Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation
Abstract
:1. Introduction
2. Gut Microbiome in Constipation
3. Potential Mechanisms by Which the Gut Microbiota Modulates Constipation
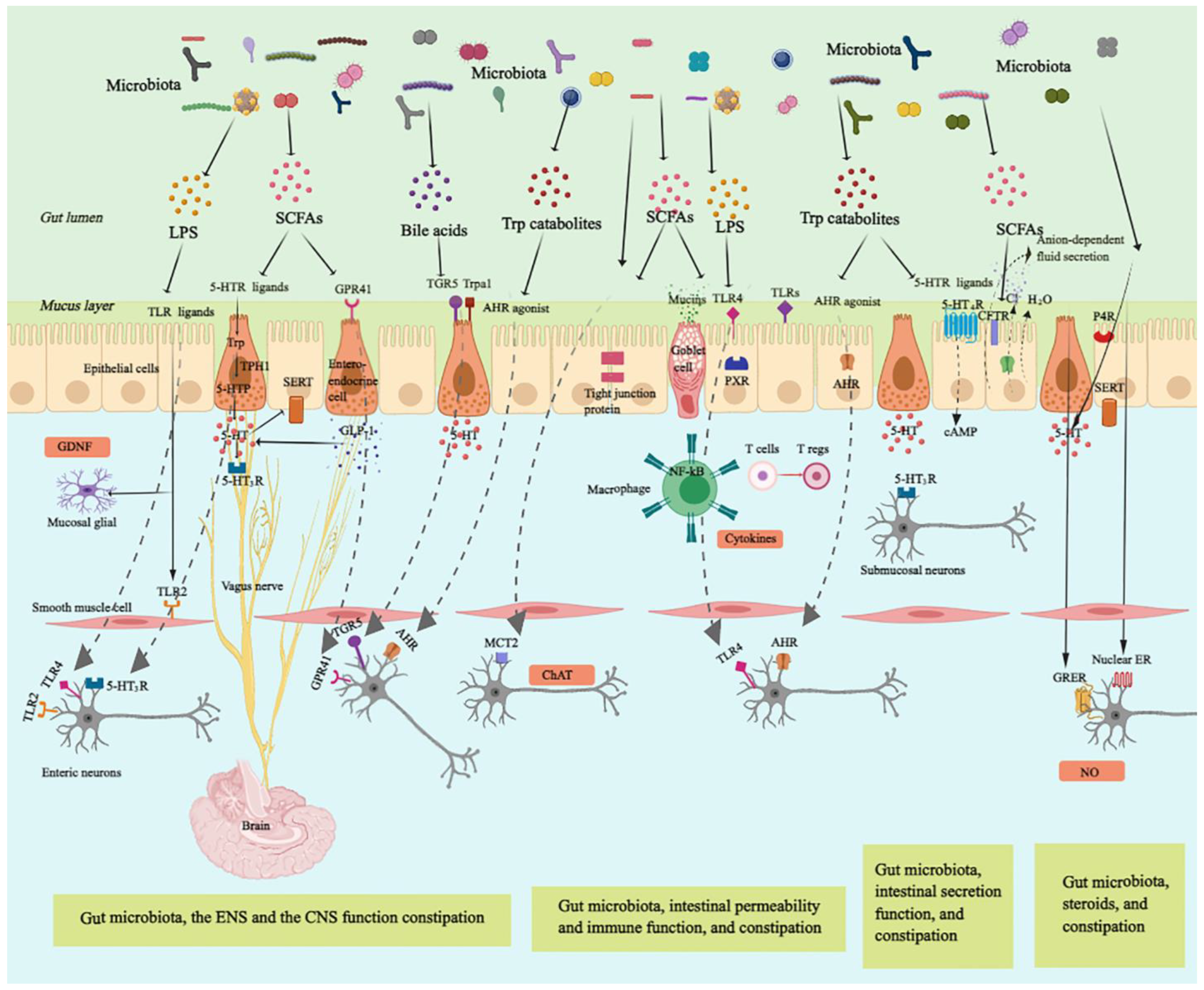
3.1. Gut Microbiota, Enteric Nervous System, and Gut Motility
| Microbial Metabolites | Effect on Gastrointestinal Physiology | Mechanism | Model Organism |
|---|---|---|---|
| Short-chain fatty acid | ENS function | Stimulation of the ENS receptor type GPCRs to regulate GLP-1 expression [38] Modulation of 5-HT biosynthesis via regulating the expression of TpH1 and SERT [39] Increase in ChAT+ neurons to improve colonic transit (Butyrate) [40] Directly acting on the colonic and ileal smooth muscle to stimulate colonic peristalsis [41] | Animal |
| CNS function | Stimulation of the mucosal receptors connected to vagal nerves and cholinergic neurons expression [47] | Animal | |
| Immune activation | Restoring Tregs populations and function [48] | Animal | |
| Intestinal barrier | Activation of AMP-activated protein kinase [49] Stimulating tight junction signaling and the expression of mucin-associated peptides [50] Modulation of goblet cells to release specific mucins, such as MUC2 [51] | Cell | |
| Animal | |||
| Animal | |||
| Intestinal secretion | Regulation of 5-HT-mediated intestinal fluid and electrolyte secretion via 5-HT3R [52] Stimulation of the absorption of water and electrolyte through sodium, water influx, and duodenal bicarbonate secretion [53] | Animal | |
| Tryptophan metabolites | ENS function | Activation of AHR inducing expression of neuron-specific effector mechanisms [45] | Animal |
| CNS function | Acting as neuronal modulators to activate Trpa1, which transmit bacterial signals to enteric and vagal nerves (Indole-3-carboxaldehyde) [54] | Animal | |
| Immune activation | Inducing innate and adaptive immune responses by acting as ligands of AHR [55] Affecting TH17/Treg balance and mucosal homeostasis via IL-22 to attenuate intestinal inflammation in an AHR-dependent manner (Indole) [56] | Animal | |
| Intestinal barrier | Promotion of barrier integrity by enhancing expression of genes contributing to maintaining the structure and function of epithelial cells (Indole) [57] Enhancement of goblet cell differentiation and mucus secretionn [58] Serving as a ligand for PXR to enhance intestinal barrier [59] | Animal | |
| Intestinal secretion | Activation of GPCR 5-HT4R expressed in the colonic epithelium to elevate amounts of cyclic AMP (cAMP) and anion-dependent fluid secretion [60] | Animal | |
| BAs (especially Chenodeoxycholate and deoxycholate) | ENS function | Activation of TGR5 to release 5-HT and alter gastrointestinal transit [61] | Animal |
| Intestinal secretion | Stimulation of colonic secretion through intracellular activation of secretory mechanisms and suppressing of apical Cl−/OH− exchange [62] | Cell | |
| Lipopolysaccharide | ENS function | Enhancement of neuronal survival via TLR4 signaling [30] | Animal |
| Immune activation | Stimulation of the macrophages to produce pro-inflammatory cytokines via TLR4/ NF-κB pathways [63] | Cell | |
| Surface components of probiotics (surface layer proteins and capsular polysaccharide) | Immune activation | Integration with specific pattern recognition receptors, such as TLRs and NF-κB, to stimulate immune activation [64] | Animal |
| Methane | ENS function | Acting as the neuromuscular transmitter to impair the neuromuscular function of the gastrointestinal tract to reduce colonic peristalsis [65] | Animal |
| Hydrogen | ENS function | Enhancement of peristaltic velocity [65] | Animal |
3.2. Gut Microbiota, the Central Nervous System, and Constipation
3.3. Gut Microbiota, the Immune System, and Constipation
3.3.1. Gut Microbiota, Intestinal Epithelium Barrier Function, and Constipation
3.3.2. Gut Microbiota, Immune Activation, and Constipation
3.4. Gut Microbiota, Intestinal Secretion, and Constipation
3.5. Gut Microbiota, Ovarian Hormones, and Constipation
4. Role of Probiotics in the Treatment of Constipation
4.1. Probiotics Relieve Constipation by Modulating the Intestinal Microenvironment
4.2. Probiotics Relieve Constipation by Modulating ENS and CNS Function
4.3. Probiotics Relieve Constipation by Modulating Intestinal Permeability and Immune Function
4.4. Probiotics Relieve Constipation by Modulating Intestinal Secretion
4.5. Probiotics Relieve Constipation by Modulating the Function of the Endocrine System
5. Clinical Applications of Probiotics in the Relief of Constipation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vriesman, M.H.; Koppen, I.J.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Kashyap, P.C. Beyond phylotyping: Understanding the impact of gut microbiota on host biology. Neurogastroenterol. Motil. 2013, 25, 358–372. [Google Scholar] [CrossRef]
- Tierney, B.T.; Yang, Z.; Luber, J.M.; Beaudin, M.; Wibowo, M.C.; Baek, C.; Mehlenbacher, E.; Patel, C.J.; Kostic, A.D. The Landscape of Genetic Content in the Gut and Oral Human Microbiome. Cell Host Microbe 2019, 26, 283–295. [Google Scholar] [CrossRef]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Consortium, H.M.P. A framework for human microbiome research. Nature 2012, 486, 215. [Google Scholar]
- Khalif, I.L.; Quigley, E.M.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef]
- Dimidi, E.; Scott, S.M.; Whelan, K. Probiotics and constipation: Mechanisms of action, evidence for effectiveness and utilisation by patients and healthcare professionals. Proc. Nutr. Soc. 2020, 79, 147–157. [Google Scholar] [CrossRef]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, 1801187. [Google Scholar] [CrossRef]
- Attaluri, A.; Jackson, M.; Paulson, J.; Rao, S.S. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am. J. Gastroenterol. 2010, 105, 1407. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wu, T.; Lu, W.; Yuan, W.; Pan, M.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W.; Zhu, J.J.M. Predicting the Role of the Human Gut Microbiome in Constipation Using Machine-Learning Methods: A Meta-Analysis. Microorganisms 2021, 9, 2149. [Google Scholar] [CrossRef]
