Vitamin D-Mediated Regulation of Intestinal Calcium Absorption
Abstract
1. Introduction
2. Vitamin D Has a Critical Physiologic Role for Protecting Bone through the Regulation of Intestinal Ca Absorption
3. Vitamin D Effects on Intestinal Ca Absorption Are Mediated through the VDR
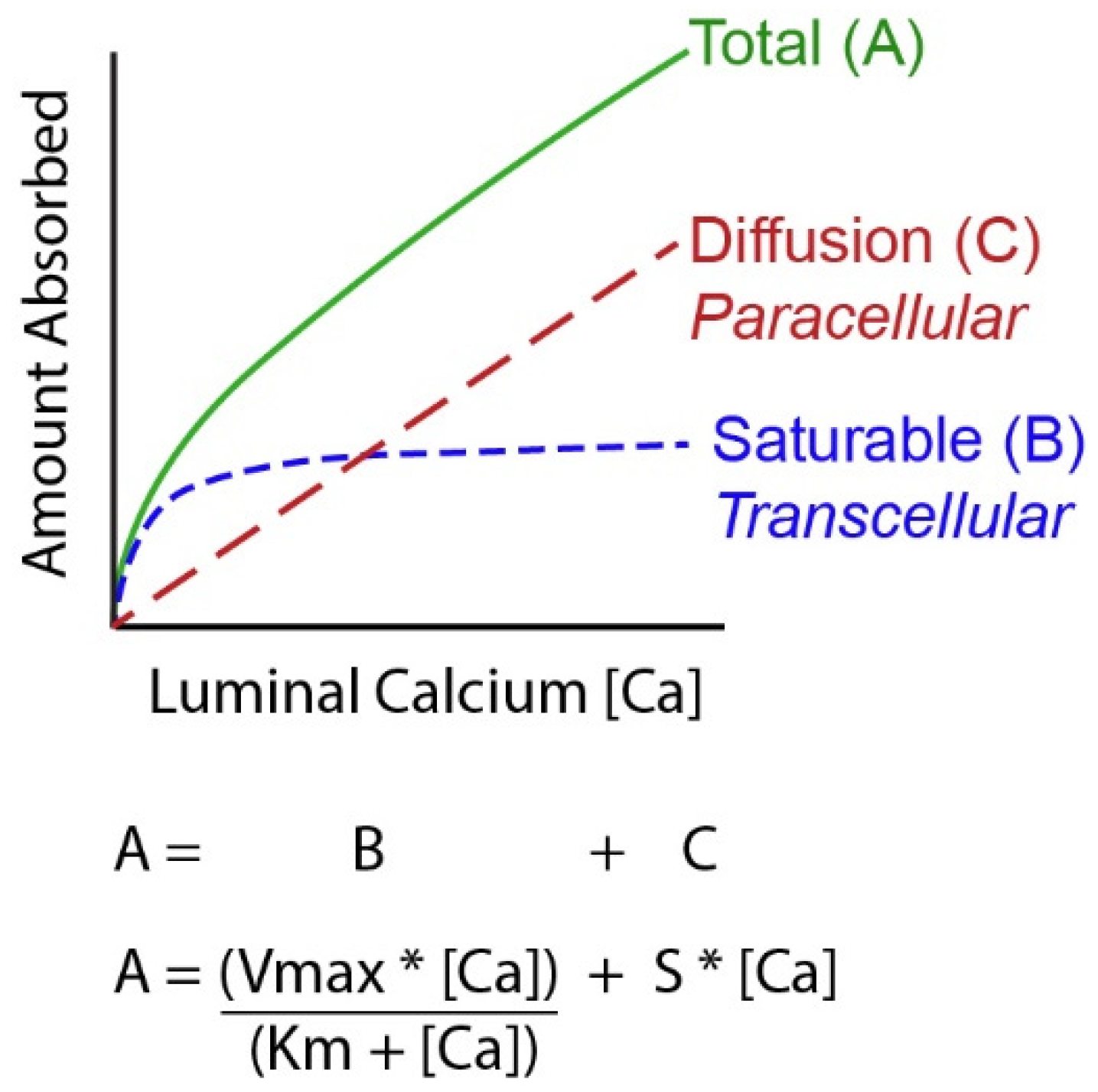
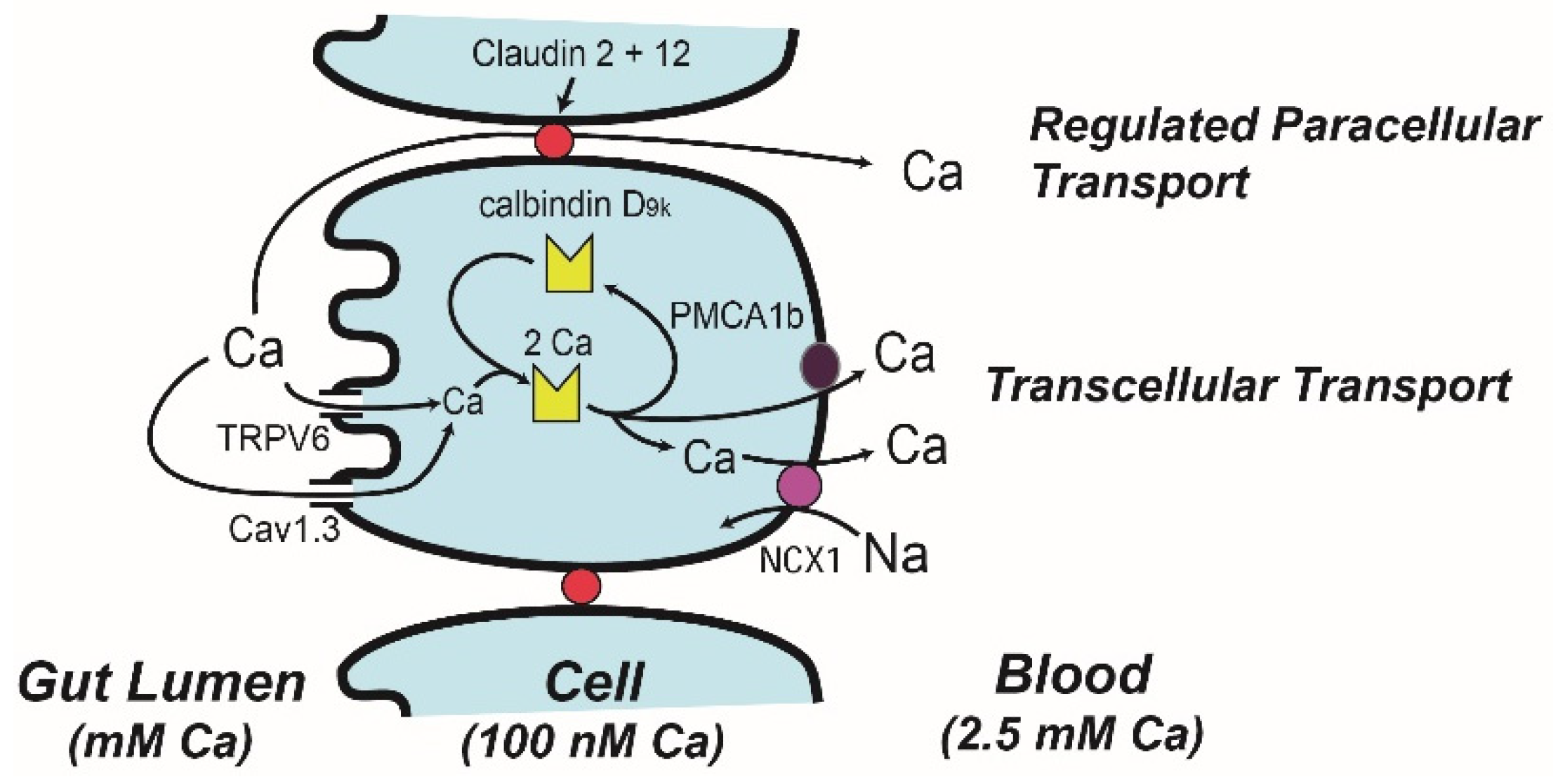
4. Molecular Models of Ca Absorption
5. Physiologic Regulation of Vitamin D-Mediated Intestinal Ca Absorption
6. Conclusions
Funding
Conflicts of Interest
References
- Nicolaysen, R. Studies upon the mode of action of vitamin D. III. the influence of vitamin D on the absorption of calcium and phosphorus in the rat. Biochem. J. 1937, 37, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Pansu, D.; Bellaton, C.; Roche, C.; Bronner, F. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am. J. Physiol. 1983, 244, G695–G700. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.S.; Ramirez, A.; Emmett, M.; Santa, A.C.; Schiller, L.R.; Fordtran, J.S. Role of vitamin D-dependent and vitamin D-independent mechanisms in absorption of food calcium. J. Clin. Investig. 1988, 81, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Schnoes, H.K.; DeLuca, H.F.; Suda, T.; Cousins, R.J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry 1971, 10, 2799–2804. [Google Scholar]
- Norman, A.W.; Myrtle, J.F.; Midgett, R.J.; Nowicki, H.G.; Williams, V.; Popjak, G. 1,25-dihydroxycholecalciferol: Identification of the proposed active form of vitamin D3 in the intestine. Science 1971, 173, 51–54. [Google Scholar] [CrossRef]
- Brumbaugh, P.F.; Haussler, M.R. Nuclear and cytoplasmic receptors for 1,25-dihydroxycholecalciferol in intestinal mucosa. Biochem. Biophys. Res. Commun. 1973, 51, 74–80. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef]
- Xue, Y.B.; Fleet, J.C. Intestinal Vitamin D Receptor Is Required for Normal Calcium and Bone Metabolism in Mice. Gastroenterology 2009, 136, 1317–1327. [Google Scholar] [CrossRef]
- Aita, R.A.D.; Hassan, S.; Hur, J.; Pellon-Cardenas, O.; Cohen, E.; Chen, L.; Shroyer, N.; Christakos, S.; Verzi, M.; Fleet, J.C. Genomic Analysis of 1,25-dihydroxyvitamin D3 Action in Mouse Intestine Reveals Compartment and Segment-Specific Gene Regulatory Effects. J. Biol. Chem. 2022, 298, 102213. [Google Scholar] [CrossRef]
- Bronner, F.; Aubert, J.P. Bone metabolism and regulation of the blood calcium level in rats. Am. J. Physiol. 1965, 209, 887–890. [Google Scholar] [CrossRef]
