Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D
Abstract
1. Introduction
- Basal nutrient status must be measured, used as an inclusion criterion for entry into study, and recorded in the report of the trial.
- The intervention (change in nutrient exposure or intake) must be large enough to change nutrient status and must be quantified.
- The change in nutrient status produced in trial participants must be measured by validated laboratory analyses and recorded in the report of the trial.
- The hypothesis to be tested must be that a change in nutrient status (not just a change in diet) produces the sought effect.
- Conutrient status must be optimized to ensure that the test nutrient is the only nutrition-related, limiting factor in the response.
2. Results
2.1. Diseases and Outcomes
2.1.1. Autoimmune Diseases
2.1.2. Cancers
Cancers—RCTs
- With findings based on vitamin D dose rather than achieved 25(OH)D concentration, enrolled participants would include those with relatively high 25(OH)D concentrations, lowering the chances of detecting reduced cancer incidence.
- Higher 25(OH)D concentration lower cancer mortality risks more strongly than it reduces cancer incidence rates [24].
- Vitamin D simply has no significant effect on cancer incidence.
Cancers—Geographical Ecological Studies
- They are easy to conduct because they can be based on publicly available data.
- They include many participants.
- No participants are omitted.
- The analysis can include many other cancer risk–modifying factors averaged at the population level.
- They can be used to locate cancer hot spots globally.
- Analyses can be performed for different ethnicities and races and can be repeated for different periods.
Cancer—Mendelian Randomization Study
2.1.3. Cardiovascular Disease
Cardiovascular Disease—RCTs
Cardiovascular Disease—Mendelian Randomization
2.1.4. COVID-19
2.1.5. Diabetes Mellitus Type 2
Diabetes Mellitus Type 2—RCTs
Diabetes Mellitus Type 2—Mendelian Randomization
2.1.6. Hypertension
Hypertension—RCTs
Hypertension—Mendelian Randomization
2.1.7. Mortality, All-Cause
2.1.8. Respiratory Tract Infections
2.1.9. Alzheimer’s Disease and Other Dementias
2.1.10. Major Depressive Disorder
2.1.11. Pregnancy Disorders and Neonatal Outcomes
Pregnancy Outcomes in Interventional Studies
3. Discussion
- Strength of association
- Consistency in findings
- Temporality, that is, the risk factor must be experienced before the event
- Biological gradient, that is, dose–response relationship.
- Plausibility, for example, mechanisms that can explain the relationship
- Coherence with known biological facts
- Experiment, for example, RCT
- Analogy with related associations
- 9.
- Accounting for confounding factors
- 10.
- Accounting for bias such as publication bias
- 11.
- Quality of study design
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, G. 100 YEARS OF VITAMIN D: Historical aspects of vitamin D. Endocr. Connect. 2022, 11, e210594. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 22–27. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Rejnmark, L.; Bislev, L.S.; Cashman, K.D.; Eiríksdottir, G.; Gaksch, M.; Grüebler, M.; Grimnes, G.; Gudnason, V.; Lips, P.; Pilz, S.; et al. Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PLoS ONE 2017, 12, e0180512. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Maretzke, F.; Bechthold, A.; Egert, S.; Ernst, J.B.; Melo van Lent, D.; Pilz, S.; Reichrath, J.; Stangl, G.I.; Stehle, P.; Volkert, D.; et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases—An Umbrella Review. Nutrients 2020, 12, 969. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism 2021, 115, 154434. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs. <20 ng/mL (150 vs. 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Vatanparast, H.; Kimball, S.M. The Association between Serum 25(OH)D Status and Blood Pressure in Participants of a Community-Based Program Taking Vitamin D Supplements. Nutrients 2017, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Dalia, T.; Ranka, S.; Sethi, P.; Oni, O.A.; Safarova, M.S.; Parashara, D.; Gupta, K.; Barua, R.S. The Effects of Vitamin D Supplementation and 25-Hydroxyvitamin D Levels on the Risk of Myocardial Infarction and Mortality. J. Endocr. Soc. 2021, 5, bvab124. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Nino, J.F.; et al. Maternal 25(OH)D concentrations ≥40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grübler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; März, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef]
- Garland, C.; Barrett-Connor, E.; Rossof, A.; Shekelle, R.; Criqui, M.; Paul, O. Dietary Vitamin D and Calcium and Risk of Colorectal Cancer: A 19-Year Prospective Study in Men. Lancet 1985, 325, 307–309. [Google Scholar] [CrossRef]
- O’Neill, C.M.; Kazantzidis, A.; Kiely, M.; Cox, L.; Meadows, S.; Goldberg, G.; Prentice, A.; Kift, R.; Webb, A.R.; Cashman, K.D. A predictive model of serum 25-hydroxyvitamin D in UK white as well as black and Asian minority ethnic population groups for application in food fortification strategy development towards vitamin D deficiency prevention. J. Steroid Biochem. Mol. Biol. 2017, 173, 245–252. [Google Scholar] [CrossRef]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC–Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef]
- Scragg, R. Limitations of vitamin D supplementation trials: Why observational studies will continue to help determine the role of vitamin D in health. J. Steroid Biochem. Mol. Biol. 2018, 177, 6–9. [Google Scholar] [CrossRef]