- Tian, H.; Ye, C.; Yang, B.; Cui, J.; Zheng, Z.; Wu, C.; Zhou, S.; Lv, X.; Qin, N.; Qin, H. Gut Metagenome as a Potential Diagnostic and Predictive Biomarker in Slow Transit Constipation. Front. Med. 2021, 8, 777961. [Google Scholar] [CrossRef]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Mangifesta, M.; Viappiani, A.; Ticinesi, A.; Nouvenne, A.; Meschi, T.; van Sinderen, D.; et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci. Rep. 2017, 7, 9879. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural changes in the gut microbiome of constipated patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Yang, F.; Liu, W.; Bai, H.; Ma, J.; Ma, X.; Zhang, J.; Fang, Y.; Miao, Y.; et al. The composition of intestinal microbiota and its association with functional constipation of the elderly patients. Future Microbiol. 2020, 15, 163–175. [Google Scholar] [CrossRef]
- de Meij, T.G.; de Groot, E.F.; Eck, A.; Budding, A.E.; Kneepkens, C.F.; Benninga, M.A.; van Bodegraven, A.A.; Savelkoul, P.H. Characterization of microbiota in children with chronic functional constipation. PLoS ONE 2016, 11, e0164731. [Google Scholar]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; Bharucha, A.E. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2015, 150, 367–379.e1. [Google Scholar] [CrossRef]
- Yarullina, D.R.; Shafigullin, M.U.; Sakulin, K.A.; Arzamastseva, A.A.; Shaidullov, I.F.; Markelova, M.I.; Grigoryeva, T.V.; Karpukhin, O.Y.; Sitdikova, G.F. Characterization of gut contractility and microbiota in patients with severe chronic constipation. PLoS ONE 2020, 15, e0235985. [Google Scholar]
- Lim, M.Y.; Song, E.-J.; Kim, S.H.; Lee, J.; Nam, Y.-D. Comparison of DNA extraction methods for human gut microbial community profiling. Syst. Appl. Microbiol. 2018, 41, 151–157. [Google Scholar] [CrossRef]
- Sartor, R.B. Optimal sampling of the intestinal microbiota for research. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 253–254. [Google Scholar]
- Mars, R.A.; Yang, Y.; Ward, T.; Houtti, M.; Priya, S.; Lekatz, H.R.; Tang, X.; Sun, Z.; Kalari, K.R.; Korem, T.J.C. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 2020, 182, 1460–1473.e17. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Verstraelen, H.; Osowska, S.; Doerffel, Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology 2008, 135, 568–579. [Google Scholar] [CrossRef]
- Durbán, A.; Abellán, J.J.; Jiménez-Hernández, N.; Salgado, P.; Ponce, M.; Ponce, J.; Garrigues, V.; Latorre, A.; Moya, A. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ. Microbiol. Rep. 2012, 4, 242–247. [Google Scholar] [CrossRef]
- Quigley, E.M.; Spiller, R.C. Constipation and the Microbiome: Lumen Versus Mucosa! Gastroenterology 2016, 150, 300–303. [Google Scholar] [CrossRef]
- Waclawikova, B.; Codutti, A.; Alim, K.; El Aidy, S. Gut microbiota-motility interregulation: Insights from in vivo, ex vivo and in silico studies. Gut Microbes 2022, 14, 1997296. [Google Scholar] [CrossRef]
- Vincent, A.D.; Wang, X.-Y.; Parsons, S.P.; Khan, W.I.; Huizinga, J.D. Abnormal absorptive colonic motor activity in germ-free mice is rectified by butyrate, an effect possibly mediated by mucosal serotonin. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G896–G907. [Google Scholar] [CrossRef]
- Obata, Y.; Pachnis, V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V.J.N. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 2015, 85, 289–295. [Google Scholar] [CrossRef]
- Anitha, M.; Vijay-Kumar, M.; Sitaraman, S.V.; Gewirtz, A.T.; Srinivasan, S. Gut Microbial Products Regulate Murine Gastrointestinal Motility via Toll-Like Receptor 4 Signaling. Gastroenterology 2012, 143, 1006–1016. [Google Scholar] [CrossRef]
- Yarandi, S.S.; Kulkarni, S.; Saha, M.; Sylvia, K.E.; Sears, C.L.; Pasricha, P.J. Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology 2020, 159, 200–213. [Google Scholar] [CrossRef]
- Turco, F.; Sarnelli, G.; Cirillo, C.; Palumbo, I.; De Giorgi, F.; D’Alessandro, A.; Cammarota, M.; Giuliano, M.; Cuomo, R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 2014, 63, 105–115. [Google Scholar] [CrossRef]
- Brun, P.; Giron, M.C.; Qesari, M.; Porzionato, A.; Caputi, V.; Zoppellaro, C.; Banzato, S.; Grillo, A.R.; Spagnol, L.; De Caro, R.; et al. Toll-Like Receptor 2 Regulates Intestinal Inflammation by Controlling Integrity of the Enteric Nervous System. Gastroenterology 2013, 145, 1323–1333. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Wichmann, A.; Allahyar, A.; Greiner, T.U.; Plovier, H.; Lundén, G.Ö.; Larsson, T.; Drucker, D.J.; Delzenne, N.M.; Cani, P.D.; Bäckhed, F.J.; et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 2013, 14, 582–590. [Google Scholar] [CrossRef]
- Tan, W.; Lee, G.; Chen, J.-H.; Huizinga, J.D. Relationships between Distention-, Butyrate- and Pellet-Induced Stimulation of Peristalsis in the Mouse Colon. Front. Physiol. 2020, 11, 109. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Hirokazu, F.; Xu, X.; Hiroto, M. Role of Gut Microbiota-Gut Hormone Axis in the Pathophysiology of Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2018, 24, 367–386. [Google Scholar]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M.J.G. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010, 138, 1772–1782.e74. [Google Scholar] [CrossRef]
- Rondeau, M.P.; Meltzer, K.; Michel, K.E.; McManus, C.M.; Washabau, R.J. Short chain fatty acids stimulate feline colonic smooth muscle contraction. J. Feline Med. Surg. 2003, 5, 167–173. [Google Scholar] [CrossRef]
- Shaidullov, I.F.; Sorokina, D.M.; Sitdikov, F.G.; Hermann, A.; Abdulkhakov, S.R.; Sitdikova, G.F. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastroenterol. 2021, 21, 37. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.J.N. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Chen, C.; Liu, L.; Wei, X.; Zeng, S. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front. Immunol. 2020, 11, 1455. [Google Scholar] [CrossRef]
