Sodium, Potassium and Iodine Intake in an Adult Population of Lithuania
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size
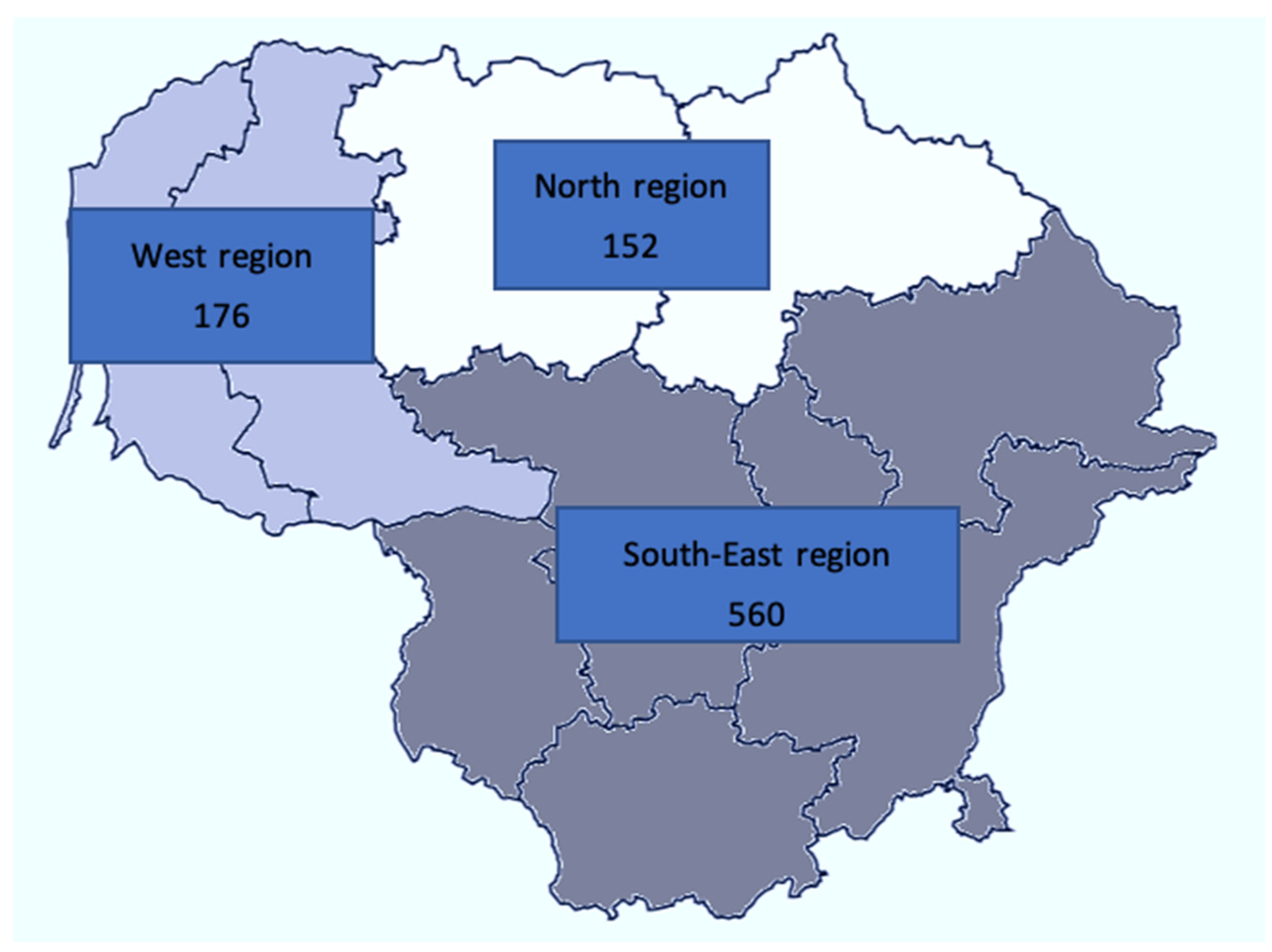
2.2. Sample Selection and Invitation Procedure
2.3. Questionnaire and Physical Data
2.4. Procedures of 24 h Urine Collection
2.5. The 24 h Urine Sample Preparation in the Laboratory
2.6. Assurance of the 24 h Urine Sample Quality
- The start and end day and time of the collection were not recorded and could not be ascertained;
- The duration of the collection, either observed by staff or self-reported, was out of range of 20–26 h;
- If self-report of missing more than one void using a questionnaire;
- If 24 h urine volume was less than 500 mL;
- If 24 h urinary creatinine excretion was outside 2 standard deviations of the sex-specific distribution (5.9–26.0 mmol/24 h for men and 4.0–16.4 mmol/24 h for women).
