Biomarker Changes in Oxygen Metabolism, Acid-Base Status, and Performance after the Off-Season in Well-Trained Cyclists
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mujika, I.; Halson, S.; Burke, L.M.; Balagué, G.; Farrow, D. An Integrated, Multifactorial Approach to Periodization for Optimal Performance in Individual and Team Sports. Int. J. Sports Physiol. Perform. 2018, 13, 538–561. [Google Scholar] [CrossRef]
- Lucía, A.; Hoyos, J.; Pardo, J.; Chicharro, J.L. Metabolic and Neuromuscular Adaptations to Endurance Training in Professional Cyclists. A Longitudinal Study. Jpn. J. Physiol. 2000, 50, 381–388. [Google Scholar] [CrossRef]
- Sassi, A.; Impellizzeri, F.; Morelli, A.; Menaspà, P.; Rampinini, E. Seasonal changes in aerobic fitness indices in elite cyclists. Appl. Physiol. Nutr. Metab. 2008, 33, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Martín, S.; Cámara, J.; James, D.V.; Fernández-López, J.R.; Artetxe-Gezuraga, X. Effects of long-term training cessation in young top-level road cyclists. J. Sports Sci. 2016, 35, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Padilla, S. Detraining: Loss of Training-Induced Physiological and Performance Adaptations. Part I. Sports Med. 2000, 30, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Padilla, S. Detraining: Loss of Training-Induced Physiological and Performance Adaptations. Part II. Sports Med. 2000, 30, 145–154. [Google Scholar] [CrossRef]
- Coyle, E.F.; Martin, W.H.; Sinacore, D.R.; Joyner, M.J.; Hagberg, J.M.; Holloszy, J.O. Time course of loss of adaptations after stopping prolonged intense endurance training. J. Appl. Physiol. 1984, 57, 1857–1864. [Google Scholar] [CrossRef]
- Coyle, E.F.; Hemmert, M.K.; Coggan, A.R. Effects of detraining on cardiovascular responses to exercise: Role of blood volume. J. Appl. Physiol. 1986, 60, 95–99. [Google Scholar] [CrossRef]
- Houmard, J.A.; Hortobágyi, T.; Johns, R.A.; Bruno, N.J.; Nute, C.C.; Shinebarger, M.H.; Welborn, J.W. Effect of Short-Term Training Cessation on Performance Measures in Distance Runners. Endoscopy 1992, 13, 572–576. [Google Scholar] [CrossRef]
- Martin, W.H.; Coyle, E.F.; Bloomfield, S.A.; Ehsani, A.A. Effects of physical deconditioning after Intense endurance training on left ventricular dimensions and stroke volume. J. Am. Coll. Cardiol. 1986, 7, 982–989. [Google Scholar] [CrossRef]
- Houston, M.E.; Bentzen, H.; Larsen, H. Interrelationships between skeletal muscle adaptations and performance as studied by detraining and retraining. Acta Physiol. Scand. 1979, 105, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Martin, W., 3rd; Bloomfield, S.A.; Lowry, O.; Holloszy, J. Effects of detraining on responses to submaximal exercise. J. Appl. Physiol. 1985, 59, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, G.; Bachl, N.; Wollein, W.; Langfy, G.; Prokop, L. Effect of training and detraining on the resting echocardiographic parameters in runners and cyclists. J. Sports Cardiol. 1986, 3, 35–45. [Google Scholar]
- Allen, G. Physiological and metabolic changes with six weeks detraining. Aust. J. Sci. Med. Sport 1989, 21, 4–9. [Google Scholar]
- Drinkwater, B.L.; Horvath, S.M. Detraining effects on young women. Med. Sci. Sports Exerc. 1972, 4, 91–95. [Google Scholar] [CrossRef]
- Miyamura, M.; Ishida, K. Adaptive changes in hypercapnic ventilatory response during training and detraining. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 353–359. [Google Scholar] [CrossRef]
- Mujika, I.; Chatard, J.-C.; Busso, T.; Geyssant, A.; Barale, F.; Lacoste, L. Effects of Training on Performance in Competitive Swimming. Can. J. Appl. Physiol. 1995, 20, 395–406. [Google Scholar] [CrossRef]
- Penny, G.D.; Wells, M.R. Heart rate, blood pressure, serum lactate, and serum cholesterol changes after the cessation of training. J. Sports Med. Phys. Fit. 1975, 15, 223–228. [Google Scholar]
- Chi, M.M.; Hintz, C.S.; Coyle, E.F.; Martin, W.H.; Ivy, J.L.; Nemeth, P.M.; Holloszy, J.O.; Lowry, O.H. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am. J. Physiol. Physiol. 1983, 244, C276–C287. [Google Scholar] [CrossRef]
- Mundial, A.M. Declaración de Helsinki de la AMM-Principios éticos para las Investigaciones Médicas en Seres Humanos. 2019. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 16 August 2022).