- Fried, S.; Wemelle, E.; Cani, P.D.; Knauf, C.J.N. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology 2021, 197, 108721. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front. Immunol. 2021, 12, 1975. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Ruan, Z.; Li, J.; Zhang, L.; Lu, H.; Xu, Z. Puerarin Rebuilding the Mucus Layer and Regulating Mucin-Utilizing Bacteria to Relieve Ulcerative Colitis. J. Agric. Food Chem. 2020, 68, 11402–11411. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Schmidt, B.A.; Linden, D.R.; Larson, E.D.; Grover, M.; Beyder, A.; Farrugia, G.; Kashyap, P.C. Human Derived Gut Microbiota Modulates Colonic Secretion in Mice by Regulating 5-HT3 Receptor Expression via Acetate Production. Ajp Gastrointest. Liver Physiol. 2017, 313, G80–G87. [Google Scholar] [CrossRef]
- Murray, R.D.; McClung, H.J.; Li, B.; Ailabouni, A. Stimulatory effects of short-chain fatty acids on colonic absorption in newborn piglets in vivo. J. Pediatr. Gastroenterol. Nutr. 1989, 8, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Bae, M.; Cassilly, C.D.; Jabba, S.V.; Thorpe, D.W.; Martin, A.M.; Lu, H.-Y.; Wang, J.; Thompson, J.D.; Lickwar, C.R.; et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 2021, 29, 179–196.e9. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.; Macchiarulo, A.; Vacca, C. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef]
- Li, S.; Qi, C.; Zhu, H.; Yu, R.; Xie, C.; Peng, Y.; Yin, S.-W.; Fan, J.; Zhao, S.; Sun, J.; et al. Lactobacillus reuteri improves gut barrier function and affects diurnal variation of the gut microbiota in mice fed a high-fat diet. Food Funct. 2019, 10, 4705–4715. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785. [Google Scholar] [CrossRef]
- Alemi, F.; Poole, D.P.; Chiu, J.; Schoonjans, K.; Cattaruzza, F.; Grider, J.R.; Bunnett, N.W.; Corvera, C.U. J The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 2013, 144, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Sarathy, J.; Domingue, J.; Alrefai, W.A.; Rao, M.C. Chenodeoxycholic acid stimulates Cl- secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am. J. Physiol.-Cell Physiol. 2013, 305, C447–C456. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Yang, Y.; Tao, L. Aloperine suppresses LPS-induced macrophage activation through inhibiting the TLR4/NF-κB pathway. Inflamm. Res. 2020, 69, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Factories 2020, 19, 23. [Google Scholar] [CrossRef]
- Jahng, J.; Jung, I.S.; Choi, E.J.; Conklin, J.L.; Park, H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol. Motil. 2012, 24, 185–190.e92. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Christl, S.U.; Murgatroyd, P.R.; Gibson, G.R.; Cummings, J.H. Production, Metabolism, and Excretion of Hydrogen in the Large-Intestine. Gastroenterology 1992, 102, 1269–1277. [Google Scholar] [CrossRef]
- Triantafyllou, K.; Chang, C.; Pimentel, M. Methanogens, Methane and Gastrointestinal Motility. J. Neurogastroenterol. Motil. 2014, 20, 31–40. [Google Scholar] [CrossRef]
- Meng, S.; Bing, L.; Yanagawa, K.; Ha, N.T.; Goel, R.; Terashima, M.; Yasui, H. Effects of low pH conditions on decay of methanogenic biomass. Water Res. 2020, 179, 115883. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Srivastava, D.; Misra, A. A randomized double-blind placebo-controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: A pilot study. Indian J. Gastroenterol. 2018, 37, 416–423. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J. Relationship between functional constipation and brain-gut-microbiota axis. Zhonghua Wei Chang Wai Ke Za Zhi = Chin. J. Gastrointest. Surg. 2017, 20, 1345–1347. [Google Scholar]
- Aktar, R.; Parkar, N.; Stentz, R.; Baumard, L.; Parker, A.; Goldson, A.; Brion, A.; Carding, S.; Blackshaw, A.; Peiris, M. Human resident gut microbe Bacteroides thetaiotaomicron regulates colonic neuronal innervation and neurogenic function. Gut Microbes 2020, 11, 1745–1757. [Google Scholar] [CrossRef]
- Yu, C.D.; Xu, Q.J.; Chang, R.B. Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 2020, 62, 133–140. [Google Scholar] [CrossRef]
- McLean, P.G.; Borman, R.A.; Lee, K. 5-HT in the enteric nervous system: Gut function and neuropharmacology. Trends Neurosci. 2007, 30, 9–13. [Google Scholar] [CrossRef]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Gastroenterology 2017, 7, 10322. [Google Scholar] [CrossRef]
- Patel, R.M.; Myers, L.S.; Kurundkar, A.R.; Maheshwari, A.; Nusrat, A.; Lin, P.W. Probiotic Bacteria Induce Maturation of Intestinal Claudin 3 Expression and Barrier Function. Am. J. Pathol. 2012, 180, 626–635. [Google Scholar] [CrossRef]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Santos, J.; Vanner, S.J.; Vergnolle, N.; Zoetendal, E.G.; Quigley, E.M. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318.e08. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Z.; Shah, N.; El-Nezami, H.J. Modulation of intestinal epithelial defense responses by probiotic bacteria. Crit. Rev. Food Sci. Nutr. 2016, 56, 2628–2641. [Google Scholar] [CrossRef]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, H.; Östlund-Lindqvist, A.-M.; Nilsson, R.; Simrén, M.; Gillberg, P.-G. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand. J. Gastroenterol. 2008, 43, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2015, 65, 57–62. [Google Scholar] [CrossRef]
- Coquoz, A.; Regli, D.; Stute, P. Impact of progesterone on the gastrointestinal tract: A comprehensive literature review. Climacteric 2022, 25, 337–361. [Google Scholar] [CrossRef]
