Abstract
Menopause is a period during which women undergo dramatic hormonal changes. These changes lead to physical and mental discomfort, are greatly afflictive, and critically affect women’s lives. However, the current safe and effective management measures for women undergoing menopause are insufficient. Several probiotic functions of lactic acid bacteria (LAB) have been recognized, including alleviation of lactose intolerance, protection of digestive tract health, activation of the immune system, protection against infections, improvement of nutrient uptake, and improvement of the microbiota. In this review, we highlight the currently available knowledge of the potential protective effects of LAB on preventing or mitigating menopausal symptoms, particularly in terms of maintaining balance in the vaginal microbiota, reducing bone loss, and regulating the nervous system and lipid metabolism. Given the increasing number of women entering menopause and the emphasis on the management of menopausal symptoms, LAB are likely to soon become an indispensable part of clinical/daily care for menopausal women. Herein, we do not intend to provide a comprehensive analysis of each menopausal disorder or to specifically judge the reliability and safety of complementary therapies; rather, we aim to highlight the potential roles of LAB in individualized treatment strategies for the clinical management of menopause.
1. Introduction
The World Health Organization defines menopause as the permanent cessation of spontaneous menses, caused by the loss of ovarian follicular activity. Compounded by the effects of ageing, social and metabolic factors, daily activity, and impaired well-being, menopausal symptoms might be extremely severe and could significantly affect the quality of life of women [1]. As life expectancy is increasing, women tend to experience menopause for more than one-third of their lives, especially in western countries [2]. According to statistics, 25% of women experience severe menopausal symptoms [3]; these include central nervous system (CNS)-associated disorders and physical changes associated with metabolic changes, musculoskeletal changes, sexual and urogenital system dysfunction, and structural changes [4]. Some menopausal symptoms, for example, hot flashes, appear even begin in the perimenopause (a period that ovarian function changing until menstruation completely disappears) [5]. Having a healthy menopause has great social benefits. A healthy menopause is a dynamic condition in which permanent loss of ovarian function occurs; it is characterized by physical, psychological, and social self-satisfaction and may include illness and disability, and this enables women to achieve the desired level of resilience and self-management [6]. Menopause is inescapable; however, for women to live a healthy and happy life in the future, the alleviation of the adverse events that accompany menopause are particularly important. Hormone replacement therapy (HRT) is currently recognized as the most effective method for treating menopausal syndrome (e.g., vasomotor dysfunction and genitourinary syndrome). However, HRT is not suitable for all menopausal women and is not effective against chronic diseases such as diabetes or cognitive decline [7]. Non-hormonal strategies, including lifestyle changes, improved diet and nutriment, non-hormonal pharmaceuticals, and the adoption of behavioural and alternative medicine therapies, have also been proposed to manage menopausal symptoms [8]. Evidence suggests that lactic acid bacteria (LAB), particularly those used as probiotics, are effective in relieving complex menopausal disorders.
LAB are a group of microorganisms first recognized in the early 19th century [9]. LAB are Gram-positive, usually peroxidase-negative, mildly aerobic, acid-tolerant non-bacilli and cocci; they are found in various habitats, including the digestive tract, oral cavity, pneogaster, and vagina in humans and in ambient ecological niches, including processed dairy and animal products and plant products. LAB are generally regarded as beneficial microorganisms, and some LAB strains, including those belonging to the genera Lactobacillus, Leuconostoc, Pediococcus, Bifidobacteria, Lactococcus, and Streptococcus, are recognized as probiotics [10]. Probiotics are defined as “live microorganisms that confer health benefits on the host when taken in sufficient quantities” [11]. LAB could produce a variety of beneficial metabolites, including antimicrobial peptides, short-chain fatty acids, and lactic acid [11]. Hence, LAB-driven fermentation preserves biological activity and has a plethora of health-enhancing effects, such as improvement of food nutritional value, prevention of intestinal infections, suppression of antibiotic-associated diarrhoea, treatment of lactose intolerance, enhancement of immunomodulation, treatment of food allergies, anti-oxidant effects, anti-anxiety effects, enhancement of lipid metabolism, and suppression of tumours [12]. Furthermore, LAB regulate imbalances in the gut microbiota composition by increasing the beneficial bacterial population, enhancing intestinal epithelial barrier function, and regulating cytokine production [13]. While the health benefits of LAB and LAB-fermented foods have been reported in multiple studies and generalized in several reviews, in the present review, we focus on the benefits of LAB in women with menopausal symptoms.
2. Menopausal Symptoms
2.1. Anxiety and Depression
Compared with men, women are more susceptible to depression and anxiety, especially during periods of hormonal fluctuations, such as during the low oestrogen phase of the menstrual cycle, after childbirth, and during menopause [14]. A prospective community-based cohort study from China showed that the prevalence of depressive symptoms increased from 14.5% (premenopausal) to 18.2% (in transition) to 19.6% (postmenopausal) along the menopause trajectory, while the incidence of anxiety symptoms increased from 3.1% (premenopausal) to 7.0% (in transition) to 7.4% (postmenopausal) [15]. In addition, anxiety and depression have been linked to sleep disorders and vasomotor symptoms in postmenopausal women [16].
2.2. Urogenital Atrophy
Urogenital atrophy is a common issue during menopause. Oestrogen loss during urogenital atrophy leads to vaginal dryness, vulvovaginal irritation and pain, dyspareunia, and recurrent urinary tract infections in 50–60% of postmenopausal women [12].
2.3. Osteoporosis
Osteoporosis is a disease characterized by deteriorated bone integrity and strength and mainly occurs in women 10–15 years after menopause [13]. Postmenopausal women with osteoporosis are more likely to experience fractures [14]. Approximately 40% of all postmenopausal women are likely to experience an osteoporotic fracture during the course of their lives [15].
2.4. Cognitive Disorders
Nearly two-thirds of all women experience subjective cognitive difficulties during menopausal transition; these difficulties mainly manifest as forgetfulness, slow thinking, and inattention [16]. Furthermore, older women have a higher risk of Alzheimer’s disease than older men, suggesting that longevity is not the reason underlying the sex-related difference [17].
2.5. Cardiovascular Risk
Menopause is recognized as a special cardiovascular risk factor in women. Early menopause has been linked to an increased risk of cardiovascular disease [18]. A previous cohort study reported that women who experienced menopause before 50 years of age had higher risks of death and cardiovascular events than those who experienced menopause after 50 years of age [19].
2.6. Metabolic Disorders
A 10-year follow-up study demonstrated that postmenopausal women had a higher body fat mass, more visceral fat, a greater fat percentage, and more central fat accumulation than premenopausal women [20]. These changes in fat distribution in menopausal women enhance insulin resistance and thereby induce an exponential increase in the incidence of diabetes. This increase, in turn, increases the risks of cardiovascular disease and death in women [21]. It is signified that metabolic disorders during menopause not only increase the risk of cardiovascular disease in menopausal women but also increase the national health system burden and affect the socio-economic development of society [22,23]. Thus, metabolic disorders in menopausal women should be considered not only as personal issues but also as socioeconomic issues [24].
3. The Mainstream Method of Managing Menopause
Decline in oestrogen levels during menopause is associated with multiple diseases in women. The understanding of menopause is increasing as various perceptions and symptomatic experiences of menopause are being described; this has promoted the medicalisation of the management of menopause-related symptoms. A positive attitude and appropriate coping strategies are necessary for a healthy menopause. Hormonal and non-hormonal strategies have also been recommended for menopausal women.
