Energy Drinks and Sleep among Adolescents
Abstract
1. Introduction
2. Materials and Methods
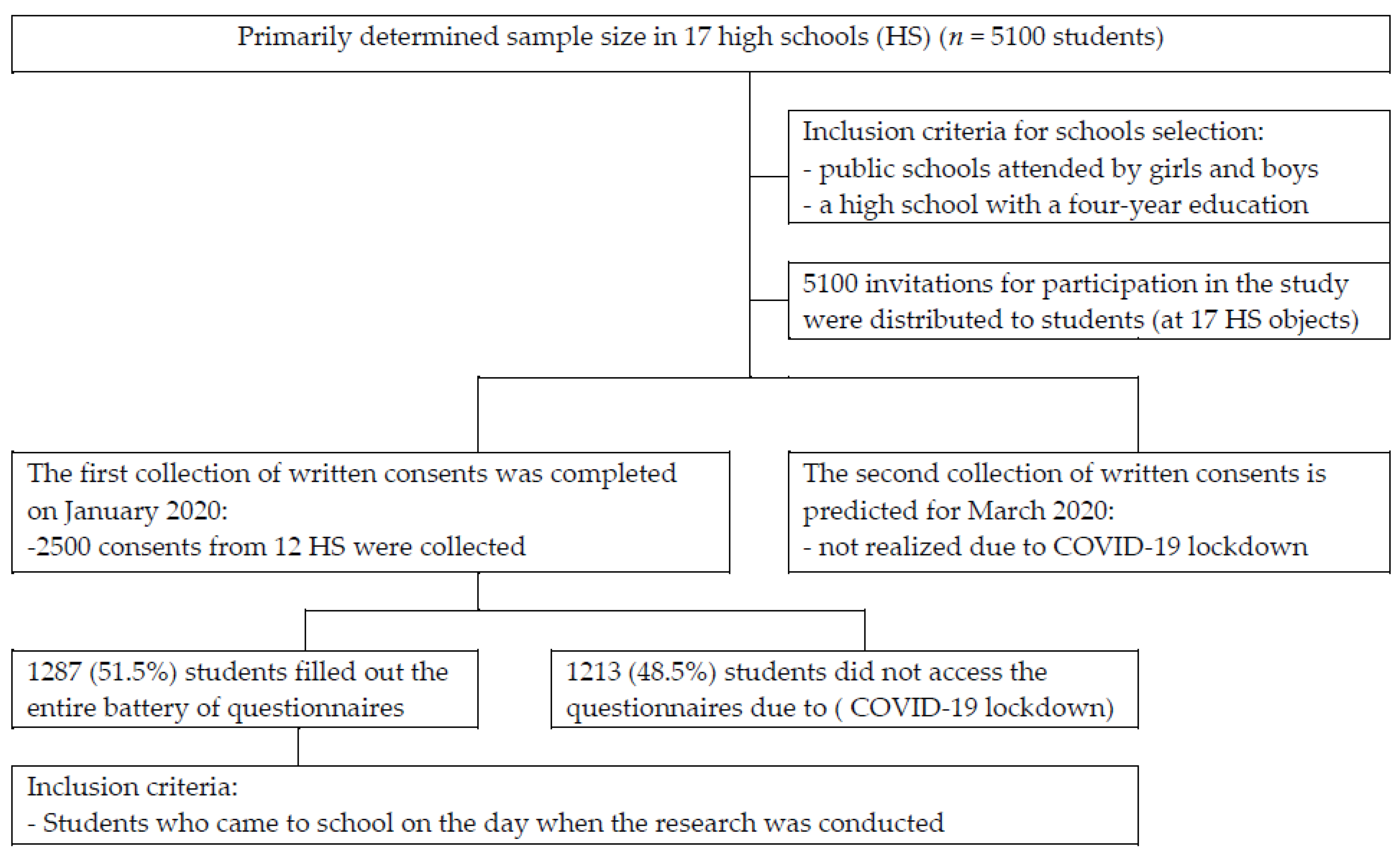
2.1. Study Design and Participants
2.2. Ethical Considerations
2.3. Assessments
2.3.1. Sociodemographic Characteristics of the Sample
2.3.2. Dietary Assessment
2.3.3. Energy Drink (ED)
2.3.4. Healthy Lifestyle Habits
2.3.5. Physical Activity
2.3.6. Sufficient Sleep (SS)
2.3.7. Unhealthy Lifestyle Habits and Mental Health Issues
2.4. Statistical Data Analysis
3. Results
3.1. General Characteristics
3.2. Dietary Factors
3.3. Physical Activity
3.4. Health-Risk Behaviors and Mental Issues
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirelli, C.; Tononi, G. Is sleep essential? PLoS Biol. 2008, 6, e216. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.B.; Simões, P.A.D.; Macedo, M.C.S.A.; Duarte, C.J.; Silva, M.D. Parents’ perception of the sleep habits and quality of preschool-aged children. Rev. Enferm. Ref. 2018, 17, 63–72. [Google Scholar] [CrossRef]
- Brand, S.; Kirov, R. Sleep and its importance in adolescence and in common adolescent somatic and psychiatric conditions. Int. J. Gen. Med. 2011, 4, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.; Kotagal, S.; Lloyd, R.M.; Malow, B.A.; Maski, K.; Nichols, C.; Quan, S.F.; et al. Consensus statement of the American Academy of Sleep Medicine on the recommended amount of sleep for healthy children: Methodology and discussion. J. Clin. Sleep Med. 2016, 12, 1549–1561. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.-B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef]
- Yang, C.K.; Kim, J.K.; Patel, S.R.; Lee, J.H. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics 2005, 115, 250–256. [Google Scholar] [CrossRef]
- Crowley, S.J.; Acebo, C.; Carskadon, M.A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007, 8, 602–612. [Google Scholar] [CrossRef]
- Owens, J.A.; Weiss, M.R. Insufficient sleep in adolescents: Causes and consequences. Minerva Pediatr. 2017, 69, 326–336. [Google Scholar] [CrossRef]
- Córdova, F.V.; Barja, S.; Brockmann, P.E. Consequences of short sleep duration on the dietary intake in children: A systematic review and metanalysis. Sleep Med. Rev. 2018, 42, 68–84. [Google Scholar] [CrossRef]