- Sovijit, W.N.; Sovijit, W.E.; Pu, S.; Usuda, K.; Inoue, R.; Watanabe, G.; Yamaguchi, H.; Nagaoka, K. Ovarian progesterone suppresses depression and anxiety-like behaviors by increasing the Lactobacillus population of gut microbiota in ovariectomized mice. Neurosci. Res. 2021, 168, 76–82. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Metwally, E.; Chughtai, M.I.; Mazhar, M.U.; Khan, S.A. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Anim. Nutr. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- García-Gómez, E.; González-Pedrajo, B.; Camacho-Arroyo, I. Role of sex steroid hormones in bacterial-host interactions. BioMed Res. Int. 2013, 2013, 928290. [Google Scholar] [CrossRef]
- Maryann, K.; Plottel, C.S.; Blaser, M.J.; Sylvia, A. The Intestinal Microbiome and Estrogen Receptor–Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar]
- Graham, M.E.; He Rbert, W.G.; Song, S.D.; Raman, H.N.; Zhu, J.E.; Gonzalez, P.E.; Walther-António, M.; Tetel, M.J. Gut and vaginal microbiomes on steroids: Implications for women’s health. Trends Endocrinol. Metab. 2021, 32, 554–565. [Google Scholar] [CrossRef]
- Nurielohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S. Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep. 2019, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Antwis, R.E.; Edwards, K.L.; Unwin, B.; Walker, S.L.; Shultz, S.J.M. Rare gut microbiota associated with breeding success, hormone metabolites and ovarian cycle phase in the critically endangered eastern black rhino. Microbiome 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Mallott, E.K.; Borries, C.; Koenig, A.; Amato, K.R.; Lu, A. Reproductive hormones mediate changes in the gut microbiome during pregnancy and lactation in Phayre’s leaf monkeys. Sci. Rep. 2020, 10, 9961. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, W.; Hua, J.; Hu, C.; Lok-Shun Lai, N.; Qian, P.-Y.; Lam, P.K.; Lam, J.C.; Zhou, B. Dysregulation of intestinal health by environmental pollutants: Involvement of the estrogen receptor and aryl hydrocarbon receptor. Environ. Sci. Technol. 2018, 52, 2323–2330. [Google Scholar] [CrossRef]
- Zielińska, M.; Fichna, J.; Bashashati, M.; Habibi, S.; Sibaev, A.; Timmermans, J.P.; Storr, M. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol. Motil. 2017, 29, e13025. [Google Scholar] [CrossRef]
- Liu, J.Y.; Lin, G.; Fang, M.; Rudd, J.A.J.G.; Endocrinology, C. Localization of estrogen receptor ERα, ERβ and GPR30 on myenteric neurons of the gastrointestinal tract and their role in motility. Gen. Comp. Endocrinol. 2019, 272, 63–75. [Google Scholar] [CrossRef]
- Xiong, W.; Jiang, Y.; Yu, T.; Zheng, Y.; Jiang, L.; Shen, X.; Tang, Y.; Lin, L. Estrogen-regulated expression of SK3 channel in rat colonic smooth muscle contraction. Life Sci. 2020, 263, 118549. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Cheng, L.; Cicala, M.; Ripetti, V.; Biancani, P.; Behar, J. Progesterone receptors and serotonin levels in colon epithelial cells from females with slow transit constipation. Neurogastroenterol. Motil. 2011, 23, 575. [Google Scholar] [CrossRef]
- Bradley, C.S.; Kennedy, C.M.; Turcea, A.M.; Rao, S.S.C.; Nygaard, I.E. Constipation in Pregnancy: Prevalence, Symptoms, and Risk Factors. Obstet. Gynecol. 2007, 110, 1351–1357. [Google Scholar] [CrossRef]
- Xiao, Z.; Pricolo, V.E.; Biancani, P.; Behar, J. Role of progesterone signaling in the regulation of G-protein levels in female chronic constipation. Gastroenterology 2005, 128, 667–675. [Google Scholar] [CrossRef]
- Cong, P.; Pricolo, V.E.; Biancani, P.; Behar, J. Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow-transit constipation. Gastroenterology 2007, 133, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Y.; Li, S.; Zhang, M.; Zhang, Z.; Shi, Y.; Zhang, X.; Zhang, S. iTRAQ-based proteomic analysis reveals the roles of progesterone receptor, inflammatory and fibrosis for slow transit constipation. J. Gastroenterol. Hepatol. 2017. [Google Scholar] [CrossRef]
- Roubaud-Baudron, C.; Ruiz, V.E.; Swan, A.M.; Vallance, B.A.; Blaser, M.J. Long-Term Effects of Early-Life Antibiotic Exposure on Resistance to Subsequent Bacterial Infection. mBio 2019, 10, e02820-19. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Fukui, H.; Eda, H.; Xu, X.; Kitayama, Y.; Hara, K.; Kodani, M.; Tomita, T.; Oshima, T.; Watari, J. Involvement of gut microbiota in association between GLP-1/GLP-1 receptor expression and gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G367–G373. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takada, T.; Shimizu, K.; Kado, Y.; Kawakami, K.; Makino, I.; Yamaoka, Y.; Hirano, K.; Nishimura, A.; Kajimoto, O.J.B.; et al. The effects of a probiotic milk product containing Lactobacillus casei strain Shirota on the defecation frequency and the intestinal microflora of sub-optimal health state volunteers: A randomized placebo-controlled cross-over study. Biosci. Microflora 2006, 25, 39–48. [Google Scholar] [CrossRef]
- Kondo, J.; Xiao, J.-Z.; Shirahata, A.; Baba, M.; Abe, A.; Ogawa, K.; Shimoda, T. Modulatory effects of Bifidobacterium longum BB536 on defecation in elderly patients receiving enteral feeding. World J. Gastroenterol. WJG 2013, 19, 2162. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Xu, Q.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacteria exerts species-specific effects on constipation in BALB/c mice. Food Funct. 2017, 8, 3587–3600. [Google Scholar] [CrossRef]
- Kusumo, P.D.; Maulahela, H.; Utari, A.P.; Surono, I.S.; Soebandrio, A.; Abdullah, M. Probiotic Lactobacillus plantarum IS 10506 supplementation increase SCFA of women with functional constipation. Iran. J. Microbiol. 2019, 11, 389–396. [Google Scholar]
- Wang, R.; Sun, J.; Li, G.; Zhang, M.; Niu, T.; Kang, X.; Zhao, H.; Chen, J.; Sun, E.; Li, Y. Effect of Bifidobacterium animalis subsp. lactis MN-Gup on constipation and the composition of gut microbiota. Benef. Microbes 2021, 12, 31–42. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Sugimura, N.; Li, Q.; Chu, E.S.H.; Lau, H.C.H.; Fong, W.; Liu, W.; Liang, C.; Nakatsu, G.; Su, A.C.Y.; Coker, O.O. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 2021, 69, 7–34. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J.J.G.M. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Saeedi, B.J.; Alam, A.; Houser, M.; Srinivasan, S.; Tansey, M.; Jones, R.; Nusrat, A.; Neish, A.S. Interactions between commensal bacteria and enteric neurons, via FPR1 induction of ROS, increase gastrointestinal motility in mice. Gastroenterology 2019, 157, 179–192.e2. [Google Scholar] [CrossRef]