3.1. HRT
HRT is the most practical method for the treatment of menopausal symptoms caused by oestrogen withdrawal. Therapeutic administration of oestrogen might eliminate almost all menopausal symptoms. HRT comprises a series of preparations of sex hormones (oestrogen alone or combined with progestogen) that could be administered orally, transdermally, intramuscularly, intranasally, subcutaneously, or locally (vaginally) [25]. Several menopausal symptoms, including vasomotor symptoms, sleep disorders, loss of sexuality, bone loss, depression, memory deficits, and cardiovascular diseases, have been shown to be resolved after HRT [26]. However, the use of HRT is not recommended over long periods or after several years following menopause as it may increase the risk of breast cancer and coronary heart disease in older women [27]. Some studies have suggested that HRT should be primarily used to prevent menopausal symptoms in younger menopausal women [28]. In addition, HRT should be avoided in women with breast or endometrial cancer, cardiovascular disease, thromboembolic disease, and active liver disease [29].
3.2. Non-Hormonal Therapy
Tibolone (TIB) is a selective tissue oestrogen activity regulator that activates hormone receptors in a tissue-specific manner. The different hormone receptor effects of TIB are determined by its three metabolites: 3α-hydroxytibolone (3α-OH-T) and 3β-hydroxytibolone (3β-OH-T) have oestrogenic effects and combine with oestrogen receptors in the breast and brain tissues to exert estrogenic effects, whereas δ4-tibolone (δ4-TIB) exhibits an affinity for progesterone and androgen receptors in the endometrium and vagina, thereby exerting progestin and androgenic properties. TIB has an ameliorative effect on several symptoms of perimenopause, including vasomotor symptoms, mood- and cognition-related symptoms, neurodegeneration, sexual health-related symptoms, and bone demineralisation [30].
Selective oestrogen receptor modulators (SERMs) act as oestrogen agonists or antagonists in a tissue-selective manner, depending on the target tissue [31]. Tamoxifen, toremifene, and raloxifene are the first three SERMs approved for clinical use [32]. Tamoxifen is the most commonly used SERM and acts as an oestrogen receptor antagonist in breast tissue but as a partial agonist in other tissues such as the endometrium and bone [32]. Although tamoxifen may treat osteoporosis and reduce the incidence of cardiovascular disease and treat breast cancer, its long-term usage unfortunately has side effects such as hot flashes and uterine cancer [33].
Selective serotonin re-uptake inhibitors (paroxetine, citalopram and venlafaxine) and serotonin–norepinephrine re-uptake inhibitors (venlafaxine) are beneficial in alleviating the frequency and severity of hot flashes during menopause [34].
3.3. Non-Pharmaceutical Treatments
3.3.1. Phytoestrogens
Non-steroidal polyphenolic plant substances are collectively known as phytoestrogens; this class mainly includes flavonoids, lignans, and stilbenes, all of which have biological activity similar to that of natural oestrogens and exert anti-oestrogenic and pro-oestrogenic effects by binding to oestrogen receptors [35]. Thus, the use of phytoestrogens is commonly recommended for alleviating menopausal symptoms. A study reported that phytoestrogens reduce menopausal hot flashes, night sweats, and other urogenital menopausal symptoms [36]. However, one study also indicated a lack of conclusive evidence proving the effects of phytoestrogen on menopausal symptoms [37].
3.3.2. Vitamin and Mineral Supplements
Vitamins might contribute to improving the quality of life in menopausal women. In particular, vitamins combined with minerals such as calcium have positive effects in perimenopausal and postmenopausal women [38].
4. Health-Promoting Benefits and Clinical Implications of LAB in Menopausal Women
4.1. LAB for Healthy Ageing
Menopause is a manifestation of ageing, and the occurrence of menopause accelerates the ageing process [39]. Thus, like in ageing, during menopause, a woman’s body inevitably undergoes immune changes and experiences oxidative stress. In 1996, Wise proposed that understanding and controlling changes in the ageing process could help develop strategies to mitigate the destructive effects of menopause [40]. As ovarian function declines, the levels of pro-inflammatory serum markers, including interleukin (IL)-1, IL-6, and tumour necrosis factor (TNF)-α increase, as does the body’s cellular response to these cytokines; moreover, the levels of cluster of differentiation (CD) 4-T cells and B-lymphocytes and the cytotoxic activity of natural killer cells decrease [41]. Elevated levels of proinflammatory cytokines in postmenopausal women reportedly contribute to the risk of chronic inflammatory disease [42]. LAB have been shown to prevent and effectively treat inflammatory bowel disease, mucositis, and even colon cancer through their immunostimulatory properties [43]. Several studies have reported that LAB modulate immunity via various mechanisms. LAB could interact with the gut-associated lymphoid tissue and thereby modulate host-immune responses [44]. For example, probiotic LAB could stimulate changes in the composition of the intestinal mucosa and influence the mucosal immune system, including inducing the change from T helper 2 (Th2) to T-helper 1 (Th1) responses, thereby promoting humoral immunity [45]. In addition, the consumption of LAB promotes intestinal barrier function by increasing mucus secretion, producing antimicrobial peptides to inhibit pathogenic growth, competing with pathogens for adhesion to the intestinal mucosa and increasing the expression of epithelial-cell-binding proteins [46].
Before menopause, adequate oestrogen serves as an antioxidant to balance oxidative stress [47]. During menopause, oestrogen production is erratic, and because of the loss of the antioxidant effects of oestrogen, reactive oxygen species are assumed to increase. Unfortunately, oxidative stress caused by the excess production of free radicals such as reactive oxygen species is a key contributor to the ageing process. Thus, levels of reactive oxygen species increase with ageing. Under the dual action of ageing and reactive oxygen species generation, the occurrence of menopausal symptoms is exacerbated. In addition to protecting the immune system, LAB respond to oxidative stress. Studies have reported that LAB have a good antioxidant capacity and protect against oxidative stress (Table 1). In vitro, many LAB strains and their metabolites have shown excellent scavenging capacity for 1,1-diphenyl-2-picrylhydrazyl, O2−, and H2O2 [48]. In addition, LAB resist oxidative stress by chelating metal ions such as Fe2+ and Cu2+. The chelation of metal ions may contribute more to the antioxidant capacity of LAB rather than to the superoxide dismutase activity, as reported by a previous study [49]. Furthermore, enzymatic regulation is another mode of action of LAB, including regulation of the auto-secretion of antioxidant enzymes, induction of the activity of host antioxidative enzymes, and regulation of certain reactive-oxygen-species-producing enzymes [50].

Table 1.
Studies presenting evidence on the antioxidant effects of LAB (in vivo).
As a dietary intervention strategy, LAB treatment seems to have great application prospects in regulating the aging immune system, combating oxidative stress, ultimately restoring immunity, preventing infection, and enhancing healthy aging. To date, although several studies have reported the effect of LAB on aging, most researchers chose males in the study of the impact of LAB on the immune system of aging animals and the possible mechanisms [51,52]. However, there are gender differences in immune response. Gonadal hormones might have contributions to this sex differential. Therefore, it is necessary to conduct targeted research on the health effects of LAB on females in terms of aging and oestrogen withdrawal [53,54].
