.; Fattori, V.; Calixto-Campos, C.; Camilios-Neto, D.; Casagrande, R.; Verri, W.A. The granulopoietic cytokine granulocyte colony-stimulating factor (G-CSF) induces pain: Analgesia by rutin. Inflammopharmacology 2019, 27, 1285–1296. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, Y.J.; Su, Y.J.; Zhou, W.W.; Yang, S.G.; Zhang, R.; Zhao, M.; Li, Y.N.; Zhang, Z.P.; Zhan, D.W.; et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology 2012, 33, 482–490. [Google Scholar] [CrossRef]
- Yeh, C.H.; Yang, J.J.; Yang, M.L.; Li, Y.C.; Kuan, Y.H. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK–NF-κB pathway. Free Radic. Biol. Med. 2014, 69, 249–257. [Google Scholar] [CrossRef]
- Chen, J.; Su, J.; Dong, L.; Bai, Z.; Liu, Y.; Bao, H.; Lu, Y.; Wu, Y. Rutin ameliorates cigarette smoke mediated lung inflammation in a mouse model of chronic obstructive pulmonary disease via regulating the levels of tumor necrosis factor-α, interleukin-8 and PAF. Arch. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Pandey, P.; Rahman, M.; Bhatt, P.C.; Beg, S.; Paul, B.; Hafeez, A.; Al-Abbasi, F.A.; Nadeem, M.S.; Baothman, O.; Anwar, F.; et al. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomedicine 2018, 13, 849–870. [Google Scholar] [CrossRef]
- Paudel, K.R.; Wadhwa, R.; Tew, X.N.; Lau, N.J.X.; Madheswaran, T.; Panneerselvam, J.; Zeeshan, F.; Kumar, P.; Gupta, G.; Anand, K.; et al. Rutin loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Life Sci. 2021, 276, 119436. [Google Scholar] [CrossRef]
- Gul, A.; Kunwar, B.; Mazhar, M.; Faizi, S.; Ahmed, D.; Shah, M.R.; Simjee, S.U. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-κB and iNOS activation. Int. Immunopharmacol. 2018, 59, 310–317. [Google Scholar] [CrossRef]
- Schumacher, M.; Cerella, C.; Reuter, S.; Dicato, M.; Diederich, M. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011, 6, 149–160. [Google Scholar] [CrossRef]
- Alzohairy, M.A. Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid.-Based Complement. Altern. Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef]
- Hossain, M.A.; Shah, M.D.; Sakari, M. Gas chromatography-mass spectrometry analysis of various organic extracts of Merremia borneensis from Sabah. Asian Pac. J. Trop. Med. 2011, 4, 637–641. [Google Scholar] [CrossRef]
- Jerobin, J.; Makwana, P.; Suresh Kumar, R.S.; Sundaramoorthy, R.; Mukherjee, A.; Chandrasekeran, N. Antibacterial activity of neem nanoemulsion and its toxicity assessment on human lymphocytes in vitro. Int. J. Nanomed. 2015, 10 (Suppl. S1), 77–86. [Google Scholar]
- Arumugam, A.; Agullo, P.; Boopalan, T.; Nandy, S.; Lopez, R.; Gutierrez, C.; Narayan, M.; Rajkumar, L. Neem leaf extract inhibits mammary carcinogenesis by altering cell proliferation, apoptosis, and angiogenesis. Cancer Biol. Ther. 2014, 15, 26–34. [Google Scholar] [CrossRef]
- Patel, S.M.; Nagulapalli Venkata, K.C.; Bhattacharyya, P.; Sethi, G.; Bishayee, A. Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2016; Volume 40, pp. 100–115. [Google Scholar]
- Oroojan, A.A.; Chenani, N.; An’aam, M. Antioxidant Effects of Eugenol on Oxidative Stress Induced by Hydrogen Peroxide in Islets of Langerhans Isolated from Male Mouse. Int. J. Hepatol. 2020, 2020, 5890378. [Google Scholar] [CrossRef]
- Omóbòwálé, T.O.; Oyagbemi, A.A.; Adejumobi, O.A.; Orherhe, E.V.; Amid, A.S.; Adedapo, A.A.; Nottidge, H.O.; Yakubu, M.A. Preconditioning with Azadirachta indica ameliorates cardiorenal dysfunction through reduction in oxidative stress and extracellular signal regulated protein kinase signalling. J. Ayurveda Integr. Med. 2016, 7, 209–217. [Google Scholar] [CrossRef]