- Wang, B.; Mao, Y.K.; Diorio, C.; Pasyk, M.; Wu, R.Y.; Bienenstock, J.; Kunze, W.A. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. Faseb J. 2010, 24, 4078–4088. [Google Scholar] [CrossRef]
- Perez-Burgos, A.; Mao, Y.K.; Bienenstock, J.; Kunze, W.A. The gut-brain axis rewired: Adding a functional vagal nicotinic “sensory synapse”. FASEB J. 2014, 28, 3064–3074. [Google Scholar] [CrossRef]
- Riezzo, G.; Chimienti, G.; Orlando, A.; D’Attoma, B.; Clemente, C.; Russo, F. Effects of long-term administration of Lactobacillus reuteri DSM-17938 on circulating levels of 5-HT and BDNF in adults with functional constipation. Benef. Microbes 2019, 10, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Iqbal Bhat, M.; Kandukuri, S.; Suman, K.; Rajeev, K. Potential probiotic Lactobacillus rhamnosus (MTCC-5897) inhibits Escherichia coliimpaired intestinal barrier function by modulating the host tight junction gene response. Probiotics Antimicrob. Proteins 2020, 12, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008, 8, 411–420. [Google Scholar] [CrossRef]
- Shi, J.; Du, P.; Xie, Q.; Wang, N.; Li, H.; Smith, E.E.; Li, C.; Liu, F.; Huo, G.; Li, B. Protective effects of tryptophan-catabolizing Lactobacillus plantarum KLDS 1.0386 against dextran sodium sulfate-induced colitis in mice. Food Funct. 2020, 11, 10736–10747. [Google Scholar] [CrossRef]
- Wang, L.; Chai, M.; Wang, G.; Zhang, H.; Zhao, J.; Chen, W. Bifidobacterium longum relieves constipation by regulating the intestinal barrier of mice. Food Funct. 2022. [Google Scholar] [CrossRef]
- Yi, R.; Peng, P.; Zhang, J.; Du, M.; Lan, L.; Qian, Y.; Zhou, J.; Zhao, X. Lactobacillus plantarum CQPC02-fermented soybean milk improves loperamide-induced constipation in mice. J. Med. Food 2019, 22, 1208–1221. [Google Scholar] [CrossRef]
- Ju Young, E.; Pei Lei, T.; Sei Mi, L.; Da Hye, C.; Seok Min, Y.; Si Young, Y.; Sae Hun, K. Laxative effect of probiotic chocolate on loperamide-induced constipation in rats. Food Res. Int. 2019, 116, 1173–1182. [Google Scholar] [CrossRef]
- Chen, C.-M.; Wu, C.-C.; Huang, C.-L.; Chang, M.-Y.; Cheng, S.-H.; Lin, C.-T.; Tsai, Y.-C. Lactobacillus plantarum PS128 Promotes Intestinal Motility, Mucin Production, and Serotonin Signaling in Mice. Probiotics Antimicrob. Proteins 2022, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Different Bifidobacterium bifidum strains change the intestinal flora composition of mice via different mechanisms to alleviate loperamide-induced constipation. Food Funct. 2021, 12, 6058–6069. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, B.; Wang, S.; Qian, X.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G.J.N. Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients 2021, 13, 1793. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Huang, F.; Zhang, Z.; Tao, X.; Wu, Q.; Qiu, L.; Wei, H. Probiotic Enterococcus faecalis Symbioflor 1 ameliorates pathobiont-induced miscarriage through bacterial antagonism and Th1-Th2 modulation in pregnant mice. Appl. Microbiol. Biotechnol. 2020, 104, 5493–5504. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Cui, S.; Lee, Y.-K.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Adhesive Bifidobacterium Induced Changes in Cecal Microbiome Alleviated Constipation in Mice. Front. Microbiol. 2019, 10, 1721. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Qi, X.; Yin, B.; Fang, D.; Gang, W.; Zhao, J.; Hao, Z.; Wei, C. Bifidobacterium adolescentis Exerts Strain-Specific Effects on Constipation Induced by Loperamide in BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 318. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, M.S.; Ji, G.E. Probiotic modulation of dendritic cells co-cultured with intestinal epithelial cells. World J. Gastroenterol. WJG 2012, 18, 1308. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S. Lactobacillus reuteri induces gut intraepithelial CD4+ CD8αα+ T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef]
- Matsuo, K.; Ota, H.; Akamatsu, T.; Sugiyama, A.; Katsuyama, T. Histochemistry of the surface mucous gel layer of the human colon. Gut 1997, 40, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Candeliere, F.; Raimondi, S.; Ranieri, R.; Musmeci, E.; Zambon, A.; Amaretti, A.; Rossi, M. β-Glucuronidase Pattern Predicted From Gut Metagenomes Indicates Potentially Diversified Pharmacomicrobiomics. Front. Microbiol. 2022, 13, 826994. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, A.; Tomizuka, K.; Aoki, R.; Nishijima, T.; Saito, Y.; Inoue, R.; Ushida, K.; Mawatari, T.; Ikeda, T. Effects of administration of Bifidobacterium animalis subsp. lactis GCL2505 on defecation frequency and bifidobacterial microbiota composition in humans. J. Biosci. Bioeng. 2012, 113, 587–591. [Google Scholar] [CrossRef]
- Tabbers, M.M.; Chmielewska, A.; Roseboom, M.G.; Crastes, N.; Perrin, C.; Reitsma, J.B.; Norbruis, O.; Szajewska, H.; Benninga, M.A. Fermented Milk Containing Bifidobacterium lactis DN-173 010 in Childhood Constipation: A Randomized, Double-Blind, Controlled Trial. Pediatr. Int. 2011, 127, e1392–e1399. [Google Scholar] [CrossRef]
- Dimidi, E.; Zdanaviciene, A.; Christodoulides, S.; Taheri, S.; Louis, P.; Duncan, P.I.; Emami, N.; Crabbé, R.; de Castro, C.A.; McLean, P.; et al. Randomised clinical trial: Bifidobacterium lactis NCC2818 probiotic vs placebo, and impact on gut transit time, symptoms, and gut microbiology in chronic constipation. Aliment. Pharmacol. Ther. 2019, 49, 251–264. [Google Scholar] [CrossRef]
- Ibarra, A.; Latreille-Barbier, M.; Donazzolo, Y.; Pelletier, X.; Ouwehand, A.C. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: A double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes 2018, 9, 236–251. [Google Scholar] [CrossRef]