4.2. Promoting Oestrogen Receptor Response
The main biological effect of oestradiol is mediated by two intracellular receptors: oestrogen receptors α and β [63]. In addition to oestrogen, phytoestrogens interact with oestrogen receptors. The effects of phytoestrogens in the body are regulated by the gut microbiota; these microbes convert the phytoestrogens into bioactive substances that are more readily absorbed and have stronger oestrogenic and antiestrogenic properties [64]. Owing to structural differences in the intestinal microbiota, the bioavailability of phytoestrogens varies from person to person. LAB might promote the production of active substances in phytoestrogens by promoting the metabolism of phytoestrogens or by modulating the balance of the microbiota [65]. In recent years, several LAB strains, including Lacticaseibacillus paracasei, Lactococcus garvieae, Ligilactobacillus salivarius, Lactobacillus gasseri, and Lactiplantibacillus plantarum have been identified as possessing an excellent ability to secrete glycosidase and convert daidzein to equol [66]. Under suitable conditions of fermentation, L. plantarum 128/2 is fully biologically active against isoflavones in soymilk and possesses a remarkable capacity to produce β-glucosidase activity [67]. In addition to using fermentation to promote the biotransformation of isoflavones, the simultaneous consumption of LAB and isoflavones could increase the production of equol in the body. Isoflavone administration in conjunction with probiotic consumption increases equol production over time [68]. When administered with isoflavones, LAB not only optimise isoflavone bioavailability by directly affecting isoflavone metabolism but also indirectly provide health benefits by affecting other bacterial species, thereby enabling the optimisation of the performance of these phytoestrogens [69]. A series of studies have demonstrated that, with the help of LAB, phytoestrogens play a critical role in the health of menopausal women. The presence of L. sporogenes increases the intestinal absorption and bioavailability of soy isoflavones that appropriately and safely alleviate sleep disorders in postmenopausal women [70]. Soy milk fermented with Lpb. plantarum 1R1.3.2 rather than unfermented soy milk significantly decreased the serum osteocalcin concentration in menopausal women [71]. In addition to helping phytoestrogens to bind to oestrogen receptors more efficiently, LAB supplements may influence the oestrogen metabolism by regulating the oestrobolome. For example, β-glucuronidase secreted by microorganisms can activate conjugated oestrogen, induce its reabsorption, and subsequently act on the oestrogen receptor [72]. Moreover, several studies involving polycystic ovarian syndrome (PCOS) showed that sex-hormone-related gut microbiota was modulated after the administration of LAB, thereby restoring abnormal sex hormone levels [73,74]. Several studies have showed that LAB is directly or indirectly involved in the regulation of hypothalamic pituitary hormones [75,76]. This provides a possibility of using LAB to regulate the sex hormone levels in the host. A recent study has shown that Lpb. plantarum CCFM1180 increased the circulating oestradiol concentration in ovariectomised mice and upregulated the expression of oestrogen receptor α in the abdominal adipose tissue [77]. This study indicated that the regulatory effect of LAB on oestrogen synthesis in extragonadal tissues will be shown when female gonad tissue function is weakened, such as the withdrawal of ovarian function in women during menopause. This provides a new idea to improve the oestrogen deficiency in women during menopause through dietary regulation. Unfortunately, the research has not been able to explain the specific mechanisms that mediates the regulation of oestrogen by this strain. With the development of omics technology, the potential targets and specific mechanism of LAB regulating gonadal hormone can be explored in future research by combining metagenomics, metabolomics, transcriptomics, and other technical means.
4.3. Role of LAB on Menopausal Osteoporosis
Bone homeostasis is maintained by the balance between bone resorption by osteoclasts and bone formation by osteoblasts [78]. The pathogenesis of osteoporosis is caused by bone resorption more than bone formation, resulting in a negative balance of bone metabolism [79]. Menopause results in a period of rapid bone loss, owing to oestrogen depletion. Higher consumption of yoghurt in older people is linked with higher bone density and greater physical function, attributable to the LAB it contains [80]. Numerous studies have shown that LAB could prevent bone resorption and bone loss (Table 2). The possible mechanism underlying bone protection by LAB regulating the intestinal microbiota has been proposed. As shown in Figure 1, LAB increase the availability of calcium by maintaining the low pH. In addition, the synthesis of vitamins and the production of short-chain fatty acids facilitated by LAB also result in increased calcium absorption [81]. Some active peptides produced by LAB can help release minerals from insoluble ions, thereby increasing mineral absorption [82]. LAB treatment acts on the immune system and attenuates increases in the osteoclastogenic factors (TNF-α and IL-1β) that are present during osteoporosis [83,84]. Regulatory T cells are protectors of bone health, and the consumption of LAB could regulate T cells to boost the transcription of the anti-osteoclastogenic factors interleukin (IL)-10 and interferon (IFN)-γ and thus enhance bone mass [85]. The lack of oestrogen during menopause leads to increased intestinal permeability [86]. Disruption of gut barrier integrity not only leads to gut inflammatory responses but also affects skeletal homeostasis. Strengthening the intestinal barrier function is one of the main roles of LAB [87]. Therefore, decreasing gut permeability is another potent mechanism of action by which LAB protect bone health during menopause [88]. Although oral LAB supplementation could be a potential alternative for preventing bone loss in menopause, more large samples and higher quality clinical trials are still needed to further verify the effectiveness of LAB in the intervention of osteoporosis or the maintenance of bone homeostasis. Moreover, considering that osteoporosis is a very common disease with a variety of causes, including lifestyle changes, genetic diseases, endocrine disorders, or the induction of other diseases, it is necessary to consider the impact of these factors on the effect of LAB intervention when designing clinical trials of menopausal osteoporosis. As shown in Table 2, in previous studies, the choice of experimental endpoint and different evaluation indicators will lead to incomparability between experimental results [83,89]. Therefore, more reliable and authoritative design and result measurement standards are needed for clinical trials targeting menopausal women with osteoporosis symptoms.

Table 2.
The regulatory effect of LAB on menopausal osteoporosis in preclinical trials or clinical trials.
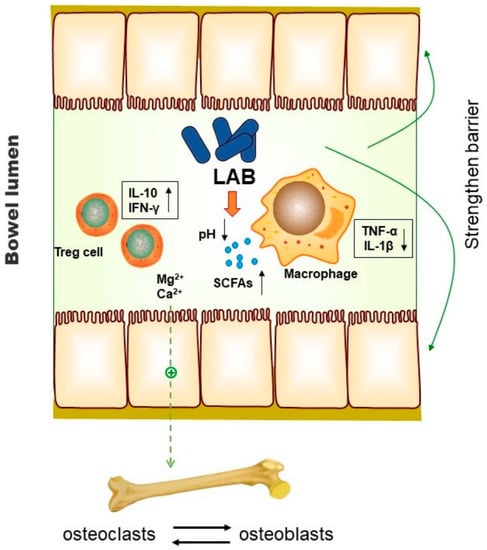
Figure 1.
Possible mechanism of action of LAB on bone through the intestinal tract. Promotion of mineral release, enhancement of calcium absorption, inhibition of inflammation, and regulation of bone metabolism balance are the important mechanisms by which LAB inhibit osteoporosis by regulating the gut microbiota [81,82,83,84,85,86,87,88].