- Wasserman, K.; Beaver, W.L.; Whipp, B.J. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 1990, 81, II14–II30. [Google Scholar]
- Denham, J.; Scott-Hamilton, J.; Hagstrom, A.D.; Gray, A.J. Cycling Power Outputs Predict Functional Threshold Power and Maximum Oxygen Uptake. J. Strength Cond. Res. 2020, 34, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Mujika, I.; Angulo, F.; Goiriena, J.J. Scientific approach to the 1-h cycling world record: A case study. J. Appl. Physiol. 2000, 89, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; King, D.S.; Thomas, R.; Hargreaves, M. Effects of Reduced Training on Muscular Power in Swimmers. Physician Sportsmed. 1985, 13, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Giada, F.; Bertaglia, E.; De Piccoli, B.; Franceschi, M.; Sartori, F.; Raviele, A.; Pascotto, P. Cardiovascular adaptations to endurance training and detraining in young and older athletes. Int. J. Cardiol. 1998, 65, 149–155. [Google Scholar] [CrossRef]
- Bosquet, L. Endurance Training: Science and Practice. S. Afr. J. Sports Med. 2012, 24, 131–132. [Google Scholar]
- Leo, P.; Mujika, I.; Lawley, J. Influence of COVID-19 Restrictions on Training and Physiological Characteristics in U23 Elite Cyclists. J. Funct. Morphol. Kinesiol. 2021, 7, 1. [Google Scholar] [CrossRef]
- Muriel, X.; Courel-Ibáñez, J.; Cerezuela-Espejo, V.; Pallarés, J.G. Training Load and Performance Impairments in Professional Cyclists During COVID-19 Lockdown. Int. J. Sports Physiol. Perform. 2021, 16, 735–738. [Google Scholar] [CrossRef]
- Santamaria, C.; Sermi, F.; Spyratos, S.; Iacus, S.M.; Annunziato, A.; Tarchi, D.; Vespe, M. Measuring the impact of COVID-19 confinement measures on human mobility using mobile positioning data. A European regional analysis. Saf. Sci. 2020, 132, 104925. [Google Scholar] [CrossRef]