2.7. Urine Biochemistry Panel
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Participants
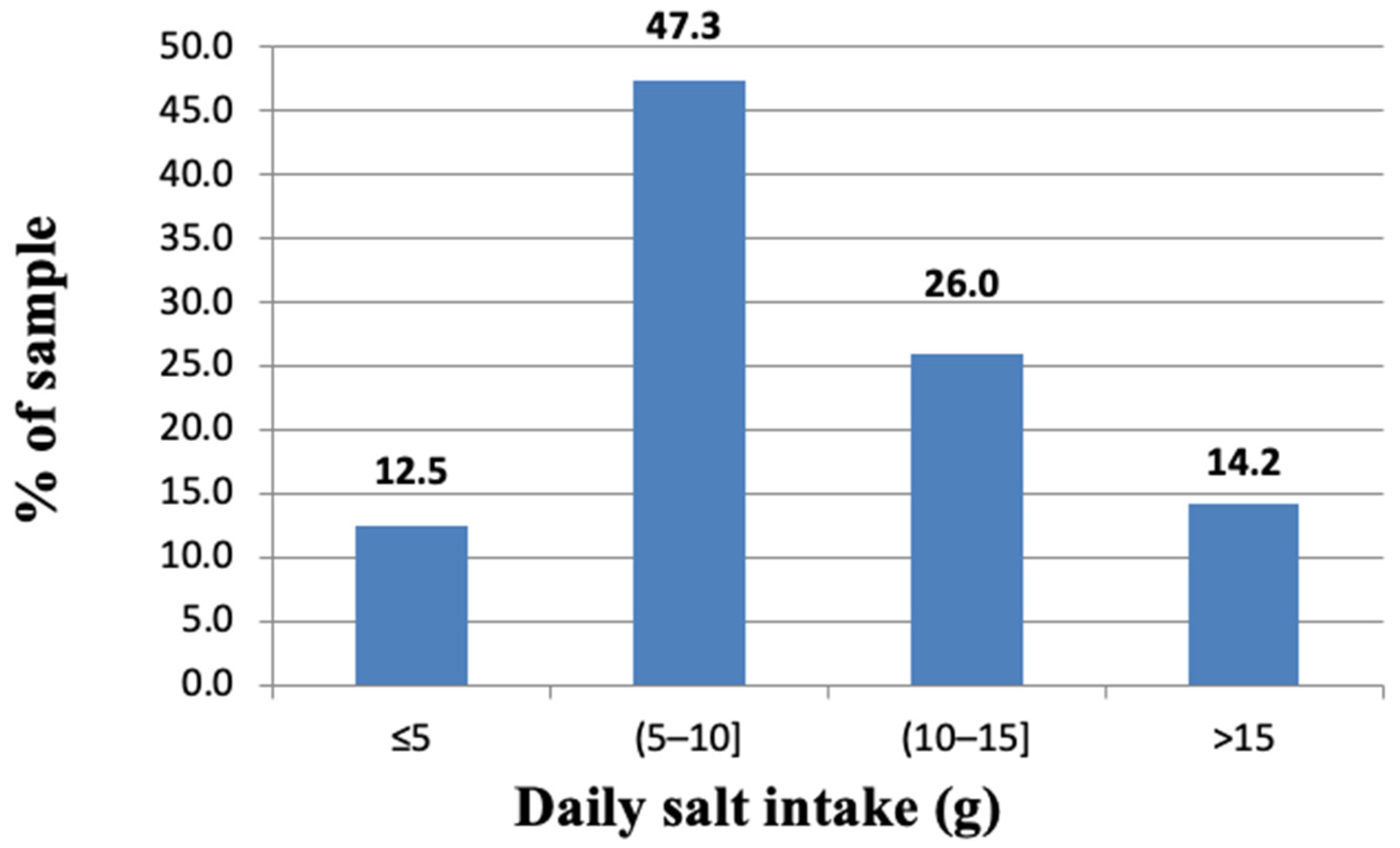
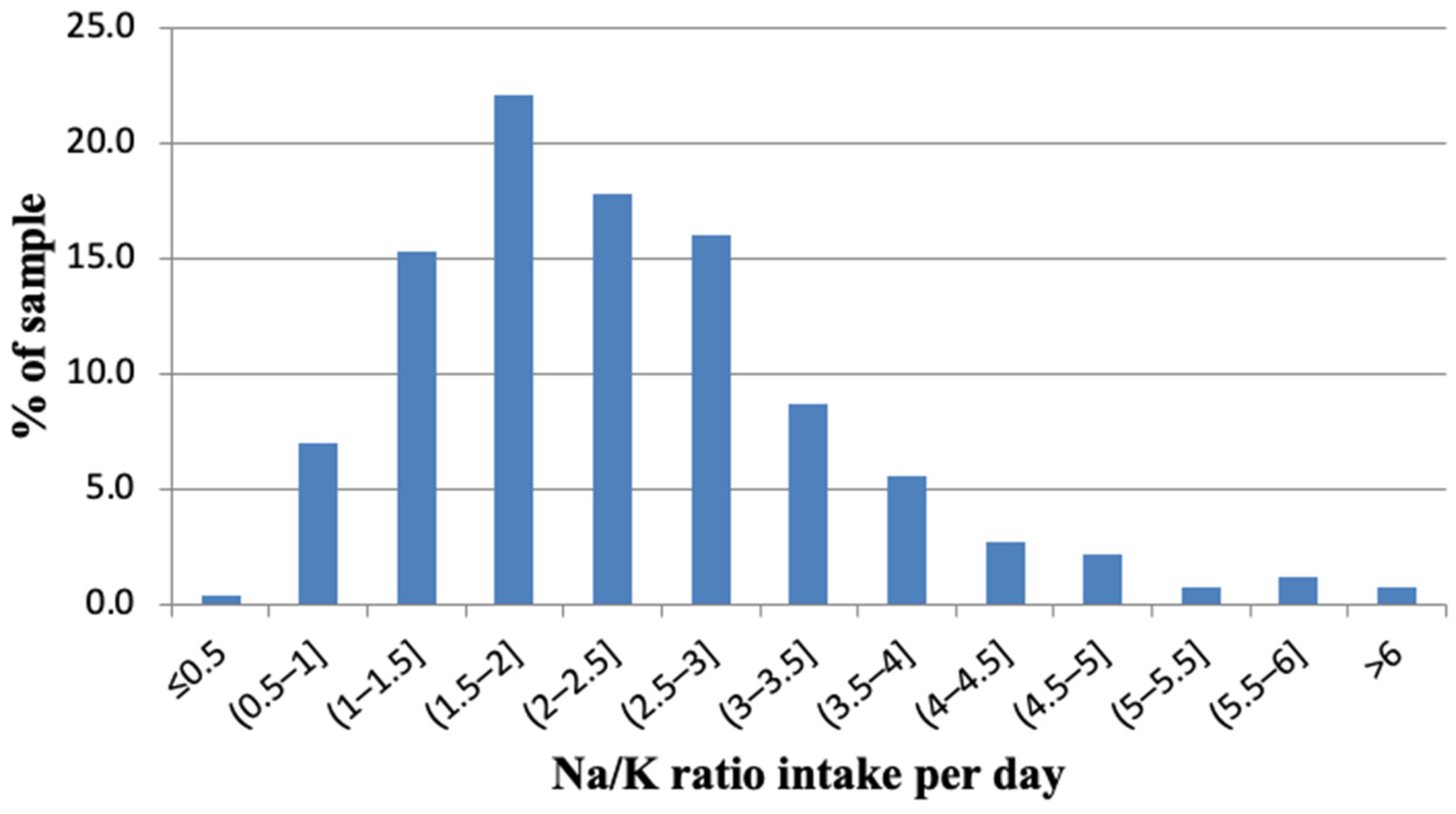
3.2. Urinary Sodium Excretion and Salt Consumption
3.3. Urinary Potassium Excretion and Potassium Intake
3.4. Daily Intake of Iodine and Use of Iodized Salt
4. Discussion
4.1. Salt Consumption
4.2. Potassium Consumption
4.3. Iodine Consumption
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013; ISBN 978-92-4-150623-6. [Google Scholar]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Sodium Consumption and Death from Cardiovascular Causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Khatib, O.M.N.; El-Guindy, M.S.; World Health Organization; Regional Office for the Eastern Mediterranean. Clinical Guidelines for the Management of Hypertension; World Health Organization: Geneva, Switzerland; Regional Office for the Eastern Mediterranean: Cairo, Egypt, 2005; ISBN 978-92-9021-407-6. [Google Scholar]
- Pastor-Barriuso, R.; Banegas, J.R.; Damin, J.; Appel, L.J.; Guallar, E. Systolic Blood Pressure, Diastolic Blood Pressure, and Pulse Pressure: An Evaluation of Their Joint Effect on Mortality. Ann. Intern. Med. 2003, 139, 731. [Google Scholar] [CrossRef] [PubMed]
- Laucevičius, A.; Rinkūnienė, E.; Petrulionienė, Ž.; Ryliškytė, L.; Jucevičienė, A.; Puronaitė, R.; Badarienė, J.; Navickas, R.; Mikolaitytė, J.; Gargalskaitė, U.; et al. Trends in Cardiovascular Risk Factor Prevalence among Lithuanian Middle-Aged Adults between 2009 and 2018. Atherosclerosis 2020, 299, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hyseni, L.; Elliot-Green, A.; Lloyd-Williams, F.; Kypridemos, C.; O’Flaherty, M.; McGill, R.; Orton, L.; Bromley, H.; Cappuccio, F.P.; Capewell, S. Systematic Review of Dietary Salt Reduction Policies: Evidence for an Effectiveness Hierarchy? PLoS ONE 2017, 12, e0177535. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Potassium Intake for Adults and Children; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150482-9. [Google Scholar]
- Cappuccio, F.; Ji, C.; Donfrancesco, C.; Palmieri, L.; Ippolito, R.; Vanuzzo, D.; Giampaoli, S.; Strazzullo, P. Geographic and Socioeconomic Variation of Sodium and Potassium Intake in Italy: Results from the MINISAL-GIRCSI Programme. BMJ Open 2015, 5, e007467. [Google Scholar] [CrossRef]