- Quelemes, P.V.; Perfeito, M.L.; Guimarães, M.A.; dos Santos, R.C.; Lima, D.F.; Nascimento, C.; Silva, M.P.; Soares, M.J.D.S.; Ropke, C.D.; Eaton, P.; et al. Effect of neem (Azadirachta indica A. Juss) leaf extract on resistant Staphylococcus aureus biofilm formation and Schistosoma mansoni worms. J. Ethnopharmacol. 2015, 175, 287–294. [Google Scholar] [CrossRef]
- Hossain, M.A.; Al-Toubi, W.A.S.; Weli, A.M.; Al-Riyami, Q.A.; Al-Sabahi, J.N. Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. J. Taibah Univ. Sci. 2013, 7, 181–188. [Google Scholar] [CrossRef]
- Soares, D.G.; Godin, A.M.; Menezes, R.R.; Nogueira, R.D.; Brito, A.M.S.; Melo, I.S.; Coura, G.M.E.; Souza, D.G.; Amaral, F.A.; Paulino, T.P.; et al. Anti-inflammatory and Antinociceptive Activities of Azadirachtin in Mice. Planta Med. 2014, 80, 630–636. [Google Scholar] [CrossRef]
- Naik, M.; Agrawal, D.; Behera, R.; Bhattacharya, A.; Dehury, S.; Kumar, S. Study of anti-inflammatory effect of neem seed oil (Azadirachta indica) on infected albino rats. J. Health Res. Rev. 2014, 1, 66. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Zhu, J.; Song, L.; Li, Z.; Lin, F.; Yu, R.; Xu, H.; Zi, J. Limonoids from seeds of Azadirachta indica A. Juss. and their cytotoxic activity. Acta Pharm. Sin. B 2018, 8, 639–644. [Google Scholar] [CrossRef]
- Tapanelli, S.; Chianese, G.; Lucantoni, L.; Yerbanga, R.S.; Habluetzel, A.; Taglialatela-Scafati, O. Transmission blocking effects of neem (Azadirachta indica) seed kernel limonoids on Plasmodium berghei early sporogonic development. Fitoterapia 2016, 114, 122–126. [Google Scholar] [CrossRef]
- Shilpa, G.; Renjitha, J.; Saranga, R.; Sajin, F.K.; Nair, M.S.; Joy, B.; Sasidhar, B.S.; Priya, S. Epoxyazadiradione Purified from the Azadirachta indica Seed Induced Mitochondrial Apoptosis and Inhibition of NFκB Nuclear Translocation in Human Cervical Cancer Cells. Phytother. Res. 2017, 31, 1892–1902. [Google Scholar] [CrossRef]
- Someya, T.; Sano, K.; Hara, K.; Sagane, Y.; Watanabe, T.; Wijesekara, R.G.S. Fibroblast and keratinocyte gene expression following exposure to extracts of neem plant (Azadirachta indica). Data Brief 2018, 16, 982–992. [Google Scholar] [CrossRef]
- Shin, V.Y.; Kwong, A. Prostaglandin and Its Receptors: Potential Targets for Gastrointestinal Inflammation and Cancer. Ther. Targets Inflamm. Cancer 2017, 295–308. [Google Scholar] [CrossRef]
- Ruslie, R.H.; Darmadi, D. Administration of neem (Azadirachta indica A. Juss) leaf extract decreases TNF-α and IL-6 expressions in dextran sodium sulfate-induced colitis in rats. J. Adv. Vet. Anim. Res. 2020, 7, 744. [Google Scholar] [CrossRef] [PubMed]
- Pasquoto-Stigliani, T.; Campos, E.V.; Oliveira, J.L.; Silva, C.M.; Bilesky-José, N.; Guilger, M.; Troost, J.; Oliveira, H.C.; Stolf-Moreira, R.; Fraceto, L.F.; et al. Nanocapsules Containing Neem (Azadirachta indica) Oil: Development, Characterization, And Toxicity Evaluation. Sci. Rep. 2017, 7, 5929. [Google Scholar] [CrossRef] [PubMed]
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.M.; McSweeney, C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Microbiol. 2013, 166, 450–457. [Google Scholar] [CrossRef]
- Chung, I.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef]
- Botcha, S.; Prattipati, S.D. Callus Extract Mediated Green Synthesis of Silver Nanoparticles, Their Characterization and Cytotoxicity Evaluation Against MDA-MB-231 and PC-3 Cells. Bionanoscience 2020, 10, 11–22. [Google Scholar] [CrossRef]
- Rasool, S.; Raza, M.A.; Manzoor, F.; Kanwal, Z.; Riaz, S.; Iqbal, M.J.; Naseem, S. Biosynthesis, characterization and anti-dengue vector activity of silver nanoparticles prepared from Azadirachta indica and Citrullus colocynthis. R. Soc. Open Sci. 2020, 7, 200540. [Google Scholar] [CrossRef]
- Curcumin as a Novel Treatment to Improve Cognitive Dysfunction in Schizophrenia—AdisInsight. Available online: https://adisinsight.springer.com/trials/700245135 (accessed on 30 June 2022).