- Murias, J.M.; Edwards, J.A.; Paterson, D.H. Effects of short-term training and detraining on VO2kinetics: Faster VO2kinetics response after one training session. Scand. J. Med. Sci. Sports 2015, 26, 620–629. [Google Scholar] [CrossRef]
- Kanstrup, I.-L.; Marving, J.; Høilund-Carlsen, P.F.; Saltin, B. Left ventricular response upon exercise with trained and detrained leg muscles. Scand. J. Med. Sci. Sports 2007, 1, 112–118. [Google Scholar] [CrossRef]
- Bosquet, L.; Berryman, N.; Dupuy, O.; Mekary, S.; Arvisais, D.; Bherer, L.; Mujika, I. Effect of training cessation on muscular performance: A meta-analysis. Scand. J. Med. Sci. Sports 2013, 23, e140–e149. [Google Scholar] [CrossRef] [PubMed]

| Start | End | p-Value | |
|---|---|---|---|
| Age (years) | 32.6 (9.12) | ||
| Height (cm) | 177.0 (6.25) | ||
| Body mass (kg) | 70.4 (6.06) | 70.7 (6.15) | 0.374 |
| BF (%) | 9.0 (1.64) | 9.0 (1.63) | 0.775 |
| Training variables | During the off-season period | ||
| Total distance (km) | 1082 (810.0) | ||
| HRAVG (bpm) | 137 (13.1) | ||
| WAVG (W) | 163.5 (32.5) | ||
| RPE | 6.3 (1.2) | ||
| Zone 1 (%) | 26.7 (17.8) | ||
| Zone 2 (%) | 24.0 (7.31) | ||
| Zone 3 (%) | 20.3 (10.1) | ||
| Zone 4 (%) | 21.7 (14.2) | ||
| Zone 5 (%) | 7.3 (6.0) | ||
| Start | End | p-Value | |
|---|---|---|---|
| Kilocalories | 2100 (529) | 2134 (449) | 0.729 |
| Carbohydrates (g) | 222 (71.5) | 248 (59.7) | 0.084 |
| Lipids (g) | 84 (24.3) | 72 (19.4) | 0.043 * |
| Proteins (g) | 115 (26.0) | 102 (24.3) | 0.177 |
| Start | End | p-Value | ES | ∆% | |
|---|---|---|---|---|---|
| Hct (%) | 49.4 (3.22) | 49.3 (2.47) | 0.980 | 0.01 | 0.19 |
| Hb (g/dL) | 16.1 (1.05) | 16.1 (0.80) | 0.939 | 0.20 | 0.12 |
| pCO2 (mmHg) | 34.1 (4.26) | 36.0 (3.12) | 0.295 | 0.28 | 7.0 |