- Clauson, K.A.; Shields, K.M.; McQueen, C.E.; Persad, N. Safety issues associated with commercially available energy drinks. J. Am. Pharm. Assoc. 2008, 48, e55–e67. [Google Scholar] [CrossRef]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef]
- Reissig, C.J.; Strain, E.C.; Griffiths, R.R. Caffeinated energy drinks: A growing problem. Drug Alcohol Depend. 2009, 99, 1–10. [Google Scholar] [CrossRef]
- Harris, J.L.; Munsell, C.R. Energy drinks and adolescents: What’s the harm? Nutr. Rev. 2015, 73, 247–257. [Google Scholar] [CrossRef]
- Wolk, B.J.; Ganetsky, M.; Babu, K.M. Toxicity of energy drinks. Curr. Opin. Pediatr. 2012, 24, 243–251. [Google Scholar] [CrossRef]
- Babu, K.M.; James, C.R.; Lewander, W. Energy drinks: The new eye-opener for adolescents. Clin. Ped. Emerg. Med. 2008, 9, 35–42. [Google Scholar] [CrossRef]
- Dobmeyer, D.J.; Stine, R.A.; Leier, C.V.; Greenberg, R.; Schaal, S.F. The arrhythmogenic effects of caffeine in human beings. N. Engl. J. Med. 1983, 308, 814–816. [Google Scholar] [CrossRef]
- Peake, S.T.; Mehta, P.A.; Dubrey, S.W. Atrial fibrillation-related cardiomyopathy: A case report. J. Med. Case Rep. 2007, 1, 111. [Google Scholar] [CrossRef]
- Worthley, M.I.; Prabhu, A.; De Sciscio, P.; Schultz, C.; Sanders, P.; Willoughby, S.R. Detrimental effects of energy drink consumption on platelet and endothelial function. Am. J. Med. 2010, 123, 184–187. [Google Scholar] [CrossRef]
- Savoca, M.R.; Evans, C.D.; Wilson, M.E.; Harshfield, G.A.; Ludwig, D.A. The association of caffeinated beverages with blood pressure in adolescents. Arch. Pediatr. Adolesc. Med. 2004, 158, 473–477. [Google Scholar] [CrossRef][Green Version]
- Vivekanandarajah, A.; Ni, S.; Waked, A. Acute hepatitis in a woman following excessive ingestion of an energy drink: A case report. J. Med. Case Rep. 2011, 5, 227. [Google Scholar] [CrossRef]
- Schaumburg, H.; Kaplan, J.; Windebank, A.; Vick, A.; Rasmus, S.; Pleasure, D.; Brown, M.J. Sensory neuropathy from pyridoxine abuse. A new megavitamin syndrome. N. Engl. J. Med. 1983, 309, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Berigan, T. An anxiety disorder secondary to energy drinks: A case report. Psychiatry (Edgmont) 2005, 2, 10. [Google Scholar] [PubMed]