- Pansu, D.; Bellaton, C.; Bronner, F. Effect of Ca intake on saturable and nonsaturable components of duodenal Ca transport. Am. J. Physiol. 1981, 240, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.A.; Fordtran, J.S.; Brinkley, L.J.; Zerwekh, J.E.; Nicar, M.J.; Strowig, S.M.; Pak, C.Y. Jejunal and ileal adaptation to alterations in dietary calcium: Changes in calcium and magnesium absorption and pathogenetic role of parathyroid hormone and 1,25-dihydroxyvitamin D. J. Clin. Investig. 1981, 67, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Favus, M.J.; Walling, M.W.; Kimberg, D.V. Effects of dietary calcium restriction and chronic thyroparathyroidectomy on the metabolism of (3H)25-hydroxyvitamin D3 and the active transport of calcium by rat intestine. J. Clin. Investig. 1974, 53, 1139–1148. [Google Scholar] [CrossRef]
- Zierold, C.; Mings, J.A.; DeLuca, H.F. Regulation of 25-hydroxyvitamin D3-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J. Cell. Biochem. 2003, 88, 234–237. [Google Scholar] [CrossRef]
- Armbrecht, H.J.; Boltz, M.A.; Hodam, T.L. PTH increases renal 25(OH)D3-1alpha -hydroxylase (CYP1alpha) mRNA but not renal 1,25(OH)2D3 production in adult rats. Am. J. Physiol. Ren. Physiol. 2003, 284, F1032–F1036. [Google Scholar] [CrossRef] [PubMed]
- Gershoff, S.N.; Hegsted, D.M. Effect of vitamin D and Ca:P ratios on chick gastrointestinal tract. Am. J. Physiol. 1956, 187, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Need, A.G.; O’Loughlin, P.D.; Morris, H.A.; Coates, P.S.; Horowitz, M.; Nordin, B.E. Vitamin D Metabolites and Calcium Absorption in Severe Vitamin D Deficiency. J. Bone Miner. Res. 2008, 23, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R.H.; Taylor, A.N. Some aspects of the intestinal absorption of calcium, with special reference to vitamin D. In Mineral Metabolism, An Advanced Treatise; Comar, C.L., Bronner, F., Eds.; Academic Press: New York, NY, USA, 1969; Volume 3, pp. 321–403. [Google Scholar]
- Heaney, R.P.; Saville, P.D.; Recker, R.R. Calcium absorption as a function of calcium intake. J. Lab. Clin. Med. 1975, 85, 881–890. [Google Scholar]
- Sheikh, M.S.; Schiller, L.R.; Fordtran, J.S. In vivo intestinal absorption of calcium in humans. Miner. Electrolyte Metab. 1990, 16, 130–146. [Google Scholar]
- Wallace, T.C.; Reider, C.; Fulgoni, V.L., 3rd. Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: Analysis of the NHANES 2001–2008 data set. J. Am. Coll. Nutr. 2013, 32, 321–330. [Google Scholar] [CrossRef]