| pO2 (mmHg) | 0.29 (0.90) | 0.26 (0.14) | 0.276 | 0.27 | 3.6 |
| sO2 (%) | 94.2 (1.36) | 95.1 (1.24) | 0.024 * | 0.56 | 0.96 |
| O2Hb (%) | 93.1 (1.34) | 93.9 (1.23) | 0.041 * | 0.51 | 0.86 |
| COHb (%) | 0.461 (0.11) | 0.505 (0.17) | 0.026 * | 0.63 | 27.7 |
| RHb (%) | 5.67 (1.35) | 4.79 (1.22) | 0.023 * | 0.57 | −12.3 |
| MetHb (%) | 0.789 (0.11) | 0.795 (0.12) | 0.971 | 0.02 | 0.90 |
| tO2 (mmol/L) | 9.43 (0.60) | 9.50 (0.51) | 0.497 | 0.16 | −4.1 |
| tCO2 (mmol/L) | 14.6 (2.54) | 16.0 (3.33) | 0.205 | 0.34 | −13.9 |
| p50 (mmHg) | 31.3 (2.96) | 30.1 (2.83) | 0.181 | 0.37 | −3.2 |
| Shunt (%) | 12.6 (3.86) | 9.79 (3.14) | 0.021 * | 0.60 | −16.1 |
| AaDpO2 (mmHg) | 25.9 (6.11) | 22.2 (4.68) | 0.032 * | 0.55 | −10.4 |
| Start | End | p-Value | ES | ∆% | |
|---|---|---|---|---|---|
| pH | 7.200 (0.05) | 7.210 (0.03) | 0.607 | 0.12 | 0.06 |
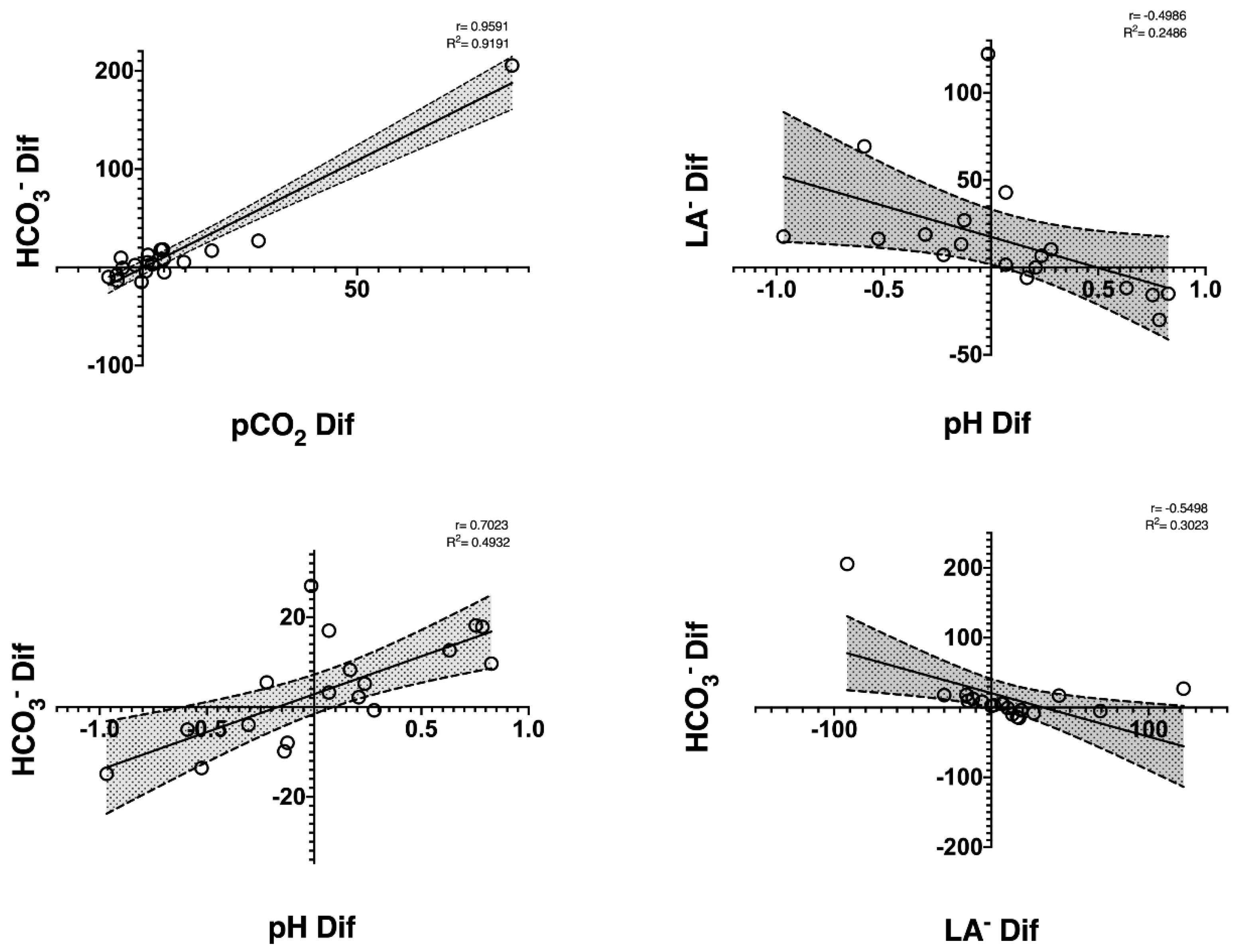
| HCO3− (mmol/L) | 13.5 (2.43) | 14.9 (3.25) | 0.241 | 0.32 | 14.5 |
| SBC (mmol/L) | 14.5 (1.77) | 15.5 (2.75) | 0.277 | 0.29 | 8.0 |
| ABE (mmol/L) | −13.4 (2.84) | −11.9 (3.75) | 0.251 | 0.31 | −6.3 |
| SBE (mmol/L) | −14.5 (3.05) | −12.8 (4.07) | 0.226 | 0.29 | −6.5 |
| La− (mmol/L) | 14.4 (3.50) | 14.8 (3.64) | 0.660 | 0.10 | 9.7 |
| Start | End | p-Value | ES | ∆% | |
|---|---|---|---|---|---|
| Na+ (mmol/L) | 146 (1.65) | 146 (2.06) | 0.881 | 0.04 | −0.03 |
| K+ (mmol/L) | 5.56 (1.60) | 4.94 (0.45) | 0.477 | 0.20 | −5.7 |
| Ca+ (mmol/L) | 1.31 (0.08) | 1.28 (0.03) | 0.176 | 0.36 | −2.0 |
| Cl− (mmol/L) | 110 (3.02) | 109 (2.26) | 0.392 | 0.27 | −0.80 |
| Anion gap (mmol/L) | 22.1 (3.66) | 22.2 (3.89) | 0.636 | 0.14 | 6.7 |
| mOsm (mmol/kg) | 298 (3.66) | 298 (4.35) | 0.740 | 0.10 | −0.01 |
| Start | End | p-Value | ES | ∆% | |
|---|---|---|---|---|---|