- Menkes, D.B. Transient psychotic relapse temporally related to ingestion of an energy drink. Med. J. Aust. 2011, 194, 206. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.B.; Pickar, D.; Kelsoe, J.; Rapaport, M.; Pato, C.; Hommer, D. Effects of the acute administration of caffeine in patients with schizophrenia. Biol. Psychiatry 1990, 28, 35–40. [Google Scholar] [CrossRef]
- Smaldone, A.; Honig, J.C.; Byrne, M.W. Sleepless in America: Inadequate sleep and relationships to health and well being of our nation’s children. Pediatrics 2007, 119, 29–37. [Google Scholar] [CrossRef]
- Bernstein, G.A.; Carroll, M.E.; Thuras, P.D.; Cosgrove, K.P.; Roth, M.E. Caffeine dependence in teenagers. Drug Alcohol Depend. 2002, 66, 1–6. [Google Scholar] [CrossRef]
- Costa, B.M.; Hayley, A.; Miller, P. Young adolescents’ perceptions, patterns, and contexts of energy drink use. A focus group study. Appetite 2014, 80, 183–189. [Google Scholar] [CrossRef]
- Holubcikova, J.; Kolarcik, P.; Geckova, A.M.; Van Dijk, J.; Reijneveld, S. Lack of parental rules increases the risk for high intake of soft and energy drinks in adolescents. Eur. J. Public Health 2016, 16, 359. [Google Scholar] [CrossRef][Green Version]
- Committee on Nutrition and the Council on Sports Medicine and Fitness. Clinical report—sports drinks and energy drinks for children and adolescents: Are they appropriate? Pediatrics 2011, 127, 1182–1189. [Google Scholar] [CrossRef]
- Hodge, J.G.; Scanlon, M., Jr.; Corbe, A.; Sorensen, A. The consumable vice: Caffeine, public health, and the law. J. Contemp. Health L 2011, 27, 76–119. [Google Scholar]
- Emond, J.A.; Sargent, J.D.; Gilbert-Diamond, D. Patterns of energy drink advertising over US television networks. J. Nutr. Educ. Behav. 2015, 47, 120–126. [Google Scholar] [CrossRef]
- Sampasa-Kanyinga, H.; Hamilton, H.A.; Chaput, J.P. Sleep duration and consumption of sugar-sweetened beverages and energy drinks among adolescents. Nutrition 2018, 48, 77–81. [Google Scholar] [CrossRef]
- Statistical Office of the Republic of Serbia. High/Secondary School Attendance. Available online: https://www.stat.gov.rs/en-us/oblasti/obrazovanje/srednje-obrazovanje/ (accessed on 14 July 2022).