- Favus, M.J.; Angeid-Backman, E.; Breyer, M.D.; Coe, F.L. Effects of trifluoperazine, ouabain, and ethacrynic acid on intestinal calcium. Am. J. Physiol. 1983, 244, G111–G115. [Google Scholar] [CrossRef]
- Favus, M.J.; Kathpalia, S.C.; Coe, F.L. Kinetic characteristics of calcium absorption and secretion by rat colon. Am. J. Physiol. 1981, 240, G350–G354. [Google Scholar] [CrossRef] [PubMed]
- Grinstead, W.C.; Pak, C.Y.C.; Krejs, G.J. Effect of 1,25-Dihydroxyvitamin-D3 on Calcium-Absorption in the Colon of Healthy Humans. Am. J. Physiol. 1984, 247, G189–G192. [Google Scholar] [CrossRef] [PubMed]
- Karbach, U.; Feldmeier, H. The cecum is the site with the highest calcium absorption in rat intestine. Dig. Dis. Sci. 1993, 38, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Veldurthy, V.; Yehia, G.; Hsaio, C.; Porta, A.; Kim, K.I.; Patel, N.; Lieben, L.; Verlinden, L.; Carmeliet, G.; et al. Transgenic Expression of the Vitamin D Receptor Restricted to the Ileum, Cecum, and Colon of Vitamin D Receptor Knockout Mice Rescues Vitamin D Receptor-Dependent Rickets. Endocrinology 2017, 158, 3792–3804. [Google Scholar] [CrossRef]
- Jiang, H.; Horst, R.L.; Koszewski, N.J.; Goff, J.P.; Christakos, S.; Fleet, J.C. Targeting 1,25(OH)2D-mediated calcium absorption machinery in proximal colon with calcitriol glycosides and glucuronides. J. Steroid Biochem. Mol. Biol. 2020, 198, 105574. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Wood, R.J. Vitamin D-regulated calcium transport in Caco-2 cells: Unique in vitro model. Am. J. Physiol. 1991, 260, G207–G212. [Google Scholar] [CrossRef]
- Fordtran, J.S.; Locklear, T.W. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am. J. Dig. Dis. 1966, 11, 503–521. [Google Scholar] [CrossRef]
- Marcus, C.S.; Lengemann, F.W. Absorption of Ca 45 and Sr 85 from solid and liquid food at various levels of the alimentary tract of the rat. J. Nut. 1962, 77, 155–160. [Google Scholar] [CrossRef]
- Duflos, C.; Bellaton, C.; Pansu, D.; Bronner, F. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J. Nutr. 1995, 125, 2348–2355. [Google Scholar] [CrossRef]
- Boyle, I.T.; Gray, R.W.; Omdahl, J.L.; DeLuca, H.F.; Schilling, R.F. The mechanism of adaptation of intestinal calcium absorption to low dietary calcium. J. Lab. Clin. Med. 1971, 78, 813. [Google Scholar]