- Boucher, B.J.; Grant, W.B. Re: Scragg–Emerging Evidence of Thresholds for Beneficial Effects from Vitamin D Supplementation. Nutrients 2019, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.P. Vitamin D and dental caries in controlled clinical trials: Systematic review and meta-analysis. Nutr. Rev. 2013, 71, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.; Rødbro, P.; Lund, M. Effect of Vitamin D on Bone Mineral Mass in Normal Subjects and in Epileptic Patients on Anticonvulsants: A Controlled Therapeutic Trial. BMJ 1973, 2, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Chen, Q.-Y.; Lee, D.H.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality by daily vs. infrequent large-bolus dosing strategies: A meta-analysis of randomised controlled trials. Br. J. Cancer 2022, 127, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.-M.; E Manson, J.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef]
- Lelieveld, J.; Klingmüller, K.; Pozzer, A.; Pöschl, U.; Fnais, M.; Daiber, A.; Münzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Birstow, S.M.; Bolland, M. Calcium and Cardiovascular Disease. Endocrinol. Metab. 2017, 32, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Hatchwell, E.; Greally, J.M. The potential role of epigenomic dysregulation in complex human disease. Trends Genet. 2007, 23, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, H.; Dermitzakis, E.T. Genetic and epigenetic contribution to complex traits. Hum. Mol. Genet. 2012, 21, R24–R28. [Google Scholar] [CrossRef]
- Wong, A.K.; Sealfon, R.S.G.; Theesfeld, C.L.; Troyanskaya, O.G. Decoding disease: From genomes to networks to phenotypes. Nat. Rev. Genet. 2021, 22, 774–790. [Google Scholar] [CrossRef]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef]
- Ong, J.S.; Dixon-Suen, S.C.; Han, X.; An, J.; Esophageal Cancer Consortium; 23 and Me Research Team; Liyanage, U.; Dusingize, J.C.; Schumacher, J.; Gockel, I.; et al. A comprehensive re-assessment of the association between vitamin D and cancer susceptibility using Mendelian randomization. Nat. Commun. 2021, 12, 246. [Google Scholar] [CrossRef]
- Navale, S.S.; Mulugeta, A.; Zhou, A.; Llewellyn, D.J.; Hyppönen, E. Vitamin D and brain health: An observational and Mendelian randomization study. Am. J. Clin. Nutr. 2022, 116, 531–540. [Google Scholar] [CrossRef]
- Zhou, A.; Selvanayagam, J.B.; Hyppönen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2022, 43, 1731–1739. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef]
- Grant, W.B. The role of geographical ecological studies in identifying diseases linked to UVB exposure and/or vitamin D. Derm.-Endocrinol. 2016, 8, e1137400. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002, 94, 1867–1875. [Google Scholar] [CrossRef]
- Grant, W.B.; Garland, C.F. The association of solar ultraviolet B (UVB) with reducing risk of cancer: Multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006, 26, 2687–2699. [Google Scholar]
- Grant, W.B.; Boucher, B.J. An Exploration of How Solar Radiation Affects the Seasonal Variation of Human Mortality Rates and the Seasonal Variation in Some Other Common Disorders. Nutrients 2022, 14, 2519. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br. J. Cancer 2005, 92, 426–429. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef]
- Prummel, M.F.; Strieder, T.; Wiersinga, W.M. The environment and autoimmune thyroid diseases. Eur. J. Endocrinol. 2004, 150, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Silman, A.J. Risk factors for the development of rheumatoid arthritis. Scand. J. Rheumatol. 2006, 35, 169–174. [Google Scholar] [CrossRef]
- Huerta, C.; Rivero, E.; Rodríguez, L.A.G. Incidence and Risk Factors for Psoriasis in the General Population. Arch. Dermatol. 2007, 143, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F. The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.; Garland, F.C.; Shaw, E.; Comstock, G.W.; Helsing, K.J.; Gorham, E.D. Serum 25-Hydroxyvitamin D and Colon Cancer: Eight-Year Prospective Study. Lancet 1989, 334, 1176–1178. [Google Scholar] [CrossRef]
- Grant, W.B.; Karras, S.N.; Bischoff-Ferrari, H.A.; Annweiler, C.; Boucher, B.J.; Juzeniene, A.; Garland, C.F.; Holick, M.F. Do studies reporting ‘U’-shaped serum 25-hydroxyvitamin D-health outcome relationships reflect adverse effects? Derm.-Endocrinol. 2016, 8, e1187349. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef]
- Grant, W.B. Effect of interval between serum draw and follow-up period on relative risk of cancer incidence with respect to 25-hydroxyvitamin D level; implications for meta-analyses and setting vitamin D guidelines. Derm.-Endocrinol. 2011, 3, 199–204. [Google Scholar] [CrossRef]
- Grant, W.B. Effect of follow-up time on the relation between prediagnostic serum 25-hydroxyvitamin D and all-cause mortality rate. Derm.-Endocrinol. 2012, 4, 198–202. [Google Scholar] [CrossRef]
- Grant, W.B. 25-hydroxyvitamin D and breast cancer, colorectal cancer, and colorectal adenomas: Case-control versus nested case-control studies. Anticancer Res. 2015, 35, 1153–1160. [Google Scholar]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The Importance of Body Weight for the Dose Response Relationship of Oral Vitamin D Supplementation and Serum 25-Hydroxyvitamin D in Healthy Volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef]