- Tomanić, M.S. Prevalence of and Risk Factors for Tinnitus among Adolescents in an Urban Environment. Ph.D. Thesis, Medical Faculty, University of Belgrade, Belgrade, Serbia, 2021. [Google Scholar]
- Harvard, T.H. Chan School of Public Health Nutrition Department’s File Download Site: Youth Adolescent Food Frequency Questionnaire. 2012. Available online: https://regepi.bwh.harvard.edu/health/KIDS/files/02.%202012%20YOUTH%20ADOLESCENT%20FOOD%20FREQUENCY%20QUESTIONNAIRE.pdf (accessed on 23 July 2022).
- Center for Disease Control and Prevention (CDC). Physical Activity Guidelines for School-Aged Children and Adolescents. Available online: https://www.cdc.gov/healthyschools/physicalactivity/guidelines.htm#:~:text=Children%20and%20adolescents%20ages%206%20through%2017%20years%20should%20do,to%2Dvigorous%20physical%20activity%20daily (accessed on 18 July 2022).
- Hysing, M.; Pallesen, S.; Stormark, K.M.; Lundervold, A.J.; Sivertsen, B. Sleep patterns and insomnia among adolescents: A population-based study. J. Sleep Res. 2013, 22, 549–556. [Google Scholar] [CrossRef]
- Gariepy, G.; Danna, S.; Gobina, I.; Rasmussen, M.; Gaspar de Matos, M.; Tynjala, J.; Janssen, I.; Kalman, M.; Villeruša, A.; Husarova, D.; et al. How are adolescents sleeping? Adolescent sleep patterns and sociodemographic differences in 24 European and North American Countries. J. Adolesc. Health 2020, 66, S81–S88. [Google Scholar] [CrossRef]
- Gradisar, M.; Gardner, G.; Dohnt, H. Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Med. 2011, 12, 110–118. [Google Scholar] [CrossRef]
- Brown, F.C.; Buboltz, W.C., Jr.; Soper, B. Relationship of sleep hygiene awareness, sleep hygiene practices, and sleep quality in university students. Behav. Med. 2002, 28, 33–38. [Google Scholar] [CrossRef]
- Forquer, L.M.; Camden, A.E.; Gabriau, K.M.; Johnson, C.M. Sleep patterns of college students at a public university. J. Am. Coll. Health 2008, 56, 563–565. [Google Scholar] [CrossRef]
- Lund, H.G.; Reider, B.D.; Whiting, A.B.; Prichard, J.R. Sleep patterns and predictors of disturbed sleep in a large population of college students. J. Adolesc. Health 2010, 46, 124–132. [Google Scholar] [CrossRef]
- Sarchiapone, M.; Mandelli, L.; Carli, V.; Iosue, M.; Wasserman, C.; Hadlaczky, G.; Hoven, C.W.; Apter, A.; Balazs, J.; Bobes, J.; et al. Hours of sleep in adolescents and its association with anxiety, emotional concerns, and suicidal ideation. Sleep Med. 2014, 15, 248–254. [Google Scholar] [CrossRef]
- Olds, T.; Blunden, S.; Petkov, J.; Forchino, F. The relationships between sex, age, geography and time in bed in adolescents: A meta-analysis of data from 23 countries. Sleep Med. Rev. 2010, 14, 371–378. [Google Scholar] [CrossRef]
- Keyes, K.M.; Maslowsky, J.; Hamilton, A.; Schulenberg, J. The great sleep recession: Changes in sleep duration among US adolescents, 1991–2012. Pediatrics 2015, 135, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Dalmases, M.; Benítez, I.D.; Mas, A.; Garcia-Codina, O.; Medina-Bustos, A.; Escarrabill, J.; Saltó, E.; Buysse, D.J.; Roure, N.; Sánchez-de-la-Torre, M.; et al. Assessing sleep health in a European population: Results of the catalan health survey 2015. PLoS ONE 2018, 13, e0194495. [Google Scholar] [CrossRef] [PubMed]