- Song, Y.; Peng, X.; Porta, A.; Takanaga, H.; Peng, J.B.; Hediger, M.A.; Fleet, J.C.; Christakos, S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 2003, 144, 3885–3894. [Google Scholar] [CrossRef]
- Abrams, S.A.; Hicks, P.D.; Hawthorne, K.M. Higher serum 25-hydroxyvitamin D levels in school-age children are inconsistently associated with increased calcium absorption. J. Clin. Endocrinol. Metab. 2009, 94, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Hawthorne, K.M.; Chen, Z. Supplementation with 1000 IU vitamin D/d leads to parathyroid hormone suppression, but not increased fractional calcium absorption, in 4-8-y-old children: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.D.; Laing, E.M.; Hill Gallant, K.M.; Hall, D.B.; McCabe, G.P.; Hausman, D.B.; Martin, B.R.; Warden, S.J.; Peacock, M.; Weaver, C.M. A randomized trial of vitamin D(3) supplementation in children: Dose-response effects on vitamin D metabolites and calcium absorption. J. Clin. Endocrinol. Metab. 2013, 98, 4816–4825. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.E.; Johnson, R.E.; Chambers, K.R.; Johnson, M.G.; Lemon, C.C.; Vo, T.N.; Marvdashti, S. Treatment of Vitamin D Insufficiency in Postmenopausal Women: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F.; Pansu, D. Nutritional aspects of calcium absorption. J. Nutr. 1999, 129, 9–12. [Google Scholar] [CrossRef]
- Sempos, C.T.; Durazo-Arvizu, R.A.; Fischer, P.R.; Munns, C.F.; Pettifor, J.M.; Thacher, T.D. Serum 25-hydroxyvitamin D requirements to prevent nutritional rickets in Nigerian children on a low-calcium diet-a multivariable reanalysis. Am. J. Clin. Nutr. 2021, 114, 231–237. [Google Scholar] [CrossRef]
- Fischer, P.R.; Almasri, N.I. Nutritional rickets-Vitamin D and beyond. J. Steroid Biochem. Mol. Biol. 2022, 219, 106070. [Google Scholar] [CrossRef]
- Matkovic, V.; Fontana, D.; Tominac, C.; Goel, P.; Chesnut, C.H. Factors that influence peak bone mass formation: A study of calcium balance and the inheritance of bone mass in adolescent females. Am. J. Clin. Nutr. 1990, 52, 878–888. [Google Scholar] [CrossRef]
- Replogle, R.A.; Li, Q.; Wang, L.; Zhang, M.; Fleet, J.C. Gene-by-Diet Interactions Influence Calcium Absorption and Bone Density in Mice. J. Bone Miner. Res. 2014, 29, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Makepeace, A.E.; Jameson, K.A.; Masterson, L.M.; Holt, R.I.; Swaminathan, R.; Javaid, M.K.; Cooper, C.; Arden, N.K. Weight in infancy and adult calcium absorption as determinants of bone mineral density in adult men: The