- Vasara, E.; Marakis, G.; Breda, J.; Skepastianos, P.; Hassapidou, M.; Kafatos, A.; Rodopaios, N.; Koulouri, A.A.; Cappuccio, F.P. Sodium and Potassium Intake in Healthy Adults in Thessaloniki Greater Metropolitan Area-The Salt Intake in Northern Greece (SING) Study. Nutrients 2017, 9, 417. [Google Scholar] [CrossRef]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.L.; Campbell, N.R.C.; Wang, M.; et al. 24-Hour Urinary Sodium and Potassium Excretion and Cardiovascular Risk. N. Engl. J. Med. 2022, 386, 252–263. [Google Scholar] [CrossRef]
- World Health Organization. Accelerating Salt Reduction in Europe: A Country Support Package to Reduce Population Salt Intake in the WHO European Region 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Effect of Increased Potassium Intake on Blood Pressure, Renal Function, Blood Lipids and Other Potential Adverse Effects; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150488-1. [Google Scholar]
- D’Elia, L.; Barba, G.; Cappuccio, F.P.; Strazzullo, P. Potassium Intake, Stroke, and Cardiovascular Disease a Meta-Analysis of Prospective Studies. J. Am. Coll. Cardiol. 2011, 57, 1210–1219. [Google Scholar] [CrossRef]
- World Health Organization. European Food and Nutrition Action Plan 2015–2020; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Cook, N.R.; Obarzanek, E.; Cutler, J.A.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Trials of Hypertension Prevention Collaborative Research Group Joint Effects of Sodium and Potassium Intake on Subsequent Cardiovascular Disease: The Trials of Hypertension Prevention Follow-up Study. Arch. Intern. Med. 2009, 169, 32–40. [Google Scholar] [CrossRef]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of Inadequate Iodine Status in UK Pregnant Women on Cognitive Outcomes in Their Children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Boelaert, K. Iodine Deficiency and Thyroid Disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Biban, B.G.; Lichiardopol, C. Iodine Deficiency, Still a Global Problem? Curr. Health Sci. J. 2017, 43, 103–111. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of Excess Iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef]