- Wamberg, L.; Kampmann, U.; Stodkilde-Jorgensen, H.; Rejnmark, L.; Pedersen, S.B.; Richelsen, B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—Results from a randomized trial. Eur. J. Intern. Med. 2013, 24, 644–649. [Google Scholar] [CrossRef]
- Michels, N.; van Aart, C.; Morisse, J.; Mullee, A.; Huybrechts, I. Chronic inflammation towards cancer incidence: A systematic review and meta-analysis of epidemiological studies. Crit. Rev. Oncol. 2021, 157, 103177. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective Study of Predictors of Vitamin D Status and Cancer Incidence and Mortality in Men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Dudenkov, D.V.; Mara, K.C.; Fischer, P.R.; Maxson, J.A.; Thacher, T.D. Serum 25-Hydroxyvitamin D and Subsequent Cancer Incidence and Mortality: A Population-Based Retrospective Cohort Study. Mayo Clin. Proc. 2021, 96, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Muzio, V.; Caini, S.; Raimondi, S.; Martinoli, C.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; et al. Vitamin D Supplementation and Cancer Mortality: Narrative Review of Observational Studies and Clinical Trials. Nutrients 2021, 13, 3285. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Zhang, J.; Huang, X.; Thabane, L.; Li, G. Effect of Vitamin D Supplementation on Risk of Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2021, 8, 655727. [Google Scholar] [CrossRef]
- Devesa, S.S.; Grauman, D.J.; Blot, W.J.; Pennelo, G.A.; Hoover, R.N.; Fraumeni, J.F., Jr. Atlas of Cancer Mortality in the United States, 1950–1994; National Institues of Health, National Cancer Institute: Bethesda, MD, USA, 1999; p. 360. [Google Scholar]
- Marti-Soler, H.; Gonseth, S.; Gubelmann, C.; Stringhini, S.; Bovet, P.; Chen, P.-C.; Wojtyniak, B.; Paccaud, F.; Tsai, D.-H.; Zdrojewski, T.; et al. Seasonal Variation of Overall and Cardiovascular Mortality: A Study in 19 Countries from Different Geographic Locations. PLoS ONE 2014, 9, e113500. [Google Scholar] [CrossRef]
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. BioMed Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.L.; Yang, W.; Ito, K.; Matte, T.D.; Shaman, J.; Kinney, P.L. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol. 2016, 1, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef]
- Zhang, R.; Li, B.; Gao, X.; Tian, R.; Pan, Y.; Jiang, Y.; Gu, H.; Wang, Y.; Wang, Y.; Liu, G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: Dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 810–819. [Google Scholar] [CrossRef]
- Ginde, A.A.; Scragg, R.; Schwartz, R.S.; Camargo, C.A., Jr. Prospective Study of Serum 25-Hydroxyvitamin D Level, Cardiovascular Disease Mortality, and All-Cause Mortality in Older U.S. Adults. J. Am. Geriatr. Soc. 2009, 57, 1595–1603. [Google Scholar] [CrossRef]
- Melamed, M.L.; Michos, E.D.; Post, W.; Astor, B. 25-Hydroxyvitamin D Levels and the Risk of Mortality in the General Population. Arch. Intern. Med. 2008, 168, 1629–1637. [Google Scholar] [CrossRef]
- Semba, R.D.; Houston, D.; Bandinelli, S.; Sun, K.; Cherubini, A.; Cappola, A.R.; Guralnik, J.M.; Ferrucci, L. Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur. J. Clin. Nutr. 2010, 64, 203–209. [Google Scholar] [CrossRef]
- Liu, L.; Chen, M.; Hankins, S.R.; Nùñez, A.E.; Watson, R.A.; Weinstock, P.J.; Newschaffer, C.J.; Eisen, H.J. Serum 25-Hydroxyvitamin D Concentration and Mortality from Heart Failure and Cardiovascular Disease, and Premature Mortality from All-Cause in United States Adults. Am. J. Cardiol. 2012, 110, 834–839. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Vanwoerkom, R.C.; Horne, B.D.; Bair, T.L.; May, H.T.; Lappe, D.L.; Muhlestein, J.B. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am. Heart J. 2011, 162, 331–339.e2. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.; Katz, R.; de Boer, I.; Hoofnagle, A.; Sarnak, M.J.; Shlipak, M.G.; Jenny, N.S.; Siscovick, D.S. Vitamin D, Parathyroid Hormone, and Cardiovascular Events Among Older Adults. J. Am. Coll. Cardiol. 2011, 58, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Chen, Y.D.; Shi, Y.; Yin, D.W.; Wang, H.; Sha, Y. Vitamin D, parathyroid hormone and risk factors for coronary artery disease in an elderly Chinese population. J. Cardiovasc. Med. 2015, 16, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Welsh, P.; Papacosta, O.; Lennon, L.; Whincup, P.H.; Sattar, N. Elevated Parathyroid Hormone, But Not Vitamin D Deficiency, Is Associated with Increased Risk of Heart Failure in Older Men with and without Cardiovascular Disease. Circ. Heart Fail. 2014, 7, 732–739. [Google Scholar] [CrossRef]
- Valcour, A.; Blocki, F.; Hawkins, D.M.; Rao, S.D. Effects of Age and Serum 25-OH-Vitamin D on Serum Parathyroid Hormone Levels. J. Clin. Endocrinol. Metab. 2012, 97, 3989–3995. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Ng, C.Y.; Wang, D.; Wang, J.; Li, G.; Liu, T. Meta-analysis of Vitamin D Deficiency and Risk of Atrial Fibrillation. Clin. Cardiol. 2016, 39, 537–543. [Google Scholar] [CrossRef]
- Kahn, R.; Robertson, R.M.; Smith, R.; Eddy, D. The Impact of Prevention on Reducing the Burden of Cardiovascular Disease. Diabetes Care 2008, 31, 1686–1696. [Google Scholar] [CrossRef]
- Farley, T.A.; Dalal, M.A.; Mostashari, F.; Frieden, T.R. Deaths Preventable in the U.S. by Improvements in Use of Clinical Preventive Services. Am. J. Prev. Med. 2010, 38, 600–609. [Google Scholar] [CrossRef]