- Bøe, T.; Hysing, M.; Stormark, K.M.; Lundervold, A.J.; Sivertsen, B. Sleep problems as a mediator of the association between parental education levels, perceived family economy and poor mental health in children. J. Psychosom. Res. 2012, 73, 430–436. [Google Scholar] [CrossRef]
- Reid, J.L.; McCrory, C.; White, C.M.; Martineau, C.; Vanderkooy, P.; Fenton, N.; Hammond, D. Consumption of caffeinated energy drinks among youth and young adults in Canada. Prev. Med. Rep. 2017, 5, 65–70. [Google Scholar] [CrossRef]
- Costa, B.M.; Hayley, A.; Miller, P. Adolescent energy drink consumption: An Australian perspective. Appetite 2016, 105, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Gambon, D.L.; Brand, H.S.; Boutkabout, C.; Levie, D.; Veerman, E.C. Patterns in consumption of potentially erosive beverages among adolescent school children in the Netherlands. Int. Dent. J. 2011, 61, 247–251. [Google Scholar] [CrossRef]
- Brunton, G.; Kneale, D.; Sowden, A.; Sutcliffe, K.; Thomas, J. Caffeinated Energy Drinks and Effects in UK Young People: A Secondary Analysis of Population-Level Datasets; EPPI-Centre, Social Science Research Unit, UCL Institute of Education: London, UK, 2019. [Google Scholar]
- Mansour, B.; Amarah, W.; Nasralla, E.; Elias, N. Energy drinks in children and adolescents: Demographic data and immediate effects. Eur. J. Pediatr. 2019, 178, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Ferreira, C.; Sousa, D.; Costa, S. Consumption Patterns of Energy Drinks in Portuguese Adolescents from A City in Northern Portugal. Acta Med. Port. 2018, 31, 207–212. [Google Scholar] [CrossRef]
- Reniers, R.L.; Murphy, L.; Lin, A.; Bartolome, S.P.; Wood, S.J. Risk perception and risk-taking behaviour during adolescence: The influence of personality and gender. PLoS ONE 2016, 11, e0153842. [Google Scholar] [CrossRef]
- Berger, L.K.; Fendrich, M.; Chen, H.-Y.; Arria, A.M.; Cisler, R.A. Sociodemographic correlates of energy drink consumption with and without alcohol: Results of a community survey. Addict. Behav. 2011, 36, 516–519. [Google Scholar] [CrossRef]
- Park, S.; Onufrak, S.; Blanck, H.M.; Sherry, B. Characteristics associated with consumption of sports and energy drinks among US adults: National Health Interview Survey, 2010. J. Acad. Nutr. Diet 2013, 113, 112–119. [Google Scholar] [CrossRef]
- Wimer, D.J.; Levant, R.F. Energy drink use and its relationship to masculinity, jock identity, and fraternity membership among men. Am. J. Mens. Health 2013, 7, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Inchley, J.; Currie, D.; Cosma, A.; Samdal, O. Health Behaviour in School-Aged Children (HBSC) Study Protocol: Background, Methodology and Mandatory Items for the 2017/2018 Survey. 2018. Available online: http://www.hbsc.org/methods/ (accessed on 28 July 2022).
- Park, S.; Lee, Y.; Lee, J.H. Association between energy drink intake, sleep, stress, and suicidality in Korean adolescents: Energy drink use in isolation or in combination with junk food consumption. Nutr. J. 2016, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; DeWolfe, J.; Story, M.; Neumark-Sztainer, D. Adolescent consumption of sports and energy drinks: Linkages to higher physical activity, unhealthy beverage patterns, cigarette smoking, and screen media use. J. Nutr. Educ. Behav. 2014, 46, 181–187. [Google Scholar] [CrossRef]