hertfordshire cohort study. Calcif. Tissue Int. 2012, 91, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Reyes Fernandez, P.C.; Replogle, R.A.; Wang, L.; Zhang, M.; Fleet, J.C. Novel Genetic Loci Control Calcium Absorption and Femur Bone Mass as Well as Their Response to Low Calcium Intake in Male BXD Recombinant Inbred Mice. J. Bone Miner. Res. 2016, 31, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Duong, T.; Cauley, J.A.; Heaney, R.P.; Wolf, R.L.; Harris, E.; Cummings, S.R. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann. Intern. Med. 2000, 132, 345–353. [Google Scholar] [CrossRef]
- Haussler, M.R.; Norman, A.W. Chromosomal receptor for a vitamin D metabolite. Proc. Natl. Acad. Sci. USA 1969, 62, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Hadad, S.; Chen, Z.; Nemirovsky, A.; Gepstein, V.; Militianu, D.; Weisman, Y.; Abrams, S.A. Calcium absorption, kinetics, bone density, and bone structure in patients with hereditary vitamin D-resistant rickets. J. Clin. Endocrinol. Metab. 2011, 96, 3701–3709. [Google Scholar] [CrossRef]
- Van Cromphaut, S.J.; Dewerchin, M.; Hoenderop, J.G.; Stockmans, I.; Van Herck, E.; Kato, S.; Bindels, R.J.; Collen, D.; Carmeliet, P.; Bouillon, R.; et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: Functional and molecular aspects. Proc. Natl. Acad. Sci. USA 2001, 98, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kato, S.; Fleet, J.C. Vitamin D Receptor (VDR) Knockout Mice Reveal VDR-Independent Regulation of Intestinal Calcium Absorption and ECaC2 and Calbindin D9k mRNA. J. Nutr. 2003, 133, 374–380. [Google Scholar] [CrossRef]
- Lieben, L.; Masuyama, R.; Torrekens, S.; Van Looveren, R.; Schrooten, J.; Baatsen, P.; Lafage-Proust, M.H.; Dresselaers, T.; Feng, J.Q.; Bonewald, L.F.; et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J. Clin. Investig. 2012, 122, 1803–1815. [Google Scholar] [CrossRef]
- Amling, M.; Priemel, M.; Holzmann, T.; Chapin, K.; Rueger, J.M.; Baron, R.; Demay, M.B. Rescue of the skeletal phenotype of vitamin D receptor ablated mice in the setting of normal mineral ion homeostasis: Formal histomorphometric and biomechanical analysis. Endocrinology 1999, 140, 4982–4987. [Google Scholar] [CrossRef]
- Lieben, L.; Verlinden, L.; Masuyama, R.; Torrekens, S.; Moermans, K.; Schoonjans, L.; Carmeliet, P.; Carmeliet, G. Extra-intestinal calcium handling contributes to normal serum calcium levels when intestinal calcium absorption is suboptimal. Bone 2015, 81, 502–512. [Google Scholar] [CrossRef]