- Koebnick, C.; Wagner, I.; Leitzmann, P.; Stern, U.; Zunft, H. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can. J. Gastroenterol. 2003, 17, 655–659. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Cha, J.M.; Oh, J.K.; Tan, P.L.; Kim, S.H.; Kwak, M.S.; Jeon, J.W.; Shin, H.P. Probiotics ameliorate stool consistency in patients with chronic constipation: A randomized, double-blind, placebo-controlled study. Dig. Dis. Sci. 2018, 63, 2754–2764. [Google Scholar] [CrossRef]
- Ling-Nan, B.U.; Chang, M.H.; Yen-Hsuan, N.I.; Chen, H.L.; Cheng, C.C. Lactobacillus casei rhamnosus Lcr35 in children with chronic constipation. Pediatr. Int. 2007, 49, 485–490. [Google Scholar]
- Wojtyniak, K.; Horvath, A.; Dziechciarz, P.; Szajewska, H. Lactobacillus casei rhamnosus Lcr35 in the Management of Functional Constipation in Children: A Randomized Trial. J. Pediatr. 2017, 184, 101–105.e1. [Google Scholar] [CrossRef]
- Chao, D.; Ge, X.; Zhang, X.; Tian, H.; Wang, H.; Gu, L.; Gong, J.; Zhu, W.; Ning, L. Efficacy of Synbiotics in Patients with Slow Transit Constipation: A Prospective Randomized Trial. Nutrients 2016, 8, 605. [Google Scholar]
- Wang, L.; Wang, L.; Tian, P.; Wang, B.; Cui, S.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Chen, W.; et al. A randomised, double-blind, placebo-controlled trial of Bifidobacterium bifidum CCFM16 for manipulation of gut microbiota and relief from chronic constipation. Food Funct. 2022, 13, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Tjokronegoro, S.D.P.; Advani, N.; Firmansyah, A. Effectiveness of Probiotics in the Management of Functional Constipation in Children: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Probiotics Prebiotics 2020, 15, 1–6. [Google Scholar]
- Kim, M.C.; Lee, S.; Park, J.K.; Park, J.; Lee, D.; Park, J.; Kim, B.-Y.; Cho, M.S.; Kim, T.-Y.; Park, H.Y. Effects of ID-HWS1000 on the Perception of Bowel Activity and Microbiome in Subjects with Functional Constipation: A Randomized, Double-Blind Placebo-Controlled Study. J. Med. Food 2021, 24, 883–893. [Google Scholar] [CrossRef]
- Venkataraman, R.; Shenoy, R.; Ahire, J.; Neelamraju, J.; Madempudi, R.J.P.; Proteins, A. Effect of Bacillus coagulans Unique IS2 with Lactulose on Functional Constipation in Adults: A Double-Blind Placebo Controlled Study. Probiotics Antimicrob. Proteins 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Yeun, Y.; Lee, J. Effect of a double-coated probiotic formulation on functional constipation in the elderly: A randomized, double blind, controlled study. Arch. Pharmacal Res. 2015, 38, 1345–1350. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Meta-analysis of randomized controlled trials of the effects of probiotics on functional constipation in adults. Clin. Nutr. 2020, 39, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.G.; Neale, E.P.; Ferreira, I. When poorly conducted systematic reviews and meta-analyses can mislead: A critical appraisal and update of systematic reviews and meta-analyses examining the effects of probiotics in the treatment of functional constipation in children. Am. J. Clin. Nutr. 2019, 110, 177–195. [Google Scholar] [CrossRef]
- Vale San Gomes, D.O.; de Morais, M.B. Gut microbiota and the use of probiotics in constipation in children and adolescents: Systematic review. Rev. Paul. Pediatr. 2020, 38, e2018123. [Google Scholar] [CrossRef]
- Tabbers, M.M.; DiLorenzo, C.; Berger, M.Y.; Faure, C.; Langendam, M.W.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, M.A. Evaluation and Treatment of Functional Constipation in Infants and Children: Evidence-Based Recommendations From ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274. [Google Scholar] [CrossRef]
- Miller, L.E.; Ouwehand, A.C.; Ibarra, A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: Systematic review and meta-analysis of randomized controlled trials. Ann. Gastroenterol. 2017, 30, 629–639. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Hungin, A.P.S.; Mitchell, C.R.; Whorwell, P.; Mulligan, C.; Cole, O.; Agreus, L.; Fracasso, P.; Lionis, C.; Mendive, J.; de Foy, J.M.P.; et al. Systematic review: Probiotics in the management of lower gastrointestinal symptoms—An updated evidence-based international consensus. Aliment. Pharmacol. Ther. 2018, 47, 1054–1070. [Google Scholar] [CrossRef]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; Andres de Paula, J.; et al. World Gastroenterology Organisation Global Guidelines Probiotics and Prebiotics October 2011. J. Clin. Gastroenterol. 2012, 46, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wilms, E.; Masclee, A.A.M.; Smidt, H. Age-dependent changes in GI physiology and microbiota: Time to reconsider? Gut 2018, 67, 2213–2222. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Xu, Z.-Y.; Han, M.; Gu, B.-H. Efficacy of pasteurised yoghurt in improving chronic constipation: A randomised, double-blind, placebo-controlled trial. Int. Dairy J. 2015, 40, 1–5. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Tian, Y.; Zuo, L.; Guo, Q.; Li, J.; Hu, Z.; Zhao, K.; Li, C.; Li, X.; Zhou, J.; Zhou, Y.; et al. Potential role of fecal microbiota in patients with constipation. Ther. Adv. Gastroenterol. 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Tian, H.; Ge, X.; Nie, Y.; Yang, L.; Ding, C.; McFarland, L.V.; Zhang, X.; Chen, Q.; Gong, J.; Li, N. Fecal microbiota transplantation in patients with slow-transit constipation: A randomized, clinical trial. PLoS ONE 2017, 12, e0171308. [Google Scholar] [CrossRef]
- Liu, J.; Gu, L.; Zhang, M.; Zhang, S.; Wang, M.; Long, Y.; Zhang, X. The Fecal Microbiota Transplantation: A Remarkable Clinical Therapy for Slow Transit Constipation in Future. Front. Cell. Infect. Microbiol. 2021, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry. Irritable Bowel Syndrome-Clinical Evaluation of Products for Treatment. 2010. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/irritable-bowel-syndrome-clinical-evaluation-products-treatment (accessed on 22 October 2012).