- Lohsoonthorn, V.; Khidir, H.; Casillas, G.; Lertmaharit, S.; Tadesse, M.G.; Pensuksan, W.C.; Rattananupong, T.; Gelaye, B.; Williams, M.A. Sleep quality and sleep patterns in relation to consumption of energy drinks, caffeinated beverages, and other stimulants among Thai college students. Sleep Breath 2013, 17, 1017–1028. [Google Scholar] [CrossRef]
- Bjorness, T.E.; Greene, R.W. Adenosine and sleep. Curr. Neuropharmacol. 2009, 7, 238–245. [Google Scholar] [CrossRef]
- Lin, F.J.; Pierce, M.M.; Sehgal, A.; Wu, T.; Skipper, D.C.; Chabba, R. Effect of taurine and caffeine on sleep-wake activity in Drosophila melanogaster. Nat. Sci. Sleep 2010, 2, 221–231. [Google Scholar] [CrossRef]
- Shilo, L.; Sabbah, H.; Hadari, R.; Kovatz, S.; Weinberg, U.; Dolev, S.; Dagan, Y.; Shenkman, L. The effects of coffee consumption on sleep and melatonin secretion. Sleep Med. 2002, 3, 271–273. [Google Scholar] [CrossRef]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Dietary factors and fluctuating levels of melatonin. Food Nutr. Res. 2012, 56, 17252. [Google Scholar] [CrossRef]
- Yan, R.; Andrew, L.; Marlow, E.; Kunaratnam, K.; Devine, A.; Dunican, I.; Christophersen, C. Dietary Fibre Intervention for Gut Microbiota, Sleep, and Mental Health in Adults with Irritable Bowel Syndrome: A Scoping Review. Nutrients 2021, 13, 2159. [Google Scholar] [CrossRef]
- Imaki, M.; Hatanaka, Y.; Ogawa, Y.; Yoshida, Y.; Tanada, S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J. Physiol. Anthropol. Appl. Human Sci. 2002, 21, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zuraikat, F.M.; Makarem, N.; Liao, M.; St-Onge, M.P.; Aggarwal, B. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the go red for women strategically focused research network. J. Am. Heart Assoc. 2020, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep symptoms associated with intake of specific dietary nutrients. J. Sleep Res. 2014, 23, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.; Mohammad, R.M.; Arshad, A.; Hashmi, I.; Yousuf, S.M.; Baig, S. Influence of Dietary Intake on Sleeping Patterns of Medical Students. Cureus 2019, 11, e4106. [Google Scholar] [CrossRef]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, J.; Du, J.; Pu, X.; Yang, X.; Yang, S.; Yang, T. Strategies of functional foods promote sleep in human being. Curr. Signal Transduct. Ther. 2014, 9, 148–155. [Google Scholar] [CrossRef]
- Gillis, B.T.; El-Sheikh, M. Sleep and adjustment in adolescence: Physical activity as a moderator of risk. Sleep Health 2019, 5, 266–272. [Google Scholar] [CrossRef]
- Kaldenbach, S.; Leonhardt, M.; Lien, L.; Bjærtnes, A.A.; Strand, T.A.; Holten-Andersen, M.N. Sleep and energy drink consumption among Norwegian adolescents—A cross-sectional study. BMC Public Health 2022, 22, 53. [Google Scholar] [CrossRef]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The efects of physical activity on sleep: A metaanalytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef]
- Angarita, G.A.; Emadi, N.; Hodges, S.; Morgan, P.T. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: A comprehensive review. Addict. Sci. Clin. Pract. 2016, 11, 1–17. [Google Scholar] [CrossRef]