- Timms, P.M.; Mannan, N.; Hitman, G.A.; Noonan, K.; Mills, P.G.; Syndercombe-Court, D.; Aganna, E.; Price, C.P.; Boucher, B.J. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: Mechanisms for inflammatory damage in chronic disorders? QJM 2002, 95, 787–796. [Google Scholar] [CrossRef]
- Rimondi, E.; Marcuzzi, A.; Casciano, F.; Tornese, G.; Pellati, A.; Toffoli, B.; Secchiero, P.; Melloni, E. Role of vitamin D in the pathogenesis of atheromatosis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 344–353. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar]
- Martineau, A.R.; Cantorna, M.T. Vitamin D for COVID-19: Where are we now? Nat. Rev. Immunol. 2022, 22, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Seal, K.H.; Bertenthal, D.; Carey, E.; Grunfeld, C.; Bikle, D.D.; Lu, C.M. Association of Vitamin D Status and COVID-19-Related Hospitalization and Mortality. J. Gen. Intern. Med. 2022, 37, 853–861. [Google Scholar] [CrossRef]
- Dissanayake, H.A.; de Silva, N.L.; Sumanatilleke, M.; de Silva, S.D.N.; Gamage, K.K.K.; Dematapitiya, C.; Kuruppu, D.C.; Ranasinghe, P.; Pathmanathan, S.; Katulanda, P. Prognostic and Therapeutic Role of Vitamin D in COVID-19: Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2022, 107, 1484–1502. [Google Scholar] [CrossRef]
- Hosseini, B.; El Abd, A.; Ducharme, F.M. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2134. [Google Scholar] [CrossRef]
- De Niet, S.; Trémège, M.; Coffiner, M.; Rousseau, A.-F.; Calmes, D.; Frix, A.-N.; Gester, F.; Delvaux, M.; Dive, A.-F.; Guglielmi, E.; et al. Positive Effects of Vitamin D Supplementation in Patients Hospitalized for COVID-19: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2022, 14, 3048. [Google Scholar] [CrossRef]
- Norman, A.W.; Frankel, B.J.; Heldt, A.M.; Grodsky, G.M. Vitamin D Deficiency Inhibits Pancreatic Secretion of Insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 92–102. [Google Scholar]
- Cheng, Q.; Boucher, B.J.; Leung, P.S. Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin–angiotensin system in mice. Diabetologia 2013, 56, 553–562. [Google Scholar] [CrossRef]
- Leung, P.S. The Potential Protective Action of Vitamin D in Hepatic Insulin Resistance and Pancreatic Islet Dysfunction in Type 2 Diabetes Mellitus. Nutrients 2016, 8, 147. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.J. Inadequate vitamin D status: Does it contribute to the disorders comprising syndrome ‘X’? Br. J. Nutr. 1998, 79, 315–327. [Google Scholar] [CrossRef]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The Role of Vitamin D and Calcium in Type 2 Diabetes. A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2013, 36, 1422–1428. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Haluza, D.; Kundi, M. 25-Hydroxyvitamin D Status and Risk for Colorectal Cancer and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Epidemiological Studies. Int. J. Environ. Res. Public Health 2017, 14, 127. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults with Prediabetes: A Secondary Analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef]
- Lu, L.; Bennett, D.A.; Millwood, I.Y.; Parish, S.; McCarthy, M.I.; Mahajan, A.; Lin, X.; Bragg, F.; Guo, Y.; Holmes, M.V.; et al. Association of vitamin D with risk of type 2 diabetes: A Mendelian randomisation study in European and Chinese adults. PLOS Med. 2018, 15, e1002566. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, Y.; Liu, J.; Wang, C.; Qu, Z.; Wei, Z.; Zhou, D. Genetically increased circulating 25(OH)D level reduces the risk of type 2 diabetes in subjects with deficiency of vitamin D: A large-scale Mendelian randomization study. Medicine 2020, 99, e23672. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Wong, M.S.K.; Delansorne, R.; Man, R.Y.K.; Svenningsen, P.; Vanhoutte, P.M. Chronic treatment with vitamin D lowers arterial blood pressure and reduces endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am. J. Physiol. Circ. Physiol. 2010, 299, H1226–H1234. [Google Scholar] [CrossRef]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of Vitamin D in Atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef]
- Ford, E.S.; Ajani, U.A.; McGuire, L.C.; Liu, S. Concentrations of Serum Vitamin D and the Metabolic Syndrome Among U.S. Adults. Diabetes Care 2005, 28, 1228–1230. [Google Scholar] [CrossRef]
- Forman, J.P.; Giovannucci, E.; Holmes, M.D.; Bischoff-Ferrari, H.A.; Tworoger, S.S.; Willett, W.C.; Curhan, G.C. Plasma 25-Hydroxyvitamin D Levels and Risk of Incident Hypertension. Hypertension 2007, 49, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, E.; Hajhashemy, Z.; Saneei, P. Serum Vitamin D Levels in Relation to Hypertension and Pre-hypertension in Adults: A Systematic Review and Dose–Response Meta-Analysis of Epidemiologic Studies. Front. Nutr. 2022, 9, 829307. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, C.; Wang, Y.; Sun, H.; Yu, S.; Xue, Y.; Liu, Y.; Li, W.; Li, X. Effect of Vitamin D on Blood Pressure and Hypertension in the General Population: An Update Meta-Analysis of Cohort Studies and Randomized Controlled Trials. Prev. Chronic Dis. 2020, 17, E03. [Google Scholar] [CrossRef]
- Liu, D.; Fernandez, B.O.; Hamilton, A.; Lang, N.N.; Gallagher, J.M.; Newby, D.E.; Feelisch, M.; Weller, R.B. UVA Irradiation of Human Skin Vasodilates Arterial Vasculature and Lowers Blood Pressure Independently of Nitric Oxide Synthase. J. Investig. Dermatol. 2014, 134, 1839–1846. [Google Scholar] [CrossRef]