4.4. Prevention of Dyslipidaemia and Obesity by LAB
In the past few years, preclinical studies investigating the effects of LAB against the development of obesity have emerged. One study showed that Lactobacillus significantly reduces the feed intake by modifying the production of satiety hormones [93]. Treatment with L. plantarum CQPC02 reduced the visceral fat and blood lipid levels in obese mice consuming a high-fat diet. The lipid-lowering effects of L. plantarum CQPC02 may depend on its regulation of obesity-related mRNA expression in the liver [94]. Similarly, L. plantarum KFY04 slowed weight gain and decreased the weight of the liver and the visceral fat by modulating the expression of the peroxisome-proliferator-activated receptor pathway and by alleviating oxidative damage and inflammation in obese mice [95]. The gut microbiota plays a special role in energy absorption and metabolism. Firmicutes are more adept at metabolising energy than Bacteroides because they possess more genes for synthesising enzymes involved in lipid and carbohydrate metabolism [96]. Obese people often have a higher abundance of Firmicutes and a lower abundance of Bacteroidetes than lean people [97]. The modulation of the gut microbiota by LAB restores the integrity of gut function and exerts beneficial effects on the gut microbiota, reversing the dysregulated state of obesity [98]. Kefir, an LAB-fermented beverage, alleviated obesity and hepatic steatosis in mice consuming a high-fat diet by modulating the gut microbiota and mycobiota and suppressing inflammatory factors [99]. The effects of LAB on the regulation of metabolic disorders caused by oestrogen deficiency are also quite remarkable. A recent study showed that L. intestinalis YT2 alleviated the increased fat mass in ovariectomised rats mimicking the condition in menopausal women [100]. Moreover, a previous study showed that Lpb. plantarum CCFM1180 increased the level of circulating oestrogen, which may play a role in alleviating metabolic disorder in ovariectomised mice [36]. This evidence suggested that the mechanism of some LAB strains to alleviate metabolic diseases caused by oestrogen withdrawal may not be limited to their general regulation on the lipid metabolism. Meanwhile, this raises another question worthy of in-depth consideration, that is, whether the anti-obesity and anti-dyslipidaemia characteristics of strains in general metabolic disorder model will also be shown in the metabolic disorder model caused by changes in sex hormones. Although the mechanism of LAB regulating metabolic disorders in menopausal women is still unclear, a series of studies have been carried out to study the effects of LAB on metabolic disorders in menopausal women, and some progress has been made. A 12-week-long clinical study showed that multispecies probiotic supplementation inhibited risk factors in a dose-dependent manner and regulated glucose metabolism, blood lipid levels, waist circumference, and visceral fat in obese postmenopausal women [101]. Several current studies have shown that the dosage and duration of LAB supplementation affects efficacy of LAB in the treatment of lipid metabolism. Therefore, these factors need to be considered in the experimental design [102,103].
4.5. LAB and Their Influence on the Vaginal Microbiome
The dominance of Lactobacillus among the microorganisms inhabiting the vagina of women receiving HRT has been reported previously; Lactobacillus is associated with the relief of vulvovaginal-atrophy-related symptoms and serum oestrone concentration [104]. Moreover, LAB play various protective roles in the vagina (Figure 2). First, Lactobacillus creates an acidic vaginal environment (pH = 4) by producing lactic acid, which has antibacterial properties [105,106]; in addition, LAB produce other antibacterial compounds, such as hydrogen peroxide, antibiotics, and target-specific bacteriocins, which may help promote the antagonistic ability of LAB and prevent foreign species from invading the vaginal microbiota [107,108,109]. Furthermore, Lactobacillus contributes to the ability of the microbiota to effectively exclude pathogenic microorganisms by modulating the epithelial innate immunity and competing for nutrients and tissue adherence [110,111]. Treatment with probiotics for vaginal dysbiosis is a relatively new concept. Several researchers have used probiotics to restore the Lactobacillus abundance in the vaginal microbiota of postmenopausal women and achieved promising results. In postmenopausal women treated with L. rhamnosus GR-1 and L. reuteri RC-14 (orally for 14 days), the vaginal flora was appropriately restored and the vaginal colonisation by potential pathogens and yeasts was reduced [111]. Moreover, it has also been reported that administration of live LAB strains in combination with low-dose oestriol has positive effects on restoring the vaginal microbiome and reducing urogenital atrophy and urinary tract infection [112,113,114]. Although recent scientific evidence shows that LAB used directly in the vagina and LAB introduced orally as adjuvant therapy may be a valuable strategy to restore the vaginal microbiota of menopausal women and protect them from genitourinary sequelae caused by oestrogen withdrawal, it should be pointed out that LAB used directly in the vagina and LAB introduced orally may play a role in the vagina through different modes and mechanisms, and researchers should put forward different requirements on the source and functional characteristics of LAB strains for oral or external use. Due to the need to ensure the outstanding ability of vaginal administrated LAB to colonize in the vagina and competitively exclude the pathogens, lactobacilli originating from the vagina of healthy women is the best candidate for LAB used directly in the vagina. In contrast, the sources of LAB introduced orally have more options. However, the difference of the mechanisms of LAB used directly in the vagina or LAB introduced orally on the vaginal microbiome and vaginal health remains unclear. Furthermore, questions remain as to how LAB introduced orally affect the vagina and how they migrate from the intestine to the vagina.
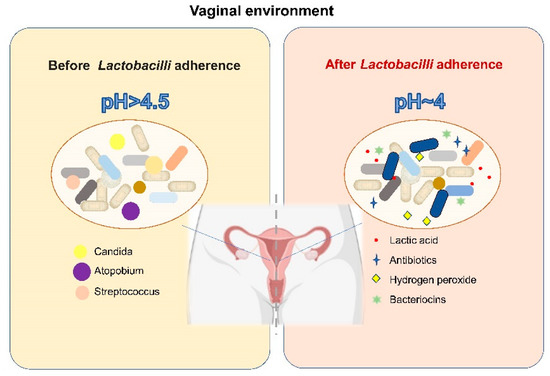
Figure 2.
The mode of action of LAB on the vaginal microbiome [105,106,107,108,109,110,111].
4.6. LAB and CNS-Related Symptoms
CNS-related symptoms are a result of neurobiochemical changes induced by ovarian function decline (e.g., vasomotor symptoms, somnipathy, anxiety and depression, migraine headaches, and cognitive impairment), and the symptoms are related [4]. It is important to control these symptoms in menopausal women. More recently, the term “psychobiotic” was linked to a series of probiotics that could influence the CNS [115]. LAB are effective in improving behaviours related to mental illness (Table 3). Indeed, some strains of psychobiotics, such as L. plantarum PS128TM and B. breve CCFM1025, have been found to be effective against a variety of mental illnesses. LAB could secrete neuro-active metabolites that affect CNS-related symptoms. γ-Aminobutyric acid, which is generated by LAB, may delay the ageing of nerve cells, inhibit neurotransmitters, and mediate most inhibitory nerve transmissions in order to treat psychiatric disorders, including menopausal syndrome and early mental disorders [116]. In addition, LAB play critical roles in CNS-related symptoms by modulating the gut microbiota, inhibiting the working of the hypothalamic–pituitary–adrenal axis, regulating key neurotransmitters, strengthening the immune system, and modulating the neural system. Unfortunately, there is no sufficient evidence to directly prove that psychobiotics can improve the mood or psychology of menopausal women. It is recommended that further research is needed to assess the efficacy of LAB treatments on the relief of CNS-related symptoms in menopausal women. Moreover, considering the influence of changes in sex hormones and various menopausal symptoms on the CNS during menopause, the mode of action by LAB on CNS-related symptoms may involve other unspecified mechanisms.