- Kwon, M.; Park, E.; Dickerson, S.S. Adolescent substance use and its association to sleep disturbances: A systematic review. Sleep Health 2019, 5, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Pieters, S.; Burk, W.J.; Van der Vorst, H.; Dahl, R.E.; Wiers, R.W.; Engels, R.C. Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. J. Youth Adolesc. 2015, 44, 379–388. [Google Scholar] [CrossRef]
- Lovato, N.; Gradisar, M. A meta-analysis and model of the relationship between sleep and depression in adolescents: Recommendations for future research and clinical practice. Sleep Med. Rev. 2014, 18, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.J.; Trinder, J.A.; Allen, N.B. Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: Implications for behavioral sleep interventions. Clin. Psychol. Rev. 2018, 63, 25–40. [Google Scholar] [CrossRef] [PubMed]
| Characteristics of the Sample | Gender (a) | Sufficient Sleep (b) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total | Male | Female | p * | Male | Female | ||||||||||||
| n | % | * | n | % | * | n | % | * | No % | Yes % | p * | No % | Yes % | p * | ||||
| Sex | Male | 483 | 37.4 | 67.4 | 32.6 | 72.4 | 27.6 | 0.058 | ||||||||||
| Female | 804 | 62.6 | ||||||||||||||||
| PE | Father | HS | 625 | 49.0 | 243 | 50.7 | 4 | 382 | 48.0 | 0.343 | 69.8 | 30.2 | 0.270 | 70.2 | 29.8 | 0.188 | ||
| College | 650 | 51.0 | 236 | 49.3 | 414 | 52.0 | 65.1 | 34.9 | 74.3 | 25.7 | ||||||||
| Mother | HS | 561 | 44.1 | 204 | 42.7 | 5 | 357 | 45.0 | 0.416 | 70.9 | 29.1 | 0.116 | 70.9 | 29.1 | 0.399 | |||
| College | 710 | 55.9 | 274 | 5.3 | 436 | 55.0 | 64.1 | 35.9 | 73.6 | 26.4 | ||||||||
| PFI | Unfulfilled needs | 92 | 7.20 | 36 | 7.5 | 1 | 56 | 7.0 | 3 | 0.748 | 66.7 | 33.3 | 0.933 | 78.6 | 21.4 | 0.291 | ||
| Fulfilled needs | 1191 | 92.8 | 446 | 92.3 | 745 | 93.0 | 67.3 | 32.7 | 72.0 | 28.0 | ||||||||
| ED | No | 1050 | 82.9 | 20 | 377 | 80.2 | 13 | 673 | 84.4 | 7 | 0.054 | 63.7 | 36.3 | 0.000 | 70.5 | 29.5 | 0.004 | |
| Yes | 217 | 17.1 | 93 | 19.8 | 124 | 15.6 | 82.8 | 17.2 | 83.1 | 16.9 | ||||||||
| SS | No | 903 | 70.5 | 6 | 322 | 67.4 | 5 | 581 | 72.4 | 1 | ||||||||
| Yes | 378 | 29.5 | 156 | 32.6 | 222 | 27.6 | ||||||||||||
| Sufficient Sleep | |||||||
|---|---|---|---|---|---|---|---|
| Dietary factors | Main Explanatory Variable: | Male | Female | ||||
| OR | 95% C.I. | pValue | OR | 95% C.I. | pValue | ||
| Energy drinks (Monthly or less) | |||||||
| Weekly or more | 0.365 | (0.205–0.651) | 0.001 | 0.488 | (0.297–0.803) | 0.005 | |
| Potential confounding variables: | |||||||
| Fresh fruits (Weekly or less) | |||||||
| Every day | 1.366 | (0.911–2.049) | 0.132 | 1.297 | (0.938–1.794) | 0.115 | |
| Fresh vegetables (Weekly or less) | |||||||
| Every day | 1.975 | (1.311–2.975) | 0.001 | 1.286 | (0.934–1.770) | 0.124 | |
| Milk (No) | |||||||
| Yes | 1.912 | (1.115–3.277) | 0.018 | 1.259 | (0.886–1.789) | 0.200 | |
| Fish (Monthly or less) | |||||||
| Weekly or more | 1.213 | (0.819–1.794) | 0.335 | 1.043 | (0.756–1.439) | 0.797 | |
| Eggs (Monthly or less) | |||||||
| Weekly or more | 0.729 | (0.441–1.206) | 0.218 | 0.999 | (0.694–1.436) | 0.994 | |
| Whole grains (No) | |||||||