| Study | Subjects | Change | Quantification Method | DNA Extraction Methods |
|---|---|---|---|---|
| Khalif, I.L., et al., 2005 [7] | 57 FC, 25 controls (adults) | Bifidobacterium ↓ Lactobacillus ↓ Escherichia coli ↑ Staphylococcus aureus ↑ | Microbial culture methods | / |
| Zhuang, M., et al., 2019 [10] | 20 FC, 20 controls (adults) | Bifidobacterium ↓ Lactobacillus ↓ Faecalibacterium ↓ Roseburia ↓ Desulfovibrionaceae ↑ | 16S rRNA sequencing (V4) | Cetyltrimethyl Ammonium Bromide (CTAB) method; without bead-beating step |
| Attaluri, A., et al., 2010 [11] | 96 CC, 106 controls (adults) | Methanogenic flora ↑ | Breath tests | / |
| Chen, Y., et al., 2021 [12] | 3056 fecal amplicon sequence data from five constipation research cohorts | Serratia ↑ Dorea ↑ Aeromonas ↑ | Machine-learning methods | Commercial kits; with or without mechanical disruption step |
| Tian, H., et al., 2021 [13] | 50 FC, 40 controls (adults) | Roseburia intestinalis ↓ Haemophilus ↓ parainfluenzae ↓ Megamonas unclassified ↓ Klebsiella pneumoniae ↓ Alistipes putredinis ↑ Parabacteroides merdae ↑ Odoribacter splanchnicus ↑ Eubacterium eligens ↑ | Shotgun metagenomics | QIAamp DNA Stool Mini kit; without additional bead-beating step |
| Mancabelli, L., et al., 2017 [14] | 68 FC, 79 controls (children and adults) | Bacteroides ↓ Roseburia ↓ Coprococcus 3 ↓ | 16S rRNA sequencing and shotgun metagenomics | QIAamp DNA Stool Mini kit; without additional bead-beating step |
| Zhu, L., et al., 2014 [15] | 8 FC, 14 controls (children) | Bifidobacteria ↔ Lactobacilli ↔ Prevotella ↓ Firmicutes ↑ | 16S rRNA sequencing (V4-V5) | DNeasy Blood and Tissue Kit; with additional bead-beating step |
| Guo, M., et al., 2020 [16] | 61 FC, 48 controls (adults) | Firmicutes ↓ Proteobacteria ↓ Bacteroides ↑ Prevotella ↑ Lactococcus ↑ Ruminococcus ↑ Butyricimonas ↑ | 16S rRNA sequencing (V3-V4) | Fast DNA SPIN extraction kit; with bead-beating step |
| de Meij, T.G., et al., 2016 [17] | 76 FC, 61 controls (children) | Bifidobacterium longum ↑ Bacteroides fragilis ↑ Bacteroides ovatus ↑ | IS-pro | Bacterial lysis method; without bead-beating step |
| Parthasarathy, G., et al., 2015 [18] | 25 CC, 25 controls (adults) | Lactococcus ↓ Butyricimonas ↑ | 16S rRNA sequencing (V3-V5) | MoBio DNA extraction kit; with bead-beating step |
| Yarullina, D.R., et al., 2020 [19] | 15 CC, 10 controls (adults) | Roseburia ↓ Coprococcus ↓ Faecalibacterium ↓ Lactobacillus ↔ Bifidobacteria ↔ | Culture-based and 16S rRNA sequencing techniques (V3-V4) | Fast DNA SPIN extraction kit; with bead-beating step |
| Probiotics | Effect on Gastrointestinal Physiology | Mechanism | Model Organism |
|---|---|---|---|
| L. casei strain Shirota | Modulation microenvironment | Elevation in Bifidobacteria and Lactobacilli abundance [105] | Adults with a stronger tendency to constipation |
| B. longum BB536 | Increase in Bifidobacteria abundance to improve the frequency of defecation [106] | Adults with low defecation frequencies | |
| B. bifidum | Increase in the ratio of Firmicutes to Bacteroidetes and the amount of Lactobacillus and decrease the levels of pathogenic bacteria [107] | Animal | |
| L. plantarum IS 10506 | Enhancement of SCFA levels to promote gut motility [108] | Adults with FC | |
| B. animalis subsp. lactis MN-Gup | Improvment of acetate levels to improve GI transit rate [109] | Animals and adults with FC | |
| L. gallinarum | Breaking down tryptophan and modulation of gut microenvironment to improve colon function [110,111] | Animal | |
| Clostridium butyricum | ENS and CNS function | Regulation of TLR2 signaling pathway to promote intestinal motility [112] | Animal |
| L. rhamnosus GG | Enhancement of the expression of choline acetyltransferase and gut motility via FPR1 [113] | Animal | |
| L.reuteri | Mediation of the excitability of myenteric neurons and interaction with the gut–brain axis by influencing afferent sensory nerves to regulate bowel movement [114] | Animal | |
| L. rhamnosus | Modulation of mesenteric vagal afferent firing [115] | Animal | |
| L.reuteri DSM-17938 | Reduction in 5-HT and BDNF levels to ameliorate constipation [116] | Adults with FC | |
| L.rhamnosus (MTCC-5897) | Intestinal permeability and immune function | Augment the expression of tight junction proteins and MUC2 gene to stimulate mucin secretion by goblet cells [117] | Animal |
| Butyrate-prodution bacteria | Enhancement of mucosal layer to alleviate constipation symptoms [118] | Animal | |
| L. plantarum KLDS 1.0386 | Augment tight junction proteins amd mucin mRNA expression and anti-inflammatory cytokine (IL-10) levels, and reduction in pro-inflammatory cytokine levels by metabolizing tryptophan [119] | Animal | |
| B. longum | Decrease in the concentrations of IL-1β and TNF-α in the colon tissue and increase in the expression of occludin to improve constipation [120] | Animal | |
| L.plantarum CQPC02 | Intestinal secretion function | Improvement of the water content in stool associated with stimulatory effects of elevated SCFAs on water and electrolyte absorption [121] | Animal |
| L.plantarum LRCC5193 | Promotion of intestinal fluid secretion in rats [122] | Animal | |
| L.plantarum PS128 | Increase in mucin production [123] | Animal | |
| Bifidobacterium (B.bifidum and B. animalis ssp.) | Modulation of 5-HT4R expression to promote colonic fluid secretion [124] | Animal | |
| Lactobacilli and bifidobacteria | Hormonal milieu | Decrease in the estrogen reabsorption rate and adjustment of the estrogen level via decreasing the relative abundance of bacteria producing β-glucuronidase [87] | Animal |
| L. plantarum 30M5 | Alteration in the levels of circulating estrogen by affecting gut microbiome and its metabolism [125] | Animal | |
| Enterococcus faecalis | Modulation of progesterone levels and Th1-Th2 homeostasis [126] | Animal |