- Lucko, A.M.; Doktorchik, C.; Woodward, M.; Cogswell, M.; Neal, B.; Rabi, D.; Anderson, C.; He, F.J.; MacGregor, G.A.; L’Abbe, M.; et al. Percentage of Ingested Sodium Excreted in 24-Hour Urine Collections: A Systematic Review and Meta-Analysis. J. Clin. Hypertens. 2018, 20, 1220–1229. [Google Scholar] [CrossRef]
- Wainwright, P.; Cook, P. The Assessment of Iodine Status—Populations, Individuals and Limitations. Ann. Clin. Biochem. 2019, 56, 7–14. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Maalouf, J.; Elliott, P.; Loria, C.M.; Patel, S.; Bowman, B.A. Use of Urine Biomarkers to Assess Sodium Intake: Challenges and Opportunities. Annu. Rev. Nutr. 2015, 35, 349–387. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- Vejbjerg, P.; Knudsen, N.; Perrild, H.; Laurberg, P.; Andersen, S.; Rasmussen, L.B.; Ovesen, L.; Jørgensen, T. Estimation of Iodine Intake from Various Urinary Iodine Measurements in Population Studies. Thyroid 2009, 19, 1281–1286. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for the Eastern Mediterranean. How to Obtain Measures of Population-Level Sodium Intake in 24-Hour Urine Samples; World Health Organization, Regional Office for the Eastern Mediterranean: Cairo, Egypt, 2018. [Google Scholar]
- World Health Organization, Regional Office for Europe. How to Obtain Measures of Population-Level Sodium Intake in 24-Hour Urine Samples: Protocol; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Pan American Health Organization/World Health Organization. Salt-Smart Americas: A Guide for Country-Level Action; Pan American Health Organization: Washington, DC, USA; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- D’Elia, L.; Obreja, G.; Ciobanu, A.; Breda, J.; Jewell, J.; Cappuccio, F.P. Sodium, Potassium and Iodine Intake, in a National Adult Population Sample of the Republic of Moldova. Nutrients 2019, 11, 2896. [Google Scholar] [CrossRef]
- D’Elia, L.; Brajović, M.; Klisic, A.; Breda, J.; Jewell, J.; Cadjenović, V.; Cappuccio, F. Sodium and Potassium Intake, Knowledge Attitudes and Behaviour Towards Salt Consumption Amongst Adults in Podgorica, Montenegro. Nutrients 2019, 11, 160. [Google Scholar] [CrossRef]