| FatMax HR (ppm) | 131 (13.70) | 127.7 (12.0) | 0.137 | 0.36 | −3.4 |
| FatMax VO2 (L·min−1) | 2.1 (0.50) | 2.0 (0.58) | 0.494 | 0.16 | −2.4 |
| FatMax VO2MAX (%) | 52.5 (7.59) | 52.4 (9.47) | 0.962 | 0.01 | 0.67 |
| FatMax FOR (g·min−1) | 20.1 (8.19) | 15.3 (10.6) | 0.026 * | 0.54 | −21.5 |
| FatMax W (watts) | 173 (38.50) | 159 (46.70) | 0.153 | 0.43 | −6.4 |
| VT1 HR (ppm) | 128 (10.90) | 129 (10.10) | 0.699 | 0.09 | 1.2 |
| VT1 VO2 (L·min−1) | 1.99 (0.40) | 2.05 (0.48) | 0.369 | 0.21 | 3.3 |
| VT1 VO2MAX (%) | 50.0 (4.78) | 52.9 (5.39) | 0.075 | 0.42 | 6.7 |
| VT1 W (watts) | 153 (33.10) | 159 (37.50) | 0.453 | 0.17 | 0.90 |
| eFTP (watts) | 279 (45.6) | 276 (42.9) | 0.388 | 0.20 | −0.67 |
| VT2 HR (ppm) | 168 (10.90) | 166 (8.13) | 0.417 | 0.19 | −0.75 |
| VT2 VO2 (L·min−1) | 3.35 (0.56) | 3.21 (0.57) | 0.055 # | 0.34 | −3.9 |
| VT2 VO2MAX (%) | 84.0 (5.70) | 82.8 (5.56) | 0.399 | 0.19 | −1.1 |
| VT2 W (watts) | 286 (49.90) | 277 (53.80) | 0.264 | 0.26 | −2.6 |
| VO2MAX HR (ppm) | 183 (8.68) | 183 (7.07) | 0.641 | 0.11 | −0.21 |
| VO2MAX (L·min−1) | 3.98 (0.63) | 3.86 (0.60) | 0.057 # | 0.45 | −2.7 |
| VO2MAX (mL·kg−1·min−1) | 57.9 (9.53) | 56.2 (8.42) | 0.097 | 0.39 | −2.5 |
| VO2MAX W (watts) | 388 (52.7) | 385 (49.6) | 0.888 | 0.04 | −0.57 |
| Time incremental test (s) | 1247 (168.00) | 1225 (177.00) | 0.185 | 0.34 | −1.73 |
| ΔsO2 | ΔpO2 | ΔpCO2 | ΔAaDpO2 | ΔpH | ΔHCO3− | ΔLa− | ΔK+ | ΔAnion Gap | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔFatMax VO2 (L·min−1) | r | 0.502 | −0.131 | −0.423 | 0.313 | 0.142 | −0.466 | 0.240 | 0.310 | 0.028 |
| p-value | 0.03 * | 0.60 | 0.07 # | 0.21 | 0.58 | 0.04 * | 0.32 | 0.20 | 0.92 | |
| ΔFatMax VO2MAX (%) | r | −0.372 | −0.081 | −0.370 | 0.200 | 0.264 | −0.396 | 0.129 | 0.286 | −0.049 |