- Forman, J.P.; Scott, J.B.; Ng, K.; Drake, B.; Suarez, E.G.; Hayden, D.L.; Bennett, G.G.; Chandler, P.; Hollis, B.W.; Emmons, K.M.; et al. Effect of Vitamin D Supplementation on Blood Pressure in Blacks. Hypertension 2013, 61, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grübler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; ó Hartaigh, B.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Theiler-Schwetz, V.; Trummer, C.; Grübler, M.R.; Keppel, M.H.; Zittermann, A.; Tomaschitz, A.; Karras, S.N.; März, W.; Pilz, S.; Gängler, S. Effects of Vitamin D Supplementation on 24-Hour Blood Pressure in Patients with Low 25-Hydroxyvitamin D Levels: A Randomized Controlled Trial. Nutrients 2022, 14, 1360. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.L.; Tikkanen, E.; Eriksson, J.; et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A mendelian randomisation study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef]
- Zittermann, A.; Iodice, S.; Pilz, S.; Grant, W.; Bagnardi, V.; Gandini, S. Vitamin D deficiency and mortality risk in the general population: A meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2012, 95, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of All-Cause Mortality According to Serum 25-Hydroxyvitamin D. Am. J. Public Health 2014, 104, e43–e50. [Google Scholar] [CrossRef]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njølstad, I.; Løchen, M.-L.; März, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar] [CrossRef]
- Afzal, S.; Brøndum-Jacobsen, P.; E Bojesen, S.; Nordestgaard, B.G. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 2014, 349, g6330. [Google Scholar] [CrossRef]
- Sofianopoulou, E.; Kaptoge, S.K.; Afzal, S.; Jiang, T.; Gill, D.; Gundersen, T.E.; Bolton, T.R.; Allara, E.; Arnold, M.G.; Mason, A.M.; et al. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021, 9, 837–846. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Shaman, J.; Kohn, M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. USA 2009, 106, 3243–3248. [Google Scholar] [CrossRef]
- Ianevski, A.; Zusinaite, E.; Shtaida, N.; Kallio-Kokko, H.; Valkonen, M.; Kantele, A.; Telling, K.; Lutsar, I.; Letjuka, P.; Metelitsa, N.; et al. Low Temperature and Low UV Indexes Correlated with Peaks of Influenza Virus Activity in Northern Europe during 2010–2018. Viruses 2019, 11, 207. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef]
- Panza, F.; La Montagna, M.; Lampignano, L.; Zupo, R.; Bortone, I.; Castellana, F.; Sardone, R.; Borraccino, L.; Dibello, V.; Resta, E.; et al. Vitamin D in the development and progression of Alzheimer’s disease: Implications for clinical management. Expert Rev. Neurother. 2021, 21, 287–301. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Shab-Bidar, S. Vitamin D status and risk of dementia and Alzheimer’s disease: A meta-analysis of dose-response. Nutr. Neurosci. 2019, 22, 750–759. [Google Scholar] [CrossRef]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: An updated meta-analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef]
- Olsson, E.; Byberg, L.; Karlström, B.; Cederholm, T.; Melhus, H.; Sjögren, P.; Kilander, L. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 2017, 105, 936–943. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Kos, K.; Henley, W.E.; Kuzma, E.; Llewellyn, D.J. Vitamin D and Dementia. J. Prev. Alzheimer’s Dis. 2016, 3, 43–52. [Google Scholar] [CrossRef]
- Feart, C.; Helmer, C.; Merle, B.; Herrmann, F.R.; Annweiler, C.; Dartigues, J.; Delcourt, C.; Samieri, C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimer’s Dement. 2017, 13, 1207–1216. [Google Scholar] [CrossRef]
- Knekt, P.; Sääksjärvi, K.; Järvinen, R.; Marniemi, J.; Männistö, S.; Kanerva, N.; Heliövaara, M. Serum 25-Hydroxyvitamin D Concentration and Risk of Dementia. Epidemiology 2014, 25, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Licher, S.; de Bruijn, R.F.; Wolters, F.J.; Zillikens, M.C.; Ikram, M.A. Vitamin D and the Risk of Dementia: The Rotterdam Study. J. Alzheimer’s Dis. 2017, 60, 989–997. [Google Scholar] [CrossRef]
- Schneider, A.L.; Lutsey, P.L.; Alonso, A.; Gottesman, R.F.; Sharrett, A.R.; Carson, K.A.; Gross, M.; Post, W.S.; Knopman, D.S.; Mosley, T.H.; et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: The ARIC Brain MRI Study. Eur. J. Neurol. 2014, 21, 1211-e70. [Google Scholar] [CrossRef]
- Karakis, I.; Pase, M.P.; Beiser, A.; Booth, S.L.; Jacques, P.F.; Rogers, G.; DeCarli, C.; Vasan, R.S.; Wang, T.J.; Himali, J.J.; et al. Association of Serum Vitamin D with the Risk of Incident Dementia and Subclinical Indices of Brain Aging: The Framingham Heart Study. J. Alzheimer’s Dis. 2016, 51, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Mokry, L.E.; Ross, S.; Morris, J.A.; Manousaki, D.; Forgetta, V.; Richards, J.B. Genetically decreased vitamin D and risk of Alzheimer disease. Neurology 2016, 87, 2567–2574. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, Y.; Zhang, H.; Zhang, Y.; Hua, J.; Jin, S.; Liu, G. Circulating Vitamin D Levels and Alzheimer’s Disease: A Mendelian Randomization Study in the IGAP and UK Biobank. J. Alzheimer’s Dis. 2020, 73, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, Z.; Ming, Y.-C.; Shen, L.; Ji, H.-F. Are micronutrient levels and supplements causally associated with the risk of Alzheimer’s disease? A two-sample Mendelian randomization analysis. Food Funct. 2022, 13, 6665–6673. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Han, Z.; Wang, L.; Zhang, Y.; Wang, L.; Liu, G. Impact of Vitamin D Binding Protein Levels on Alzheimer’s Disease: A Mendelian Randomization Study. J. Alzheimer’s Dis. 2020, 74, 991–998. [Google Scholar] [CrossRef]