- Stamler, J.; Rose, G.; Stamler, R.; Elliott, P.; Dyer, A.; Marmot, M. INTERSALT Study Findings. Public Health and Medical Care Implications. Hypertension 1989, 14, 570–577. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for Europe. STEPS Prevalence of Noncommunicable Disease Risk Factors in Ukraine 2019; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Brussels, Belgium, 2020. [Google Scholar]
- Trieu, K.; Ospanova, F.; Tazhibayev, S.; Jewell, J.; Breda, J.; Santos, J.A.; Webster, J. Sodium and Potassium Intakes in the Kazakhstan Population Estimated Using 24-h Urinary Excretion: Evidence for National Action. Eur. J. Nutr. 2021, 60, 1537–1546. [Google Scholar] [CrossRef]
- Tan, M.; He, F.J.; Wang, C.; MacGregor, G.A. Twenty-Four-Hour Urinary Sodium and Potassium Excretion in China: A Systematic Review and Meta-Analysis. JAHA 2019, 8, e012923. [Google Scholar] [CrossRef]
- Laatikainen, T.; Pietinen, P.; Valsta, L.; Sundvall, J.; Reinivuo, H.; Tuomilehto, J. Sodium in the Finnish Diet: 20-Year Trends in Urinary Sodium Excretion among the Adult Population. Eur. J. Clin. Nutr. 2006, 60, 965–970. [Google Scholar] [CrossRef]
- Madar, A.A.; Heen, E.; Hopstock, L.A.; Carlsen, M.H.; Meyer, H.E. Iodine Intake in Norwegian Women and Men: The Population-Based Tromsø Study 2015–2016. Nutrients 2020, 12, 3246. [Google Scholar] [CrossRef]
- Hulthén, L.; Aurell, M.; Klingberg, S.; Hallenberg, E.; Lorentzon, M.; Ohlsson, C. Salt Intake in Young Swedish Men. Public Health Nutr. 2010, 13, 601. [Google Scholar] [CrossRef] [PubMed]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, Regional and National Sodium Intakes in 1990 and 2010: A Systematic Analysis of 24 h Urinary Sodium Excretion and Dietary Surveys Worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef]
- Cappuccio, F.P. Cardiovascular and Other Effects of Salt Consumption. Kidney Int. Suppl. 2013, 3, 312–315. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Jousilahti, P.; Rastenyte, D.; Moltchanov, V.; Tanskanen, A.; Pietinen, P.; Nissinen, A. Urinary Sodium Excretion and Cardiovascular Mortality in Finland: A Prospective Study. Lancet 2001, 357, 848–851. [Google Scholar] [CrossRef]
- Ma, H.; Xue, Q.; Wang, X.; Li, X.; Franco, O.H.; Li, Y.; Heianza, Y.; Manson, J.E.; Qi, L. Adding Salt to Foods and Hazard of Premature Mortality. Eur. Heart J. 2022, 43, 2878–2888. [Google Scholar] [CrossRef]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef]