- Song, Y.; Fleet, J.C. Intestinal Resistance to 1,25 Dihydroxyvitamin D in Mice Heterozygous for the Vitamin D Receptor Knockout Allele. Endocrinology 2007, 148, 1396–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ames, S.K.; Ellis, K.J.; Gunn, S.K.; Copeland, K.C.; Abrams, S.A. Vitamin D receptor gene Fok1 polymorphisms predicts calcium absorption and bone mineral density in children. J. Bone Miner. Res. 1999, 14, 740–746. [Google Scholar] [CrossRef]
- Jurutka, P.W.; Remus, L.S.; Whitfield, K.; Thompson, P.D.; Hsieh, J.C.; Zitzer, H.; Tavakkoli, P.; Galligan, M.A.; Dang, H.T.L.; Haussler, C.A.; et al. The polymorphic N terminus in human vitamin D receptor isoforms influences trascriptional activity by modulating interaction with transcription factor IIB. Mol. Endocrinol. 2000, 14, 401–420. [Google Scholar] [CrossRef]
- Huang, Z.W.; Dong, J.; Piao, J.H.; Li, W.D.; Tian, Y.; Xu, J.; Yang, X.G. Relationship between the absorption of dietary calcium and the Fok I polymorphism of VDR gene in young women. Zhonghua Yu Fang Yi Xue Za Zhi 2006, 40, 75–78. [Google Scholar] [PubMed]
- Chandra, S.; Fullmer, C.S.; Smith, C.A.; Wasserman, R.H.; Morrison, G.H. Ion microscopic imaging of calcium transport in the intestinal tissue of vitamin D-deficient and vitamin D-replete chickens: A 44Ca stable isotope study. Proc. Natl. Acad. Sci. USA 1990, 87, 5715–5719. [Google Scholar] [CrossRef] [PubMed]
- Fullmer, C.S.; Chandra, S.; Smith, C.A.; Morrison, G.H.; Wasserman, R.H. Ion microscopic imaging of calcium during 1,25-dihydroxyvitamin D-mediated intestinal absorption. Histochem. Cell. Biol. 1996, 106, 215–222. [Google Scholar] [CrossRef]
- Bronner, F.; Pansu, D.; Stein, W.D. An analysis of intestinal calcium transport across the rat intestine. Am. J. Physiol. 1986, 250, G561–G569. [Google Scholar] [CrossRef]
- Peng, J.B.; Chen, X.Z.; Berger, U.V.; Vassilev, P.M.; Tsukaguchi, H.; Brown, E.M.; Hediger, M.A. Molecular cloning and characterization of a channel-like transporter mediated intestinal calcium absorption. J. Biol. Chem. 1999, 274, 22739–22746. [Google Scholar] [CrossRef]
- Meyer, M.B.; Zella, L.A.; Nerenz, R.D.; Pike, J.W. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J. Biol. Chem. 2007, 282, 22344–22352. [Google Scholar] [CrossRef]