- Capsaicin Nanoparticle in Patient with Painful Diabetic Neuropathy. Available online: https://clinicaltrials.gov/ct2/show/NCT01125215 (accessed on 1 July 2022).
- Curcumin in Management of Chronic Obstructive Pulmonary Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT04687449 (accessed on 1 July 2022).
- In-vivo Effects of E-cigarette Aerosol on Innate Lung Host Defense. Available online: https://clinicaltrials.gov/ct2/show/NCT03700892 (accessed on 1 July 2022).
- Human Safety of Capsaicin Inhalation Challenge Testing for Young and Older Men. Available online: https://clinicaltrials.gov/ct2/show/NCT01621685 (accessed on 1 July 2022).


| α-Boswellic Acid | β-Boswellic Acid |
|---|---|
| Acetyl-α-boswellic acid | Acetyl-β-boswellic acid |
| Lupeolic acid | 11-Keto-β-boswellic acid |
| Acetyl-lupeolic acid | Acetyl-11-keto-β-boswellic acid |
| 11-Dehydro-α-boswellic acid | 9.11-Dehydro-β-boswellic acid |
| Acetyl-9-11-dehydro-α-boswellic acid | Acetyl-9-11-dehydro-β-boswellic acid |
 |  |
   | |
 |  |
 |   |
 |    |
| Functional Food | Drug Delivery Involved/Type | Clinical Trial Number | Description |
|---|---|---|---|
| Curcumin | Curcumin Nanoparticles | NCT02104752 | The clinical study was aimed to test if the curcumin nanoparticles were able to improve the behavioral measures, as well as act as biomarkers of cognition and neuroplasticity, in patients who had been diagnosed with schizophrenia and were already receiving a stable dose of antipsychotic [236]. |
| Capsaicin | Capsaicin Nanoparticles | NCT01125215 | This study tested the efficacy and safety of a 0.75% topical capsaicin nanoparticle preparation against a placebo for patients with painful diabetic neuropathy [237]. |
| Functional Food | Drug Delivery Involved/Type | Clinical Trial Number | Description |
|---|---|---|---|
| Curcumin | Daily Oral Consumption | NCT04687449 | A double-blind placebo-controlled trial on 120 COPD patients who were tested at random with curcumin or a placebo for 90 days. The outcomes were compared among other study arms. No dose escalation was used [238]. |
| Cinnamaldehyde | Inhalation | NCT03700892 | This clinical research looked into identifying the effects of cinnamaldehyde-containing e-cigarettes on airway epithelial cell ciliary function, as well as airway immune cells, in humans [239]. |
| Capsaicin | Inhalation | NCT01621685 | This study tested the effects of capsaicin inhalation on human safety, specifically on young and older men who had been diagnosed with COPD [240]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarence, D.D.; Paudel, K.R.; Manandhar, B.; Singh, S.K.; Devkota, H.P.; Panneerselvam, J.; Gupta, V.; Chitranshi, N.; Verma, N.; Saad, S.; et al. Unravelling the Therapeutic Potential of Nano-Delivered Functional Foods in Chronic Respiratory Diseases. Nutrients 2022, 14, 3828. https://doi.org/10.3390/nu14183828
Clarence DD, Paudel KR, Manandhar B, Singh SK, Devkota HP, Panneerselvam J, Gupta V, Chitranshi N, Verma N, Saad S, et al. Unravelling the Therapeutic Potential of Nano-Delivered Functional Foods in Chronic Respiratory Diseases. Nutrients. 2022; 14(18):3828. https://doi.org/10.3390/nu14183828
Chicago/Turabian StyleClarence, Dvya Delilaa, Keshav Raj Paudel, Bikash Manandhar, Sachin Kumar Singh, Hari Prasad Devkota, Jithendra Panneerselvam, Vivek Gupta, Nitin Chitranshi, Nitin Verma, Sonia Saad, and et al. 2022. "Unravelling the Therapeutic Potential of Nano-Delivered Functional Foods in Chronic Respiratory Diseases" Nutrients 14, no. 18: 3828. https://doi.org/10.3390/nu14183828
APA StyleClarence, D. D., Paudel, K. R., Manandhar, B., Singh, S. K., Devkota, H. P., Panneerselvam, J., Gupta, V., Chitranshi, N., Verma, N., Saad, S., Gupta, G., Hansbro, P. M., Oliver, B. G., Madheswaran, T., Dua, K., & Chellappan, D. K. (2022). Unravelling the Therapeutic Potential of Nano-Delivered Functional Foods in Chronic Respiratory Diseases. Nutrients, 14(18), 3828. https://doi.org/10.3390/nu14183828