- Okayama, A.; Okuda, N.; Miura, K.; Okamura, T.; Hayakawa, T.; Akasaka, H.; Ohnishi, H.; Saitoh, S.; Arai, Y.; Kiyohara, Y.; et al. Dietary Sodium-to-Potassium Ratio as a Risk Factor for Stroke, Cardiovascular Disease and All-Cause Mortality in Japan: The NIPPON DATA80 Cohort Study. BMJ Open 2016, 6, e011632. [Google Scholar] [CrossRef]
- Petrauskaite, A.; Imbrasienė, A.; Bartkeviciute, R. The Evaluation of Iodine Excretion between the Lithuania School Children. Medicina 1995, 3, 714–716. [Google Scholar]
- Iacone, R.; Iaccarino Idelson, P.; Formisano, P.; Russo, O.; Lo Noce, C.; Donfrancesco, C.; Macchia, P.E.; Palmieri, L.; Galeone, D.; di Lenarda, A.; et al. Iodine Intake Estimated by 24 h Urine Collection in the Italian Adult Population: 2008–2012 Survey. Nutrients 2021, 13, 1529. [Google Scholar] [CrossRef]
- Vandevijvere, S.; Ruttens, A.; Wilmet, A.; Marien, C.; Hautekiet, P.; Van Loco, J.; Moreno-Reyes, R.; Van der Heyden, J. Urinary Sodium and Iodine Concentrations among Belgian Adults: Results from the First National Health Examination Survey. Eur. J. Clin. Nutr. 2021, 75, 689–696. [Google Scholar] [CrossRef]
- Ittermann, T.; Albrecht, D.; Arohonka, P.; Bilek, R.; de Castro, J.J.; Dahl, L.; Filipsson Nystrom, H.; Gaberscek, S.; Garcia-Fuentes, E.; Gheorghiu, M.L.; et al. Standardized Map of Iodine Status in Europe. Thyroid 2020, 30, 1346–1354. [Google Scholar] [CrossRef]
- Bartkevičiūtė, R.; Bulotaitė, G.; Stukas, R.; Butvila, M.; Drungilas, V.; Barzda, A. Dietary Habits of Lithuanian Adult Population and Dietary Habits Trends. Visuomenės Sveik. 2020, 90, 1–84. [Google Scholar]
- García Ascaso, M.T.; Pérez, P.R.; Alcol, E.C.; López, A.L.; de Lucas Collantes, C.; Santos, I.M.; Tessier, E.; Segura, S.A. Nutritional Status of Iodine in Children: When Appropriateness Relies on Milk Consumption and Not Adequate Coverage of Iodized Salt in Households. Clin. Nutr. ESPEN 2019, 30, 52–58. [Google Scholar] [CrossRef]
- Vandevijvere, S.; Mourri, A.B.; Amsalkhir, S.; Avni, F.; Van Oyen, H.; Moreno-Reyes, R. Fortification of Bread with Iodized Salt Corrected Iodine Deficiency in School-Aged Children, but Not in Their Mothers: A National Cross-Sectional Survey in Belgium. Thyroid 2012, 22, 1046–1053. [Google Scholar] [CrossRef]
- Esche, J.; Thamm, M.; Remer, T. Contribution of Iodized Salt to Total Iodine and Total Salt Intake in Germany. Eur. J. Nutr. 2020, 59, 3163–3169. [Google Scholar] [CrossRef]