| Yes | 1.429 | (0.945–2.161) | 0.091 | 0.985 | (0.714–1.359) | 0.926 | |
| Daily water intake (<2 L) | |||||||
| ≥ than 2 L | 1.830 | (1.225–2.734) | 0.003 | 1.179 | (0.787–1.768) | 0.425 | |
| Sodas (Monthly or less) | |||||||
| Weekly or more | 0.868 | (0.586–1.287) | 0.481 | 0.772 | (0.566–1.054) | 0.104 | |
| Daily coffee intake (Two or less) | |||||||
| Three or more | 1.285 | (0.521–3.167) | 0.586 | 0.997 | (0.435–2.285) | 0.994 | |
| Snacks (Monthly or less) | |||||||
| Weekly | 1.140 | (0.752–1.730) | 0.537 | 1.060 | (0.752–1.493) | 0.739 | |
| Fast food (Monthly or less) | |||||||
| Weekly | 0.875 | (0.592–1.292) | 0.501 | 1.294 | (0.941–1.780) | 0.113 | |
| Extra salting food (No) | |||||||
| Yes | 0.738 | (0.474–1.151) | 0.180 | 1.036 | (0.726–1.478) | 0.847 | |
| Physical activity | Physically active (No) | ||||||
| Yes | 1.621 | (1.051–2.499) | 0.029 | 1.283 | (0.760–2.166) | 0.350 | |
| Harmful health behavioral habits and self-reported mental issues | Smoking (No) | ||||||
| Yes | 0.564 | (0.328–0.969) | 0.038 | 1.004 | 0.671–1.502 | 0.985 | |
| Alcohol use (No) | |||||||
| Yes | 0.733 | (0.494–1.089) | 0.180 | 0.978 | (0.712–1.343) | 0.889 | |
| Drugs use (No) | |||||||
| Yes | 1.033 | (0.347–3.076) | 0.953 | 0.578 | (0.124–2.695) | 0.485 | |
| Sedatives use (No) | |||||||
| Yes | 0.115 | (0.015–0.869) | 0.036 | 0.264 | 0.104–0.673 | 0.005 | |
| Anxious-depressive sympt. (No) | |||||||
| Yes | 0.547 | (0.285–0.776) | 0.070 | 0.572 | (0.375–0.874) | 0.010 | |
| Sufficient Sleep | ||||||
|---|---|---|---|---|---|---|
| Model I | Model II | |||||
| Male | Female | |||||
| Variables | AOR | (95% C.I.) | p | AOR | (95% C.I.) | p |
| Energy drinks (No) Yes | 0.448 | (0.243–0.824) | 0.010 | 0.556 | (0.331–0.934) | 0.026 |
| Vegetables (Weekly or less) Every day | 1.883 | (1.177–2.856) | 0.007 | |||
| Milk (Do not drink); Drink | 1.632 | (0.910–2.927) | 0.100 | |||
| Water intake (<2 L/day); 2 L/day or more | 1.716 | (1.095–2.690) | 0.019 | |||
| Physically active (No); Yes | 1.137 | (0.694–1,863) | 0.610 | |||
| Smoking cigarettes (No) Yes | 0.854 | (0.471–1.549)) | 0.603 | |||
| Sedative use (No) Yes | 0.164 | (0.021–1.305) | 0.088 | 0.376 | (0.143–0.989) | 0.048 |
| Anxious- depressive symptoms (No) Yes | 0.687 | 0.443–1.065 | 0.093 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomanic, M.; Paunovic, K.; Lackovic, M.; Djurdjevic, K.; Nestorovic, M.; Jakovljevic, A.; Markovic, M. Energy Drinks and Sleep among Adolescents. Nutrients 2022, 14, 3813. https://doi.org/10.3390/nu14183813
Tomanic M, Paunovic K, Lackovic M, Djurdjevic K, Nestorovic M, Jakovljevic A, Markovic M. Energy Drinks and Sleep among Adolescents. Nutrients. 2022; 14(18):3813. https://doi.org/10.3390/nu14183813
Chicago/Turabian StyleTomanic, Milena, Katarina Paunovic, Maja Lackovic, Katarina Djurdjevic, Milica Nestorovic, Ana Jakovljevic, and Milos Markovic. 2022. "Energy Drinks and Sleep among Adolescents" Nutrients 14, no. 18: 3813. https://doi.org/10.3390/nu14183813
APA StyleTomanic, M., Paunovic, K., Lackovic, M., Djurdjevic, K., Nestorovic, M., Jakovljevic, A., & Markovic, M. (2022). Energy Drinks and Sleep among Adolescents. Nutrients, 14(18), 3813. https://doi.org/10.3390/nu14183813