Table 3.
Evidence from animal experiments and human clinical trials of LAB treatment for improving mental symptoms.
5. Summary and Future Perspectives
When menopause occurs, women not only face problems related to ageing but also experience the adverse consequences of oestrogen withdrawal. Thus, the management of menopausal symptoms is very important. HRT is recognized as the most effective strategy to deal with menopausal symptoms; however, there are some concerns with its safety that limit its applicability. Additionally, HRT is not recommended for long-term usage. Thus, alternative therapies are needed for patients who are concerned about the safety of HRT or for those for whom HRT is not suitable. The strong evidence collected and reported in this review indicates that LAB supplementation has great prospects in the management of menopausal health. On the one hand, as a dietary intervention, LAB not only relay on their own salutary function to produce beneficial metabolites, fight oxidative stress, and enhance immunity but also manipulate the effects of the intestinal microbiota of the host via a two-way relationship with it. On the other hand, LAB that were thoroughly studied prior to application showed no adverse events during consumption, indicating that their use is safe. Therefore, when LAB are used as a mild dietary intervention, no risk level assessment is required.
Although LAB have many potential benefits for menopausal management, researchers still have a large amount of work to do, and extensive and systematic studies are necessary to demonstrate the effectiveness of LAB. Symptoms that occur during menopause, such as osteoporosis, depression, anxiety, and insomnia, are common. When designing a LAB intervention for women with menopausal symptoms, it is important not only to pay attention to the relief of general symptoms, but also to take into account whether LAB strains could address the root causes of the disease, such as menopausal sex hormone changes and immune aging. The regulation of LAB on the host’s gonadal axis, central nervous system, and immune system is not only related to the strain itself, but also may benefit from the gut microbiome and its metabolites regulated by LAB, such as short-chain fatty acids, bile acids, and amino acids. Therefore, genomics, metabolomics, transcriptomics, and other omics technologies should be fully combined to better understand the mechanism of LAB in relieving menopausal symptoms. Up until now, different standards have been adopted in clinical trials of LAB intervention for menopausal women. We suggest that the same standards should be adopted, and the experimental design should be based on authoritative guidelines, such as Good Clinical Practice guidelines of the International Council for Harmonization (GCP/ICH). Meanwhile, different endpoint judgment criteria should be designed for different menopausal symptoms. Furthermore, in order to improve the comparability between studies, clinical trial designers also need to consider the possible impact of diet, disease status, menopause, menstruation, fertility status, and even the race of menopausal subjects.
When adequately documented, we recommend that obstetricians and gynaecologists incorporate LAB interventions into perimenopausal and menopausal management strategies. After quantifying the symptoms and signs of menopause using standardized scales, the corresponding menopause management plan should be assigned to the patient according to the symptoms and severity. For those who want to enter menopause naturally, it is recommended that they supplement LAB therapy with immune protective, antioxidant, and mineral (e.g., calcium) supplements. LAB strains with symptom-targeted function may be recommended to prevent the recurrence of menopausal symptoms in those who have benefited from HRT and are ready to discontinue the treatment or reduce its dose. For patients on non-hormonal therapy, specific strains of LAB could be used in combination with medicines, such as selective serotonin re-uptake inhibitors, to alter tryptophan metabolism and relieve serious menopausal depressive symptoms.
Author Contributions
Conceptualization, G.W., X.L. and H.C.; methodology, Q.C.; software, Q.C.; validation, J.Z. and G.W.; data curation, H.W. and J.Z.; writing—original draft preparation, Q.C.; writing—review and editing, G.W., X.L. and H.C.; visualization, Q.C.; supervision, J.Z. and G.W.; project administration, G.W., X.L. and H.C.; funding acquisition, G.W., H.W., H.C. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Nature Science Foundation of China (no. 31972052, 31820103010, 32021005), the Fundamental Research Funds for the Central Universities (JUSRP22006, JUSRP51501), the Program of Collaborative Innovation Centre of Food Safety and Quality Control in Jiangsu Province, the Appropriate Technology Promotion Project of Wuxi Municipal Health Commission (FYTG202006), and the scientific research project plan of Wuxi Municipal Health Commission (no. M202007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Honour, J.W. Biochemistry of the menopause. Ann. Clin. Biochem. 2018, 55, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A.; Gompel, A. Management of menopause: A view towards prevention. Lancet Diabetes Endocrinol. 2022, 10, 457–470. [Google Scholar] [CrossRef]
- Lee, P.-S.; Lee, C.-L. Prevalence of symptoms and associated factors across menopause status in Taiwanese women. Menopause 2020, 28, 182–188. [Google Scholar] [CrossRef]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Greendale, G.A.; Lee, N.P.; Arriola, E.R. The menopause. Lancet 1999, 353, 571–580. [Google Scholar] [CrossRef]
- Jaspers, L.; Daan, N.M.; van Dijk, G.M.; Gazibara, T.; Muka, T.; Wen, K.-X.; Meun, C.; Zillikens, M.C.; van Lennep, J.E.R.; Roos-Hesselink, J.W.; et al. Health in middle-aged and elderly women: A conceptual framework for healthy menopause. Maturitas 2015, 81, 93–98. [Google Scholar] [CrossRef]
- Mehta, J.; Kling, J.M.; Manson, J.E. Risks, benefits, and treatment modalities of menopausal hormone therapy: Current concepts. Front. Endocrinol. 2021, 12, 564781. [Google Scholar] [CrossRef]
- E Nappi, R.; Chedraui, P.; Lambrinoudaki, I.; Simoncini, T. Menopause: A cardiometabolic transition. Lancet Diabetes Endocrinol. 2022, 10, 442–456. [Google Scholar] [CrossRef]
- Stiles, M.E.; Holzapfel, W.H. Holzapfel, Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 1997, 36, 1–29. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer activity of lactic acid bacteria. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Naumova, I.; Castelo-Branco, C. Current treatment options for postmenopausal vaginal atrophy. Int. J. Women’s Health 2018, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Gosset, A.; Pouillès, J.-M.; Trémollieres, F. Menopausal hormone therapy for the management of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101551. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Ashraf, A.; Zeynali, N.; Ebrahimi, B.; A Jehu, D. Balance and functional mobility predict low bone mineral density among postmenopausal women undergoing recent menopause with osteoporosis, osteopenia, and normal bone mineral density: A cross-sectional study. Geriatr. Nurs. 2021, 42, 33–36. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Karlamangla, A.S.; Maki, P.M. The menopause transition and cognition. JAMA 2020, 323, 1495–1496. [Google Scholar] [CrossRef]