- World Health Organization. Salt Reduction and Iodine Fortification Strategies in Public Health; World Health Organization: Geneva, Switzerland, 2014; pp. 1–34. [Google Scholar]

| Analyte (Urine) | Method | Traceability to Reference Material |
|---|---|---|
| Creatinine | Photometric, enzymatic | NIST SRM 967 |
| Sodium, potassium | Indirect potentiometry (indirect ion-selective electrode (ISE)) | NIST SRM 918 and NIST SRM 919 |
| Iodine | Spectrophotometric, Sandell–Kolthoff method | NIST RM2670a, SRM3668 |
| Variables | Total | Men | Women | p-Value |
|---|---|---|---|---|
| 888 | 422 | 466 | - | |
| Age, year | 47.4 (12.1) | 46.1 (12.3) | 48.6 (11.7) | 0.82 |
| Height, cm | 173.2 (9.4) | 180.4 (6.8) | 166.8 (6.2) | <0.001 |
| Weight, kg | 79.7 (16.7) | 88.5 (14.8) | 71.9 (14.4) | <0.001 |
| BMI, kg/m2 | 26.5 (4.7) | 27.2 (4.2) | 25.9 (5) | <0.001 |
| Systolic BP, mmHg | 124.1 (22.5) | 129.7 (28.1) | 119.1 (14.1) | <0.001 |
| Diastolic BP, mmHg | 77.9 (9.3) | 80.4 (8.9) | 75.7 (10.1) | <0.001 |
| Pulse rate, bpm | 72.5 (10.0) | 72.3 (10,1) | 72.8 (9.9) | 0.55 |
| Hypertension, n (%) | 312 (35.5) | 177 (42.5) | 135 (29.2) | <0.001 * |
| Current smokers, n (%) | 151 (17.0) | 93 (22.0) | 58 (12.4) | <0.001 * |
| Variables | Total | Men | Women | p-Value |
|---|---|---|---|---|
| n | 888 | 422 | 466 | |
| Volume, ml/24 h | 2045 (799) 1920 (1472; 2530) | 2057 (823) 1920 (1487; 2559) | 2035 (778) 1920 (1470; 2553) | 0.68 |
| Sodium, mmol/24 h | 162.4 (86) 146.1 (104.8; 198.6) | 191.3 (94.6) 171.2 (131.8; 236.3) | 136.3 (67.5) 123.7 (89.7; 167.4) | <0.001 |
| Potassium, mmol/24 h | 73.8 (29.6) 69.9 (52.9; 88.4) | 80.1 (32.7) 75 (56.9; 98.1) | 68.1 (25.2) 65.6 (49; 82.6) | <0.001 |
| Sodium-to-potassium ratio | 2.3 (1.1) 2.1 (1.6; 2.9) | 2.6 (1.2) 2.4 (1.8; 3.2) | 2.1 (0.9) 1.9 (1.4; 2.7) | <0.001 |
| Creatinine, mmol/24 h | 12.9 (4.5) 12.3 (9.5; 15.8) | 16 (4.1) 15.9 (13.3; 18.7) | 10.2 (2.6) 10.1 (8.3; 11.9) | <0.001 |
| Salt intake, g/24 h | 10 (5.3) 9 (6.4; 12.2) | 11.7 (5.8) 10.5 (8.1; 14.5) | 8.4 (4.1) 7.6 (5.5; 10.3) | <0.001 |
| Potassium intake, g/24 h | 3.3 (1.3) 3.1 (2.4; 4) | 3.6 (1.5) 3.4 (2.6; 4.4) | 3.1 (1.1) 2.9 (2.2; 3.7) | <0.001 |
| Salt < 5 g/24 h, (n%) | 111 (12.5) | 18 (4.3) | 93 (20) | <0.001 * |