| Study | Population | Probiotic | Intervention | Main Outcome |
|---|---|---|---|---|
| Ishizuka A, T.K., et al., 2012 [133] | 17 adults with FC | B. animalis subsp. lactis GCL2505 | Four consecutive 2-week periods (1010 CFU/d) | Supplementation with GCL2505 increased the defecation frequency (+0.5 times/week, p < 0.05) and there was no significant change in stool quantity (p < 0.1). |
| Tabbers, M.M., et al., 2011 [134] | 159 children with FC | B. lactis DN-173 010 | Twice a day for 3 weeks (8.5 × 109 CFU/d) | There was no statistically significant change in the stool frequency (4.5 times/week vs. 3.9 times/week, p = 0.31) and stool consistency between probiotic group and placebo (mean score of 3.3 vs. 3.5, p = 0.07). |
| Dimidi E, Zdanaviciene A, et al., 2019 [135] | 79 adults with FC | B. lactis NCC2818 | 4 weeks (1.5 × 1010 CFU/d) | There was no statistically significant change in the gut transit time, stool frequency, stool output, symptoms, stool consistency, or quality of life and Bifidobacterium concentrations (p < 0.05) between B. lactis NCC2818 treatment group and placebo group. |
| Ibarra, A., et al., 2018 [136] | 228 adults with FC | B. animalis subsp. lactis HN019 | 4 weeks (1 × 109 or 1 × 1010 CFU/d) | There was no statistically significant differences in constipation symptoms after interventions (p < 0.05); B. animalis subsp. lactis HN019 administration improved the BMF in patients with low stool frequency (≤3 times/week) (high dose: +2 times/week, low lose: +1.7 times/week, placebo: +0.8 times/week, p= 0.01). |
| Koebnick, C., et al., 2003 [137] | 70 adults with CC | L. casei Shirota (LcS) | 4 weeks (65 mL/d of a probiotic beverage containing LcS) | Treatment with LcS increased defecation frequency by 3 times/week (p = 0.04), increased percentage of treatment success (Lcs: 89%, placebo: 56%, p = 0.003), reduced the incidence of severe constipation (Lcs: 34%, placebo: 83%, p < 0.001). |
| Yoon, J.Y., et al., 2018 [138] | 171 adults with CC | Streptococcus thermophilus MG510 and L. plantarum LRCC5193 | 4 weeks (3.0 × 108 CFU/g Streptococcus thermophilus MG510 and 1.0 × 108 CFU/g L. plantarum LRCC5193) | Probiotics improved stool consistency indicated by the Bristol Stool Form Scale in the probiotic group compared with placebo group (3.7 ± 1.1 vs. 3.1 ± 1.1, p = 0.002) and quality of life (p = 0.049). |
| Ling-Nan, B.U., et al., 2007 [139] | 45 children with CC | L. casei rhamnosus Lcr35 | Once daily for 4 weeks (8 × 108 CFU/d) | Administration of L. casei rhamnosus Lcr35 significantly increased defecation frequency (0.57 ± 0.17 times/day vs. 0.37 ± 0.1 times/day, p = 0.03), reduced the incidence of hard stools (22.4 ± 7.9% vs. 75.5 ± 6.1%, p = 0.03), and the percentage of treatment success compared to the placebo group (77.8% vs. 11.1%, p = 0.002). |
| Wojtyniak, K., et al., 2017 [140] | 94 children with FC | L. casei rhamnosus Lcr35 | Twice daily for 4 weeks (1.6 × 109 CFU/d) | The defecation frequency in the placebo group was significantly greater than in the Lcr35 group (+4 times/week vs. +2 times/week, p < 0.01). |
| Chao, D., et al., 2016 [141] | 100 adults with FC | Bifid triple | Twice daily for 12 weeks (0.63 g of bifid triple viable capsules and 8 g of soluble dietary fiber) | Synbiotic intake dramatically enhanced clinical remission rates (64.6% vs. 29.2%, p < 0.01), reduced colonic transit time (49.3 ± 11.7 vs. 70.5 ± 12.1, p = 0.03), improved the stool consistency score (3.5 ± 1.1 vs. 2.4 ± 0.8, p < 0.001). |
| Wang, L., et al., 2022 [142] | 103 adults with CC | B. bifidum CCFM16 | 4 weeks (2 × 109 CF U/d) | Treatment of B. bifidum CCFM16 increased SBMs (+0.736 SBMs peer week vs. +0.36 SBMs peer week, p = 0.116) and obviously improved BSFS (+0.925 vs. +0.2, p = 0.0019) compared with placebo. |
| Tjokronegoro, S.D.P., et al., 2020 [143] | 78 children with FC | L. acidophilus, B. longum, and S. thermophylus | Twice a day for 4 weeks (2 × 109 CFU/d) | Probiotics treatment significantly improved stool consistency (27/39 vs. 17/39, p = 0.022) and difficulty of defecation (31/39 vs. 20/39, p = 0.009) compared with placebo. Overall, relief of constipation with probiotics was better than placebo (31/39 vs. 18/39, p = 0.002). |
| Kim, M.C., et al., 2021 [144] | 30 adults with FC | ID-HWS1000 contained six types of probiotics and xylooligosaccharide | 4 weeks (one packet a day) | ID-HWS1000 greatly ameliorated the discomfort related to bowel movements, including number of irritable bowel movements compared with placebo (p < 0.001). |
| Venkataraman, R., et al., 2021 [145] | 150 adults with FC | B. coagulans Unique IS2 and lactulose | 4 weeks (B. coagulans Unique IS2, 2 × 109 spores) with lactulose (10 g) | There was significant improvement in number of bowel movements in synbiotic groups compared to lactulose or probiotics treatment alone at 3 weeks (p < 0.001), while the difference was insignificant at 4 weeks. Probiotics combined with lactulose were significantly more effective and required less time to achieve normal fecal consistency than lactulose (p < 0.001). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. https://doi.org/10.3390/nu14183704
Pan R, Wang L, Xu X, Chen Y, Wang H, Wang G, Zhao J, Chen W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients. 2022; 14(18):3704. https://doi.org/10.3390/nu14183704
Chicago/Turabian StylePan, Ruili, Linlin Wang, Xiaopeng Xu, Ying Chen, Haojue Wang, Gang Wang, Jianxin Zhao, and Wei Chen. 2022. "Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation" Nutrients 14, no. 18: 3704. https://doi.org/10.3390/nu14183704
APA StylePan, R., Wang, L., Xu, X., Chen, Y., Wang, H., Wang, G., Zhao, J., & Chen, W. (2022). Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients, 14(18), 3704. https://doi.org/10.3390/nu14183704