- Andrew, M.K.; Tierney, M.C. The puzzle of sex, gender and Alzheimer’s disease: Why are women more often affected than men? Women’s Health 2018, 14, 1745506518817995. [Google Scholar] [CrossRef]
- El Khoudary, S.R. Age at menopause onset and risk of cardiovascular disease around the world. Maturitas 2020, 141, 33–38. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D.; Wang, M.; Sun, J.-Y.; Liu, J.; Qi, Y.; Hao, Y.-C.; Deng, Q.-J.; Liu, J.; Liu, J.; et al. Combined effect of menopause and cardiovascular risk factors on death and cardiovascular disease: A cohort study. BMC Cardiovasc. Disord. 2021, 21, 109. [Google Scholar] [CrossRef]
- Razmjou, S.; Abdulnour, J.; Bastard, J.-P.; Fellahi, S.; Doucet, É.; Brochu, M.; Lavoie, J.-M.; Rabasa-Lhoret, R.; Prud’Homme, D. Body composition, cardiometabolic risk factors, physical activity, and inflammatory markers in premenopausal women after a 10-year follow-up: A MONET study. Menopause 2018, 25, 89–97. [Google Scholar] [CrossRef]
- Woodward, M. Cardiovascular disease and the female disadvantage. Int. J. Environ. Res. Public Health 2019, 16, 1165. [Google Scholar] [CrossRef]
- Slopien, R.; Wender-Ozegowska, E.; Rogowicz-Frontczak, A.; Meczekalski, B.; Zozulinska-Ziolkiewicz, D.; Jaremek, J.D.; Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; et al. Menopause and diabetes: EMAS clinical guide. Maturitas 2018, 117, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Paraoxonase-1 as a regulator of glucose and lipid homeostasis: Impact on the onset and progression of metabolic disorders. Int. J. Mol. Sci. 2019, 20, 4049. [Google Scholar] [CrossRef] [PubMed]
- Kozakowski, J.; Gietka-Czernel, M.; Leszczyńska, D.; Majos, A. Obesity in menopause–our negligence or an unfortunate inevitability? Prz. Menopauzalny = Menopause Rev. 2017, 16, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Fait, T. Menopause hormone therapy: Latest developments and clinical practice. Drugs Context 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Vigneswaran, K.; Hamoda, H. Hormone replacement therapy-current recommendations. Best Pract. Res. Clin. Obstet. Gynaecol. 2021. [Google Scholar] [CrossRef]
- Pan, M.; Pan, X.; Zhou, J.; Wang, J.; Qi, Q.; Wang, L. Update on hormone therapy for the management of postmenopausal women. BioScience. Trends 2022, 16, 46–57. [Google Scholar] [CrossRef]
- Chester, R.C.; Kling, J.M.; Manson, J.E. What the Women’s Health Initiative has taught us about menopausal hormone therapy. Clin. Cardiol. 2018, 41, 247–252. [Google Scholar]
- Papadakis, G.; Hans, D.; Gonzalez-Rodriguez, E.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P.M.; Lamy, O. The benefit of menopausal hormone therapy on bone density and microarchitecture persists after its withdrawal. J. Clin. Endocrinol. Metab. 2016, 101, 5004–5011. [Google Scholar] [CrossRef]
- Del Río, J.P.; Molina, S.; Hidalgo-Lanussa, O.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone as hormonal therapy and neuroprotective agent. Trends Endocrinol. Metab. 2020, 31, 742–759. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Conner, E.A. Beyond estrogen: Advances in tissue selective estrogen complexes and selective estrogen receptor modulators. Climacteric 2019, 22, 140–147. [Google Scholar] [CrossRef]
- Gómez-Coronado, D.; Lasunción, M.A.; Martínez-Botas, J.; Fernández-Suárez, M.E. Role of cholesterol metabolism in the anticancer pharmacology of selective estrogen receptor modulators. Semin. Cancer Biol. 2020, 73, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar]
- Stubbs, C.; Mattingly, L.; A Crawford, S.; A Wickersham, E.; Brockhaus, J.L.; McCarthy, L.H. Do SSRIs and SNRIs reduce the frequency and/or severity of hot flashes in menopausal women. J. Okla. State Med Assoc. 2017, 110, 272–274. [Google Scholar] [PubMed]
- Gorzkiewicz, J.; Bartosz, G.; Sadowska-Bartosz, I. The Potential Effects of Phytoestrogens: The Role in Neuroprotection. Molecules 2021, 26, 2954. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef]
- Saghafi, N.; Ghazanfarpour, M.; Sadeghi, R.; Najarkolaei, A.H.; Omid, M.G.; Azad, A.; Najarkolaei, E.H. Effects of Phytoestrogens in Alleviating the Menopausal Symptoms: A Systematic Review and Meta-Analysis. IJPR 2017, 16, 99–111. [Google Scholar] [CrossRef]
- Vitale, S.G.; Caruso, S.; Rapisarda, A.M.C.; Cianci, S.; Cianci, A. Isoflavones, calcium, vitamin D and inulin improve quality of life, sexual function, body composition and metabolic parameters in menopausal women: Result from a prospective, randomized, placebo-controlled, parallel-group study. Menopausal Rev. 2018, 17, 32–38. [Google Scholar] [CrossRef]
- Lobo, R.A. Menopause and aging. In Yen and Jaffe’s Reproductive Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 322–356.e9. [Google Scholar]
- Wise, P.M.; Krajnak, K.M.; Kashon, M.L. Menopause: The aging of multiple pacemakers. Science 1996, 273, 67–70. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Han, A.; Kim, J.Y.; Kwak-Kim, J.; Lee, S.K. Menopause is an inflection point of age-related immune changes in women. J. Reprod. Immunol. 2021, 146, 103346. [Google Scholar] [CrossRef]
- Levit, R.; De Giori, G.S.; Leblanc, A.D.M.D.; Leblanc, J.G. Folate-producing lactic acid bacteria reduce inflammation in mice with induced intestinal mucositis. J. Appl. Microbiol. 2018, 125, 1494–1501. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Shi, J.; Zhu, J.; Shao, D.; Huang, Q.; Yang, H.; Jin, M. Capacity of lactic acid bacteria in immunity enhancement and cancer prevention. Appl. Microbiol. Biotechnol. 2016, 101, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, Z.; Tian, P.; Wang, G. Lactic acid bacteria and host immunity. In Lactic Acid Bacteria; Springer: Berlin/Heidelberg, Germany, 2019; pp. 261–296. [Google Scholar]
- LeBlanc, J.G.; Levit, R.; de Giori, G.S.; LeBlanc, A.D.M.D. Application of vitamin-producing lactic acid bacteria to treat intestinal inflammatory diseases. Appl. Microbiol. Biotechnol. 2020, 104, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Menopause as risk factor for oxidative stress. Menopause 2012, 19, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-C.; Kwong, H.K.; Chen, H.-Y.; Hsu, H.-Y.; Yu, S.-H.; Hsieh, C.-W.; Lin, H.-W.; Chu, Y.-L.; Cheng, K.-C. Enhanced antioxidant activity of Chenopodium formosanum Koidz. by lactic acid bacteria: Optimization of fermentation conditions. PLoS ONE 2021, 16, e0249250. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, K.-T.; Chung, M.-Y.; Cho, D.-H.; Park, C.-S. Resistance of Lactobacillus casei KCTC 3260 to Reactive Oxygen Species (ROS): Role for a Metal Ion Chelating Effect. J. Food Sci. 2005, 70, m388–m391. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Sharma, R.; Kapila, R.; Kapasiya, M.; Saliganti, V.; Dass, G.; Kapila, S. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr. Res. 2014, 34, 968–981. [Google Scholar] [CrossRef]