- De Haan, P.; Klein, H.C.; Hart, B.A. Autoimmune Aspects of Neurodegenerative and Psychiatric Diseases: A Template for Innovative Therapy. Front. Psychiatry 2017, 8, 46. [Google Scholar] [CrossRef]
- Kouba, B.R.; Camargo, A.; Gil-Mohapel, J.; Rodrigues, A.L.S. Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. Int. J. Mol. Sci. 2022, 23, 7077. [Google Scholar] [CrossRef]
- Luthold, R.V.; Fernandes, G.R.; Franco-De-Moraes, A.C.; Folchetti, L.G.; Ferreira, S.R.G. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef]
- Xie, F.; Huang, T.; Lou, D.; Fu, R.; Ni, C.; Hong, J.; Ruan, L. Effect of vitamin D supplementation on the incidence and prognosis of depression: An updated meta-analysis based on randomized controlled trials. Front. Public Health 2022, 10, 903547. [Google Scholar] [CrossRef]
- Li, H.; Sun, D.; Wang, A.; Pan, H.; Feng, W.; Ng, C.H.; Ungvari, G.S.; Tao, L.; Li, X.; Wang, W.; et al. Serum 25-Hydroxyvitamin D Levels and Depression in Older Adults: A Dose–Response Meta-Analysis of Prospective Cohort Studies. Am. J. Geriatr. Psychiatry 2019, 27, 1192–1202. [Google Scholar] [CrossRef]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’Neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The effect of vitamin D supplementation on depressive symptoms in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Luo, C.-W.; Chen, S.-P.; Chiang, C.-Y.; Wu, W.-J.; Chen, C.-J.; Chen, W.-Y.; Kuan, Y.-H. Association between Ultraviolet B Exposure Levels and Depression in Taiwanese Adults: A Nested Case–Control Study. Int. J. Environ. Res. Public Health 2022, 19, 6846. [Google Scholar] [CrossRef]
- Mulugeta, A.; Lumsden, A.; Hyppönen, E. Relationship between Serum 25(OH)D and Depression: Causal Evidence from a Bi-Directional Mendelian Randomization Study. Nutrients 2020, 13, 109. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef]
- Aguilar-Cordero, M.; Lasserrot-Cuadrado, A.; Mur-Villar, N.; León-Ríos, X.; Rivero-Blanco, T.; Pérez-Castillo, I. Vitamin D, preeclampsia and prematurity: A systematic review and meta-analysis of observational and interventional studies. Midwifery 2020, 87, 102707. [Google Scholar] [CrossRef]
- Gernand, A.D.; Bodnar, L.M.; A Klebanoff, M.; Parks, W.T.; Simhan, H.N. Maternal serum 25-hydroxyvitamin D and placental vascular pathology in a multicenter US cohort. Am. J. Clin. Nutr. 2013, 98, 383–388. [Google Scholar] [CrossRef]
- Merewood, A.; Mehta, S.D.; Chen, T.C.; Bauchner, H.; Holick, M.F. Association between Vitamin D Deficiency and Primary Cesarean Section. J. Clin. Endocrinol. Metab. 2009, 94, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Chen, X.; Stein, P. Maternal Vitamin D Status and Delivery by Cesarean. Nutrients 2012, 4, 319–330. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Wang, X.; You, L.; Xu, P.; Cui, X.; Zhu, L.; Ji, C.; Guo, X.; Wen, J. Maternal Vitamin D Status and Risk of Gestational Diabetes: A Meta-Analysis. Cell. Physiol. Biochem. 2018, 45, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Abbasi, F.; Mousavi, S.M.; Esmaillzadeh, A. Maternal vitamin D status and risk of gestational diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Clin. Nutr. 2021, 40, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Baggerly, C.; McDonnell, S.; Hamilton, S.; Winkler, J.; Warner, G.; Rodriguez, C.; Shary, J.; Smith, P.; Hollis, B. Post-hoc comparison of vitamin D status at three timepoints during pregnancy demonstrates lower risk of preterm birth with higher vitamin D closer to delivery. J. Steroid Biochem. Mol. Biol. 2015, 148, 256–260. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Tao, Y.-H.; Huang, K.; Zhu, B.-B.; Tao, F.-B. Vitamin D and risk of preterm birth: Up-to-date meta-analysis of randomized controlled trials and observational studies. J. Obstet. Gynaecol. Res. 2017, 43, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, A.J.O.; Holt, B.J.; Serralha, M.; Holt, P.G.; Kusel, M.M.H.; Hart, P.H. Maternal Serum Vitamin D Levels During Pregnancy and Offspring Neurocognitive Development. Pediatrics 2012, 129, 485–493. [Google Scholar] [CrossRef]
- Garcia-Serna, A.M.; Morales, E. Neurodevelopmental effects of prenatal vitamin D in humans: Systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 2468–2481. [Google Scholar] [CrossRef]
- Shi, D.; Wang, D.; Meng, Y.; Chen, J.; Mu, G.; Chen, W. Maternal vitamin D intake during pregnancy and risk of asthma and wheeze in children: A systematic review and meta-analysis of observational studies. J. Matern. Neonatal Med. 2021, 34, 653–659. [Google Scholar] [CrossRef]
- Robinson, C.J.; Alanis, M.C.; Wagner, C.L.; Hollis, B.W.; Johnson, D.D. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am. J. Obstet. Gynecol. 2010, 203, 366.e1–366.e6. [Google Scholar] [CrossRef]
- Serrano-Diaz, N.C.; Gamboa-Delgado, E.M.; Dominguez-Urrego, C.L.; Vesga-Varela, A.L.; Serrano-Gomez, S.E.; Quintero-Lesmes, D.C. Vitamin D and risk of preeclampsia: A systematic review and meta-analysis. Biomedica 2018, 38 (Suppl. 1), 43–53. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; McDermid, J.M.; Al-Nimr, R.I.; Hakeem, R.; Moreschi, J.M.; Pari-Keener, M.; Stahnke, B.; Papoutsakis, C.; Handu, D.; Cheng, F.W. Vitamin D Supplementation during Pregnancy: An Evidence Analysis Center Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2020, 120, 898–924.e4. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Kostiuk, L.K.; Pena-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Saha, S. A comparison of the risk of cesarean section in gestational diabetes mellitus patients supplemented antenatally with vitamin D containing supplements versus placebo: A systematic review and meta-analysis of double-blinded randomized controlled trials. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 201–212. [Google Scholar] [CrossRef]