| p-value | 0.12 | 0.75 | 0.12 | 0.43 | 0.29 | 0.09 | 0.60 | 0.24 | 0.85 | |
| ΔFatMax FOR (g·min−1) | r | −0.575 | −0.123 | −0.395 | 0.306 | −0.145 | −0.347 | −0.099 | 0.420 | 0.356 |
| p-value | 0.01 * | 0.63 | 0.10 | 0.22 | 0.57 | 0.15 | 0.69 | 0.07 # | 0.16 | |
| ΔFatMax W (watts) | r | −0.689 | −0.244 | −0.324 | 0.393 | 0.053 | −0.363 | 0.236 | 0.189 | 0.333 |
| p-value | <0.01 * | 0.33 | 0.18 | 0.11 | 0.83 | 0.13 | 0.33 | 0.44 | 0.19 | |
| ΔVT1 VO2 (L·min−1) | r | −0.212 | −0.239 | −0.196 | 0.340 | 0.003 | −0.212 | −0.025 | 0.272 | −0.017 |
| p-value | 0.38 | 0.34 | 0.42 | 0.17 | 1.00 | 0.38 | 0.92 | 0.26 | 0.66 | |
| ΔVT1 VO2MAX (%) | r | −0.043 | −0.136 | −0.150 | 0.174 | 0.067 | −0.139 | −0.124 | 0.275 | −0.221 |
| p-value | 0.86 | 0.59 | 0.54 | 0.49 | 0.79 | 0.57 | 0.61 | 0.26 | 0.39 | |
| ΔVT1 W (watts) | r | −0.339 | −0.291 | −0.083 | 0.277 | −0.143 | −0.099 | 0.004 | 0.260 | −0.037 |
| p-value | 0.16 | 0.24 | 0.74 | 0.27 | 0.57 | 0.69 | 1.00 | 0.28 | 0.89 | |
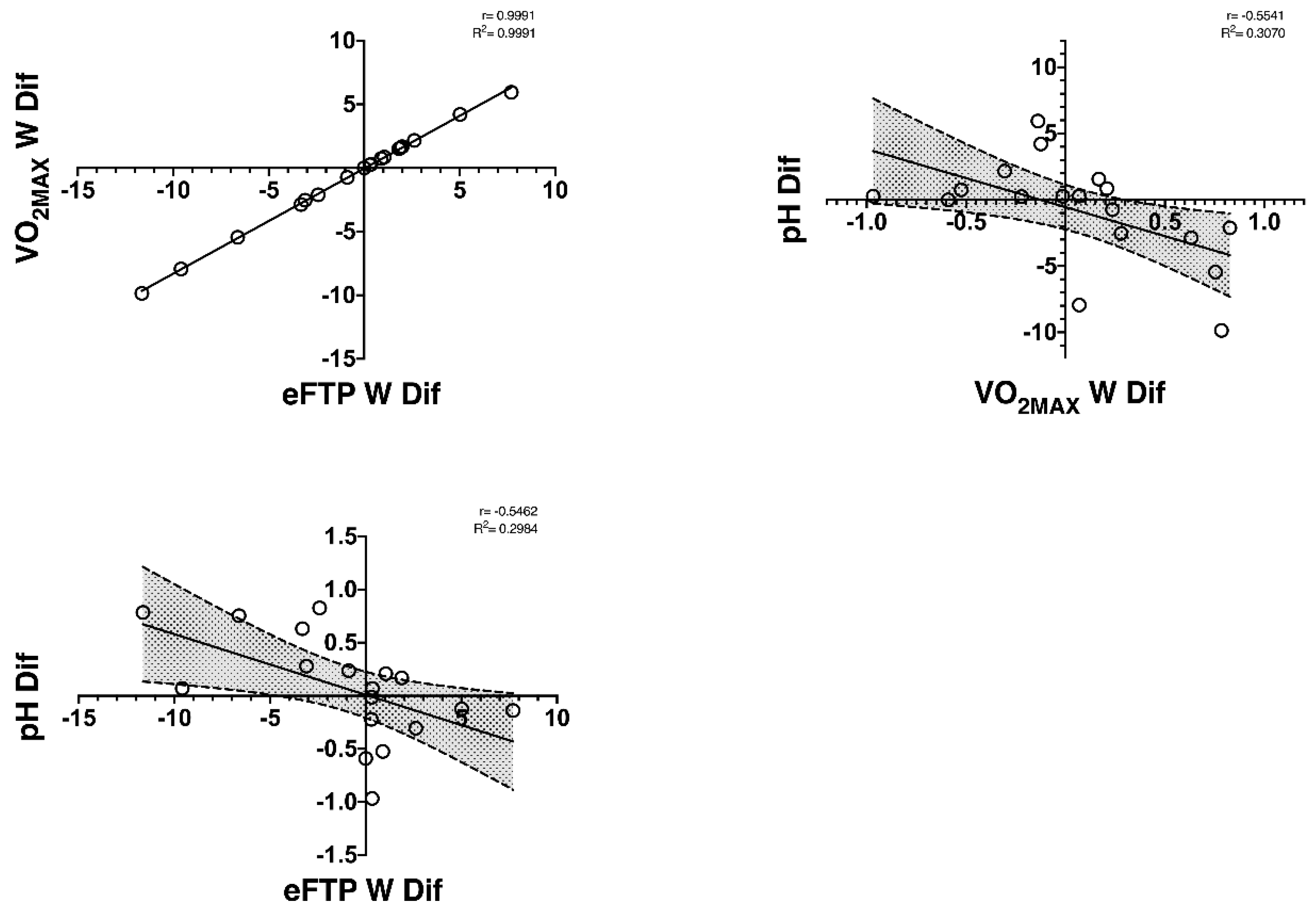
| ΔeFTP W (watts) | r | −0.104 | −0.100 | 0.063 | 0.098 | −0.546 | 0.015 | 0.231 | −0.026 | 0.207 |
| p-value | 0.67 | 0.69 | 0.80 | 0.70 | 0.02 * | 0.95 | 0.34 | 0.91 | 0.43 | |
| ΔVT2 VO2 (L·min−1) | r | −0.546 | −0.241 | −0.171 | 0.263 | −0.071 | −0.192 | 0.020 | 0.191 | −0.109 |
| p-value | 0.02 | 0.34 | 0.48 | 0.29 | 0.78 | 0.43 | 0.94 | 0.43 | 0.68 | |
| ΔVT2 VO2MAX (%) | r | −0.305 | −0.129 | −0.060 | −0.003 | 0.159 | −0.037 | −0.223 | 0.160 | −0.313 |
| p-value | 0.20 | 0.61 | 0.81 | 1.00 | 0.53 | 0.88 | 0.36 | 0.51 | 0.22 | |
| ΔVT2 W (watts) | r | −0.489 | −0.243 | −0.140 | 0.181 | −0.107 | −0.174 | −0.049 | 0.216 | −0.189 |
| p-value | 0.03 | 0.33 | 0.57 | 0.47 | 0.67 | 0.48 | 0.844 | 0.37 | 0.47 | |
| VO2MAX (L·min−1) | r | −0.345 | −0.155 | −0.139 | 0.319 | −0.255 | −0.187 | 0.270 | 0.051 | 0.172 |