- Pennell, L.M.; Galligan, C.L.; Fish, E.N. Sex affects immunity. J. Autoimmun. 2012, 38, J282–J291. [Google Scholar] [CrossRef]
- Moxley, G.; Posthuma, D.; Carlson, P.; Estrada, E.; Han, J.; Benson, L.L.; Neale, M.C. Sexual dimorphism in innate immunity. Arthritis Care Res. 2002, 46, 250–258. [Google Scholar] [CrossRef]
- Abbès, S.; Ben Salah-Abbès, J.; Jebali, R.; Ben Younes, R.; Oueslati, R. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid bacteria. J. Immunotoxicol. 2015, 13, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U. Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch. Microbiol. 2021, 203, 1221–1229. [Google Scholar] [PubMed]
- Chen, Q.; Kong, Q.; Tian, P.; He, Y.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactic acid bacteria alleviate di-(2-ethylhexyl) phthalate-induced liver and testis toxicity via their bio-binding capacity, antioxidant capacity and regulation of the gut microbiota. Environ. Pollut. 2022, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Zhang, Q.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. The synergistic effect of Lactobacillus plantarum CCFM242 and zinc on ulcerative colitis through modulating intestinal homeostasis. Food Funct. 2019, 10, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, S.; Mei, C.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Capabilities of bio-binding, antioxidant and intestinal environmental repair jointly determine the ability of lactic acid bacteria to mitigate perfluorooctane sulfonate toxicity. Environ. Int. 2022, 166, 107388. [Google Scholar] [PubMed]
- Vaghef-Mehrabany, E.; Rad, A.H.; Alipour, B.; Sharif, S.; Vaghef-Mehrabany, L.; Alipour-Ajiry, S. Effects of Probiotic Supplementation on Oxidative Stress Indices in Women with Rheumatoid Arthritis: A Randomized Double-Blind Clinical Trial. J. Am. Coll. Nutr. 2015, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, L.; Alipour, B.; Jafarabadi, M.A.; Behrooz, M.; Gargari, B.P. Daily consumption effects of probiotic yogurt containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 on oxidative stress in metabolic syndrome patients. Clin. Nutr. ESPEN 2020, 41, 136–142. [Google Scholar] [CrossRef]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of Probiotics on Lipid Profile, Glycemic Control, Insulin Action, Oxidative Stress, and Inflammatory Markers in Patients with Type 2 Diabetes: A Clinical Trial. Iran. J. Med Sci. 2013, 38, 38–43. [Google Scholar]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar]
- Hameed, A.S.S.; Rawat, P.S.; Meng, X.; Liu, W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol. Adv. 2020, 43, 107576. [Google Scholar] [CrossRef]
- Stojanov, S.; Kreft, S. Gut Microbiota and the Metabolism of Phytoestrogens. Rev. Bras. Farm. 2020, 30, 145–154. [Google Scholar] [CrossRef]
- Kwon, J.E.; Lim, J.; Kim, I.; Kim, D.; Kang, S.C. Isolation and identification of new bacterial stains producing equol from Pueraria lobata extract fermentation. PLoS ONE 2018, 13, e0192490. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, J.; Barañano, L.; Castañón, S.; Alkorta, I.; Quirós, L.; Garbisu, C. Optimization of the Bioactivation of Isoflavones in Soymilk by Lactic Acid Bacteria. Processes 2021, 9, 963. [Google Scholar] [CrossRef]
- Ribeiro, A.E.; Monteiro, N.E.S.; De Moraes, A.V.G.; Costa-Paiva, L.H.; Pedro, A.O. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause 2018, 26, 643–652. [Google Scholar] [CrossRef]
- Monteiro, N.E.S.; Queirós, L.D.; Lopes, D.B.; Pedro, A.O.; Macedo, G.A. Impact of microbiota on the use and effects of isoflavones in the relief of climacteric symptoms in menopausal women—A review. J. Funct. Foods 2018, 41, 100–111. [Google Scholar] [CrossRef]
- De Franciscis, P.; Grauso, F.; Luisi, A.; Schettino, M.T.; Torella, M.; Colacurci, N. Adding Agnus Castus and Magnolia to Soy Isoflavones Relieves Sleep Disturbances Besides Postmenopausal Vasomotor Symptoms-Long Term Safety and Effectiveness. Nutrients 2017, 9, 129. [Google Scholar] [CrossRef]
- Desfita, S.; Sari, W.; Yusmarini, Y.; Pato, U.; Zakłos-Szyda, M.; Budryn, G. Effect of Fermented Soymilk-Honey from Different Probiotics on Osteocalcin Level in Menopausal Women. Nutrients 2021, 13, 3581. [Google Scholar] [CrossRef]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Connecting microbiome and menopause for healthy ageing. Nat. Microbiol. 2022, 7, 354–358. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Li, X.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020, 11, 5192–5204. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic Bifidobacterium lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems 2019, 4, e00017-19. [Google Scholar] [CrossRef]
- Poutahidis, T.; Springer, A.D.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Probiotic Microbes Sustain Youthful Serum Testosterone Levels and Testicular Size in Aging Mice. PLoS ONE 2014, 9, e84877. [Google Scholar] [CrossRef] [PubMed]
- Erdman, S.; Poutahidis, T. Probiotic ‘glow of health’: It’s more than skin deep. Benef. Microbes 2014, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, B.; Wang, S.; Qian, X.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients 2021, 13, 1793. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z. Ovarian aging and osteoporosis. Aging Aging-Relat. Dis. 2018, 199–215. [Google Scholar]
- Binda, S.; Ouwehand, A.C. Chapter 12: Lactic Acid Bacteria for Fermented Dairy Products. In Lactic Acid Bacteria: Microbiological and Functional Aspects; CRC Press: Boca Raton, FL, USA, 2019; pp. 175–198. [Google Scholar]
- Campbell, J.M.; Fahey, J.G.C.; Wolf, B.W. Selected Indigestible Oligosaccharides Affect Large Bowel Mass, Cecal and Fecal Short-Chain Fatty Acids, pH and Microflora in Rats. J. Nutr. 1997, 127, 130–136. [Google Scholar] [CrossRef]
- Matar, C.; Amiot, J.; Savoie, L.; Goulet, J. The Effect of Milk Fermentation by Lactobacillus helveticus on the Release of Peptides During In Vitro Digestion. J. Dairy Sci. 1996, 79, 971–979. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Djafarian, K.; Fazeli, M.R.; Yekaninejad, M.S.; Rostamian, A.; Keshavarz, S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 497–506. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics Protect Mice from Ovariectomy-Induced Cortical Bone Loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.L.; Rios-Arce, N.D.; Atkinson, S.; Bierhalter, H.; Schoenherr, D.; Bazil, J.N.; McCabe, L.R.; Parameswaran, N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol. Rep. 2017, 5, e13263. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Q.; de Haan, B.J.; Faas, M.M.; Zhang, H.; de Vos, P. Protective effects of lactic acid bacteria on gut epithelial barrier dysfunction are Toll like receptor 2 and protein kinase C dependent. Food Funct. 2020, 11, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-A.; Curiac, D.; Ahrén, I.L.; Hansson, F.; Niskanen, T.M.; Sjögren, K.; Ohlsson, C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019, 1, e154–e162. [Google Scholar] [CrossRef]