- Fogacci, S.; Fogacci, F.; Banach, M.; Michos, E.D.; Hernandez, A.V.; Lip, G.Y.; Blaha, M.J.; Toth, P.P.; Borghi, C.; Cicero, A.F.G. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020, 39, 1742–1752. [Google Scholar] [CrossRef]
- Kinshella, M.-L.; Omar, S.; Scherbinsky, K.; Vidler, M.; Magee, L.; von Dadelszen, P.; Moore, S.; Elango, R. The PRECISE Conceptual Framework Working Group Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map. Nutrients 2021, 13, 472. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Costello, P.M.; Lillycrop, K.A. Development, Epigenetics and Metabolic Programming. Prev. Asp. Early Nutr. 2016, 85, 71–80. [Google Scholar]
- Moon, R.J.; Curtis, E.M.; Woolford, S.J.; Ashai, S.; Cooper, C.; Harvey, N.C. The importance of maternal pregnancy vitamin D for offspring bone health: Learnings from the MAVIDOS trial. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211006979. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Toner, C.D.; Davis, C.D.; Milner, J.A. The Vitamin D and Cancer Conundrum: Aiming at a Moving Target. J. Am. Diet. Assoc. 2010, 110, 1492–1500. [Google Scholar] [CrossRef]
- Sempos, C.T.; Durazo-Arvizu, R.A.; Dawson-Hughes, B.; Yetley, E.A.; Looker, A.C.; Schleicher, R.L.; Cao, G.; Burt, V.; Kramer, H.; Bailey, R.L.; et al. Is There a Reverse J-Shaped Association Between 25-Hydroxyvitamin D and All-Cause Mortality? Results from the U.S. Nationally Representative NHANES. J. Clin. Endocrinol. Metab. 2013, 98, 3001–3009. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hulsey, T.C.; Fanning, D.; Ebeling, M.; Hollis, B.W. High-Dose Vitamin D3 Supplementation in a Cohort of Breastfeeding Mothers and Their Infants: A 6-Month Follow-Up Pilot Study. Breastfeed. Med. 2006, 1, 59–70. [Google Scholar] [CrossRef]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Potischman, N.; Weed, D.L. Causal criteria in nutritional epidemiology. Am. J. Clin. Nutr. 1999, 69, 1309S–1314S. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer? An examination using Hill’s criteria for causality. Derm.-Endocrinol. 2009, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Gorham, E.D.; Alcaraz, J.E.; Kane, C.I.; Macera, C.A.; Parsons, J.K.; Wingard, D.L.; Garland, C.F. Does the evidence for an inverse relationship between serum vitamin D status and breast cancer risk satisfy the Hill criteria? Derm.-Endocrinol. 2012, 4, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Robsahm, T.E.; Schwartz, G.G.; Tretli, S. The Inverse Relationship between 25-Hydroxyvitamin D and Cancer Survival: Discussion of Causation. Cancers 2013, 5, 1439–1455. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast, Colorectal, Prostate, and Overall Cancer. Anticancer Res. 2020, 40, 491–499. [Google Scholar] [CrossRef]
- Weyland, P.G.; Grant, W.B.; Howie-Esquivel, J. Does Sufficient Evidence Exist to Support a Causal Association between Vitamin D Status and Cardiovascular Disease Risk? An Assessment Using Hill’s Criteria for Causality. Nutrients 2014, 6, 3403–3430. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.B.; McCartney, D.M.; Laird, É.; McCarroll, K.; Byrne, D.G.; Healy, M.; O’Shea, P.M.; Kenny, R.A.; Faul, J.L. Understanding a Low Vitamin D State in the Context of COVID-19. Front. Pharmacol. 2022, 13, 835480. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C. Vitamin D in dementia prevention. Ann. N. Y. Acad. Sci. 2016, 1367, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and pancreas: The role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 3472–3488. [Google Scholar] [CrossRef]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008, 93, 512–517. [Google Scholar] [CrossRef]
- Giovannoni, G.; Ebers, G. Multiple sclerosis: The environment and causation. Curr. Opin. Neurol. 2007, 20, 261–268. [Google Scholar] [CrossRef]
- Hanwell, H.E.; Banwell, B. Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Murererehe, J.; Ineza, M.C.; Harelimana, E.I.; Nsabimana, U.; Uwambaye, P.; Gatarayiha, A.; Haq, A.; Razzaque, M.S. Effects of vitamin D status on oral health. J. Steroid Biochem. Mol. Biol. 2018, 175, 190–194. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J. Are Hill’s criteria for causality satisfied for vitamin D and periodontal disease? Derm.-Endocrinol. 2010, 2, 30–36. [Google Scholar] [CrossRef]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef]


| Study | Change in 25(OH)D | Incidence, RR or HR (95% CI) | Mortality, RR or HR (95% CI) | Author |
|---|---|---|---|---|
| Observational Studies | ||||
| Harvard Health Professionals Follow-Up Study, all cancer | 10 ng/mL | RR = 0.83 (0.74–0.92) | RR = 0.71 (0.60–0.83) | Giovannucci et al., [65] |
| Adult patients living in Olmsted County, Minnesota, all less skin cancer | <12 ng/mL | HR = 1.56 (1.03–2.36) | HR = 2.35 (1.01–5.48) | Johnson et al., [66] |
| Meta-analysis, breast cancer | High vs. low | RR = 0.92 (0.83–1.02) | RR = 0.58 (0.40–0.85) | Kim et al., [67] |
| VITAL, exclude first 2 years | Vitamin D treatment vs. placebo | HR = 0.94 (0.83–1.06) | HR = 0.75 (0.59–0.96) | Manson et al., [8] |