| p-value | 0.15 | 0.54 | 0.57 | 0.20 | 0.31 | 0.44 | 0.26 | 0.84 | 0.51 | |
| VO2MAX (mL·kg−1·min−1) | r | −0.285 | −0.112 | −0.177 | 0.287 | −0.263 | −0.226 | 0.316 | 0.059 | 0.103 |
| p-value | 0.24 | 0.66 | 0.47 | 0.25 | 0.29 | 0.35 | 0.19 | 0.81 | 0.69 | |
| VO2MAX W (watts) | r | −0.099 | −0.101 | 0.070 | 0.098 | −0.554 | 0.019 | 0.235 | −0.035 | 0.213 |
| p-value | 0.69 | 0.69 | 0.78 | 0.70 | 0.02 * | 0.94 | 0.33 | 0.89 | 0.41 | |
| Time incremental test (s) | r | −0.601 | −0.247 | −0.151 | 0.276 | −0.123 | −0.156 | −0.003 | 0.193 | 0.363 |
| p-value | 0.01 * | 0.32 | 0.54 | 0.27 | 0.63 | 0.52 | 1.00 | 0.43 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Noguera, F.J.; Marín-Pagán, C.; Chung, L.H.; Alcaraz, P.E. Biomarker Changes in Oxygen Metabolism, Acid-Base Status, and Performance after the Off-Season in Well-Trained Cyclists. Nutrients 2022, 14, 3808. https://doi.org/10.3390/nu14183808
Martínez Noguera FJ, Marín-Pagán C, Chung LH, Alcaraz PE. Biomarker Changes in Oxygen Metabolism, Acid-Base Status, and Performance after the Off-Season in Well-Trained Cyclists. Nutrients. 2022; 14(18):3808. https://doi.org/10.3390/nu14183808
Chicago/Turabian StyleMartínez Noguera, Francisco Javier, Cristian Marín-Pagán, Linda H. Chung, and Pedro E. Alcaraz. 2022. "Biomarker Changes in Oxygen Metabolism, Acid-Base Status, and Performance after the Off-Season in Well-Trained Cyclists" Nutrients 14, no. 18: 3808. https://doi.org/10.3390/nu14183808
APA StyleMartínez Noguera, F. J., Marín-Pagán, C., Chung, L. H., & Alcaraz, P. E. (2022). Biomarker Changes in Oxygen Metabolism, Acid-Base Status, and Performance after the Off-Season in Well-Trained Cyclists. Nutrients, 14(18), 3808. https://doi.org/10.3390/nu14183808