- Chen, C.; Dong, B.; Wang, Y.; Zhang, Q.; Wang, B.; Feng, S.; Zhu, Y. The role of Bacillus acidophilus in osteoporosis and its roles in osteocyte proliferation and differentiation. J. Clin. Lab. Anal. 2019, 34, e23471. [Google Scholar]
- Kim, D.E.; Kim, J.K.; Han, S.K.; Jang, S.E.; Han, M.J.; Kim, D.H. Lactobacillus plantarum NK3 and Bifidobacterium longum NK49 alleviate bacterial vaginosis and osteoporosis in mice by suppressing NF-κ B-Linked TNF-α expression. J. Med. Food 2019, 22, 1022–1031. [Google Scholar] [CrossRef]
- Nilsson, A.G.; Sundh, D.; Bäckhed, F.; Lorentzon, M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef]
- Belguesmia, Y.; Domenger, D.; Caron, J.; Dhulster, P.; Ravallec, R.; Drider, D.; Cudennec, B. Novel probiotic evidence of lactobacilli on immunomodulation and regulation of satiety hormones release in intestinal cells. J. Funct. Foods 2016, 24, 276–286. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Yi, S.; Li, X.; Guo, Z.; Zhou, X.; Mu, J.; Yi, R. Lactobacillus plantarum CQPC02 prevents obesity in mice through the PPAR-α signaling pathway. Biomolecules 2019, 9, 407. [Google Scholar]
- Long, X.; Zeng, X.; Tan, F.; Yi, R.; Pan, Y.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus plantarum KFY04 prevents obesity in mice through the PPAR pathway and alleviates oxidative damage and inflammation. Food Funct. 2020, 11, 5460–5472. [Google Scholar] [CrossRef] [PubMed]
- Kallus, S.J.; Brandt, L.J. The Intestinal Microbiota and Obesity. J. Clin. Gastroenterol. 2012, 46, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-Y.; Zhao, C.-N.; Xu, X.-Y.; Tang, G.-Y.; Corke, H.; Gan, R.-Y.; Li, H.-B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How effective are they in the fight against obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, H.; Jeong, D.; Kang, I.-B.; Chon, J.-W.; Kim, H.-S.; Song, K.-Y.; Seo, K.-H. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: Targeted and untargeted community analysis with correlation of biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef]
- Lim, E.; Song, E.-J.; Kim, J.; Jung, S.; Lee, S.-Y.; Shin, H.; Nam, Y.-D.; Kim, Y. Lactobacillus intestinalis YT2 restores the gut microbiota and improves menopausal symptoms in ovariectomized rats. Benef. Microbes 2021, 12, 503–516. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women—A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients 2018, 10, 1672. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Musazadeh, V.; Faghfouri, A.H.; Sarmadi, B.; Jamilian, P.; Jamilian, P.; Tutunchi, H.; Dehghan, P. Probiotic therapy, a novel and efficient adjuvant approach to improve glycemic status: An umbrella meta-analysis. Pharmacol. Res. 2022. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Srinivasan, S.; Zhan, X.; Wu, M.C.; Reed, S.; Guthrie, K.A.; LaCroix, A.Z.; Fiedler, T.; Munch, M.; Liu, C.; et al. Vaginal microbiota and genitourinary menopausal symptoms: A cross-sectional analysis. Menopause 2017, 24, 1160–1166. [Google Scholar] [CrossRef]
- Kumar, N.; Behera, B.; Sagiri, S.S.; Pal, K.; Ray, S.S.; Roy, S. Bacterial vaginosis: Etiology and modalities of treatment-A brief note. J. Pharm. Bioallied Sci. 2011, 3, 496. [Google Scholar] [PubMed]
- Selle, K.; Klaenhammer, T.R. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L.; Krohn, M.A.; Rabe, L.K.; Klebanoff, S.J.; Eschenbach, D.A. The Normal Vaginal Flora, H2O2-Producing Lactobacilli, and Bacterial Vaginosis in Pregnant Women. Clin. Infect. Dis. 1993, 16 (Suppl. 4), S273–S281. [Google Scholar] [CrossRef] [PubMed]
- Vallor, A.C.; Antonio, M.A.D.; Hawes, S.E.; Hillier, S.L. Factors Associated with Acquisition of, or Persistent Colonization by, Vaginal Lactobacilli: Role of Hydrogen Peroxide Production. J. Infect. Dis. 2001, 184, 1431–1436. [Google Scholar] [CrossRef]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal Microbiome: Rethinking Health and Disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef]
- McMillan, A.; Dell, M.; Zellar, M.P.; Cribby, S.; Martz, S.; Hong, E.; Fu, J.; Abbas, A.; Dang, T.; Miller, W.; et al. Disruption of urogenital biofilms by lactobacilli. Colloids Surf. B Biointerfaces 2011, 86, 58–64. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Seney, S.; McMillan, A.; Vongsa, R.; Koenig, D.; Wong, L.; Dvoracek, B.; Gloor, G.; Sumarah, M.; Ford, B.; et al. A Systems Biology Approach Investigating the Effect of Probiotics on the Vaginal Microbiome and Host Responses in a Double Blind, Placebo-Controlled Clinical Trial of Post-Menopausal Women. PLoS ONE 2014, 9, e104511. [Google Scholar] [CrossRef]
- Özkinay, E.; Terek, M.C.; Yayci, M.; Kaiser, R.; Grob, P.; Tuncay, G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 234–240. [Google Scholar] [CrossRef]
- Uehara, S.; Monden, K.; Nomoto, K.; Seno, Y.; Kariyama, R.; Kumon, H. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int. J. Antimicrob. Agents 2006, 28, 30–34. [Google Scholar] [CrossRef]
- Capobianco, G.; Wenger, J.M.; Meloni, G.B.; Dessole, M.; Cherchi, P.; Dessole, S. Triple therapy with Lactobacilli acidophili, estriol plus pelvic floor rehabilitation for symptoms of urogenital aging in postmenopausal women. Arch. Gynecol. Obstet. 2013, 289, 601–608. [Google Scholar] [CrossRef]
- Visñuk, D.P.; de Giori, G.S.; LeBlanc, J.G.; de LeBlanc AD, M. Neuroprotective effects associated with immune modulation by selected lactic acid bacteria in a Parkinson’s disease model. Nutrition 2020, 79, 110995. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, P.; Dang, H. Lactic acid bacteria and γ-aminobutyric acid and diacetyl. In Lactic Acid Bacteria; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–19. [Google Scholar]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-I.; Wu, C.-C.; Tsai, P.-J.; Cheng, L.-H.; Hsu, C.-C.; Shan, I.-K.; Chan, P.-Y.; Lin, T.-W.; Ko, C.-J.; Chen, W.-L.; et al. Psychobiotic Supplementation of PS128TM Improves Stress, Anxiety, and Insomnia in Highly Stressed Information Technology Specialists: A Pilot Study. Front. Nutr. 2021, 8, 614105. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.S.; Chang, H.C.; Weng, Y.H.; Chen, C.C.; Kuo, Y.S.; Tsai, Y.C. The add-on effect of Lactobacillus plantarum PS128 in patients with Parkinson’s disease: A pilot study. Front. Nutr. 2021, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Chen, J.L.; Liao, J.F.; Chen, Y.H.; Chieu, M.W.; Ke, Y.Y.; Hsu, C.-C.; Tsai, Y.-J.; Hsieh-Li, H.M. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer’s disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement. Med. Ther. 2021, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2021, 100, 233–241. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Administration of Bifidobacterium breve Improves the Brain Function of Aβ1-42-Treated Mice via the Modulation of the Gut Microbiome. Nutrients 2021, 13, 1602. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).