| RCTs | ||||
| RCTs, meta-analysis | All participants | (12 RCTs) SRR = 0.99 (0.04–1.03) | (6 RCT) SRR = 0.92 (0.82–1.03) | Keum et al., [24] |
| RCTs, meta-analysis | Normal-weight individuals | (1 RCT) SRR = 0.76 (0.60–0.94) | Keum et al., [24] |
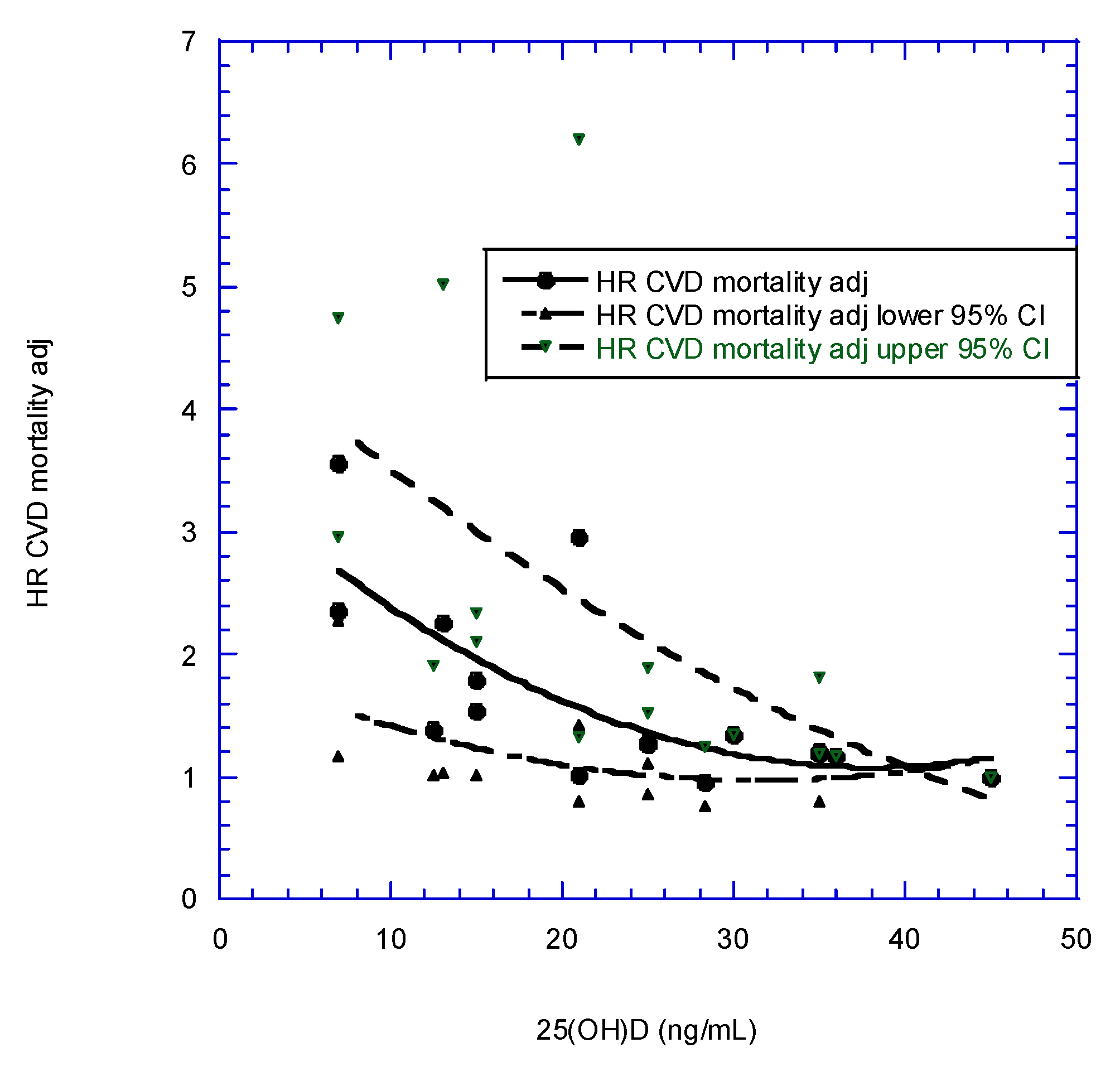
| Mean 25(OH)D Concentration (ng/mL) | aHR | aHR Adjusted | aHR Adjusted, 95% CI Low | aHR Adjusted, 95% CI High | Author |
|---|---|---|---|---|---|
| 12.5 | 1.20 | 1.39 | 1.01 | 1.90 | Melamed et al., [79] |
| 21.0 | 0.88 | 1.02 | 0.80 | 1.32 | |
| 28.3 | 0.83 | 0.96 | 0.75 | 1.24 | |
| 36.0 | 1.00 | 1.16 | 1.16 | 1.16 | |
| 7.00 | 2.36 | 1.17 | 4.75 | Ginde et al. [78] | |
| 15.0 | 1.54 | 1.01 | 2.34 | ||
| 25.0 | 1.26 | 0.85 | 1.88 | ||
| 35.0 | 1.20 | 0.79 | 1.81 | ||
| 45.0 | 1.00 | 1.00 | 1.00 | ||
| 7.00 | 2.64 | 3.55 | 2.27 | 2.96 | Semba et al., [80] |
| 13.0 | 1.68 | 2.26 | 1.03 | 5.02 | |
| 21.0 | 2.19 | 2.95 | 1.42 | 6.20 | |
| 30.0 | 1.00 | 1.35 | 1.35 | 1.35 | |
| 15.0 | 1.52 | 1.79 | 1.52 | 2.11 | Liu et al., [81] |
| 25.0 | 1.09 | 1.29 | 1.11 | 1.51 | |
| 35.0 | 1.00 | 1.18 | 1.18 | 1.18 |
| Country | Mean 25(OH)D (ng/mL) | Follow-Up (yrs) | Vascular Dementia, HR (95% CI) for 10 ng/mL Increase | Alzheimer’s, HR (95% CI) for 10 ng/mL Increase | Author |
|---|---|---|---|---|---|
| US | 12 | 5.6 | 0.57 (0.34–0.97) | 0.61 (0.41–0.93) | Littlejohns et al., [141] |
| France | 14 | 11.4 | 0.60 (0.47–0.78) | 0.60 (0.47–0.78) | Feart et al., [142] |
| Finland | 16 | 17.0 | 0.77 (0.62–0.92) | Knekt et al., [143] | |
| Denmark | 16 | 21.0 | 0.91 (0.82–1.02) | Afzal et al., [130] | |
| Netherlands | 20 | 13.3 | 0.77 (0.63–0.95) | 0.73 (0.59–0.93) | Licher et al., [144] |
| US | 22 | 16.6 | 0.93 (0.79–1.07) | Schneider et al., [145] | |
| US | 25 | 9.0 | 1.01 (0.88–1.14) | 1.09 (0.95–1.12) | Karakis et al., [146] |
| Sweden | 28 | 12.0 | 1.04 (0.93–1.17) | 0.95 (0.81–1.12) | Olsson et al., [140] |
| Outcome | Setting | Outcome | Finding | Author |
|---|---|---|---|---|
| Birth weight | ||||
| Cesarean delivery, primary | Maternal 25(OH)D < 15 vs. >15 ng/mL | aOR = 3.8 (95% CI, 1.7–8.6) | Merewood et al., [162] | |
| Cesarean delivery, primary | Maternal 25(OH)D < 15 vs. >15 ng/mL | aOR = 2.0 (95% CI, 1.2–3.3) | Scholl et al., [163] | |
| Gestational diabetes | Meta-analysis, 29 studies | <20 vs. >20 ng/mL | OR = 1.39 (95% CI, 1.20–1.60) | Hu et al., [164] |
| Gestational diabetes | Meta-analysis, 27 studies | >20 vs. >30 ng/mL | OR = 1.26 (95% CI, 1.13–1.41) | Milajerdi et al., [165] |
| Preeclampsia | Hospital study | Early-onset severe preeclampsia, 10-ng/mL increase in 25(OH)D | aOR = 0.37 (95% CI, 0.22–0.62) | Robinson et al., [171] |
| Preeclampsia | Meta-analysis, 13 studies | Comparison of 25(OH)D | OR = 0.57 (95% CI, 0.51–0.65) | Serrano-Diaz et al., [172] |
| Preeclampsia | Meta-analysis, 11 studies | 25(OH)D < 30 vs. >30 ng/mL | OR = 1.44 (95% CI, 1.26–1.64) | Aguilar-Cordero et al., [160] |
| Preterm delivery | Hospital study | 25(OH)D < 20 vs. >40 ng/mL, <16 wks | OR = 3.8 (95% CI, 1.4–10.7) | Wagner et al., [166] |
| Preterm delivery | Meta-analysis, 16 studies | 25(OH)D < 20 vs. >20 ng/mL | OR = 1.25 (95% CI, 1.13–1.38) | Zhou et al., [167] |
| Preterm delivery | Open-label vitamin D supplementation | 25(OH)D > 40 vs. >20 ng/mL | SES adjusted OR = 0.41 (95% CI, 0.24–0.72) | McDonnell et al., [14] |
| Infant outcomes | ||||
| Brain dysfunction | Language impairment in childhood vs. maternal 25(OH)D at 18 weeks pregnancy | 6-18 vs. 29-62 ng/mL | aOR = 1.97 (95% CI, 1.00–3.93, p < 0.05) | Whitehouse et al., [168] |
| Brain dysfunction | Risk of ADHD, meta-analysis, 5 studies | High vs. low 25(OH)D | OR/RR = 0.72 (95% CI, 0.59–0.89) | Garcia-Serna et al., [169] |
| Respiratory dysfunction | Risk of asthma vs. maternal 25(OH)D, 11 studies | High vs. low 25(OH)D | OR = 0.78 (95% CI, 0.69–0.89) | Shi et al., [170] |
| Respiratory dysfunction | Risk of wheeze vs. maternal 25(OH)D, 14 studies | High vs. low 25(OH)D | OR = 0.65 (95% CI, 0.54–0.79) | Shi et al., [170] |
| Outcome | Setting | Finding | Author |
|---|---|---|---|
| Birth weight, low | Review of 5 RCTs | RR = 0.55 (95% CI, 0.35–0.87) | Palacios et al., [174] |
| Birth weight | Review of 11 RCTs | Increased weight, mean difference = 114 g (95% CI, 63–165 g) | Gallo et al., [173] |
| Cesarean delivery, primary | Review of 6 RCTs | OR = 0.9 (95% CI, 0.7–1.2) | Gallo et al., [173] |
| Cesarean delivery in Iran | Review of 5 RCTs | RR = 0.61 (95% CI, 0.44–0.83) | Saha and Saha, [175] |
| Gestational diabetes | Review of 4 RCTs | RR = 0.51 (95% CI, 0.27–0.97) | Palacios et al., [174] |
| Preeclampsia | Review of 27 RCTs | RR = 0.37 (95% CI, 0.26–0.52) | Fogacci et al., [176] |
| Preterm delivery | Review of 17 RCTs | RR = 0.70 (95% CI, 0.49–1.00) | Kinshella et al., [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022, 14, 3811. https://doi.org/10.3390/nu14183811
Grant WB, Boucher BJ, Al Anouti F, Pilz S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients. 2022; 14(18):3811. https://doi.org/10.3390/nu14183811
Chicago/Turabian StyleGrant, William B., Barbara J. Boucher, Fatme Al Anouti, and Stefan Pilz. 2022. "Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D" Nutrients 14, no. 18: 3811. https://doi.org/10.3390/nu14183811
APA StyleGrant, W. B., Boucher, B. J., Al Anouti, F., & Pilz, S. (2022). Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients, 14(18), 3811. https://doi.org/10.3390/nu14183811