| Potassium > 90 mmol/24 h, (n%) | 205 (23.1) | 130 (30.8) | 75 (16.1) | <0.001 * |
| Variables | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Association with 24 h sodium excretion (mmol/24 h) | |||
| Male sex | 21.04123 | (9.88; 32.21) | <0.01 |
| Body weight (kg) | 1.132687 | (0.78; 1.49) | <0.01 |
| Potassium excretion | 1.103706 | (0.94; 1.27) | <0.01 |
| Systolic blood pressure | 0.389656 | (0.04; 0.74) | 0.029 |
| Iodine Status (Iodine Concentrations, μg/L) | N (%) |
|---|---|
| All participants | 679 |
| Insufficient (<100): | 355 (52.3) |
| Severe insufficiency (<20) | 75 (11) |
| Moderate insufficiency (20; 50) | 88 (13) |
| Mild insufficiency (50; 100) | 192 (28.3) |
| Adequate consumption (100; 200) | 223 (32.8) |
| Above requirement (200; 300) | 70 (10.3) |
| Excessive consumption (≥300) | 31 (4.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakauskiene, U.; Macioniene, E.; Zabuliene, L.; Sukackiene, D.; Linkeviciute-Dumce, A.; Banys, V.; Bratcikoviene, N.; Karosiene, D.; Slekiene, V.; Kontrimas, V.; et al. Sodium, Potassium and Iodine Intake in an Adult Population of Lithuania. Nutrients 2022, 14, 3817. https://doi.org/10.3390/nu14183817
Zakauskiene U, Macioniene E, Zabuliene L, Sukackiene D, Linkeviciute-Dumce A, Banys V, Bratcikoviene N, Karosiene D, Slekiene V, Kontrimas V, et al. Sodium, Potassium and Iodine Intake in an Adult Population of Lithuania. Nutrients. 2022; 14(18):3817. https://doi.org/10.3390/nu14183817
Chicago/Turabian StyleZakauskiene, Urte, Ernesta Macioniene, Lina Zabuliene, Diana Sukackiene, Ausra Linkeviciute-Dumce, Valdas Banys, Nomeda Bratcikoviene, Dovile Karosiene, Virginija Slekiene, Virginijus Kontrimas, and et al. 2022. "Sodium, Potassium and Iodine Intake in an Adult Population of Lithuania" Nutrients 14, no. 18: 3817. https://doi.org/10.3390/nu14183817
APA StyleZakauskiene, U., Macioniene, E., Zabuliene, L., Sukackiene, D., Linkeviciute-Dumce, A., Banys, V., Bratcikoviene, N., Karosiene, D., Slekiene, V., Kontrimas, V., Simanauskas, K., Utkus, A., Brazdziunaite, D., Migline, V., Makarskiene, I., Zurlyte, I., Rakovac, I., Breda, J., Cappuccio, F. P., & Miglinas, M. (2022). Sodium, Potassium and Iodine Intake in an Adult Population of Lithuania. Nutrients, 14(18), 3817. https://doi.org/10.3390/nu14183817