- Fleet, J.C.; Eksir, F.; Hance, K.W.; Wood, R.J. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am. J. Physiol. 2002, 283, G618–G625. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Riley, E.M.; Meyer, M.B.; Benkusky, N.A.; Plum, L.A.; DeLuca, H.F.; Pike, J.W. 1,25-Dihydroxyvitamin D3 Controls a Cohort of Vitamin D Receptor Target Genes in the Proximal Intestine That Is Enriched for Calcium-regulating Components. J. Biol. Chem. 2015, 290, 18199–18215. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Watanuki, M.; Kim, S.; Shevde, N.K.; Pike, J.W. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol. Endocrinol. 2006, 20, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Kutuzova, G.D.; Sundersingh, F.; Vaughan, J.; Tadi, B.P.; Ansay, S.E.; Christakos, S.; DeLuca, H.F. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 19655–19659. [Google Scholar] [CrossRef] [PubMed]
- Benn, B.S.; Ajibade, D.; Porta, A.; Dhawan, P.; Hediger, M.; Peng, J.B.; Jiang, Y.; Oh, G.T.; Jeung, E.B.; Lieben, L.; et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 2008, 149, 3196–3205. [Google Scholar] [CrossRef]
- Woudenberg-Vrenken, T.E.; Lameris, A.L.; Weissgerber, P.; Olausson, J.; Flockerzi, V.; Bindels, R.J.; Freichel, M.; Hoenderop, J.G. Functional TRPV6 channels are crucial for transepithelial Ca2+ absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G879–G885. [Google Scholar] [CrossRef]
- Cui, M.; Li, Q.; Johnson, R.; Fleet, J.C. Villin promoter-mediated transgenic expression of transient receptor potential cation channel, subfamily V, member 6 (TRPV6) increases intestinal calcium absorption in wild-type and vitamin D receptor knockout mice. J. Bone Miner. Res. 2012, 27, 2097–2107. [Google Scholar] [CrossRef]
- Kellett, G.L. Alternative perspective on intestinal calcium absorption: Proposed complementary actions of Ca(v)1.3 and TRPV6. Nutr. Rev. 2011, 69, 347–370. [Google Scholar] [CrossRef]
- Reyes-Fernandez, P.C.; Fleet, J.C. Luminal glucose does not enhance active intestinal calcium absorption in mice: Evidence against a role for Ca(v)1.3 as a mediator of calcium uptake during absorption. Nutr. Res. 2015, 35, 1009–1015. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Ferries, I.K.; Jiang, L.; Desta, M.Z.; Yu, X.; Yang, Z.; Duncan, R.L.; Turner, C.H. Skeletal phenotype of mice with a null mutation in Cav 1.3 L-type calcium channel. J. Musculoskelet. Neuronal Interact. 2010, 10, 180–187. [Google Scholar]
- Nakkrasae, L.I.; Thongon, N.; Thongbunchoo, J.; Krishnamra, N.; Charoenphandhu, N. Transepithelial calcium transport in prolactin-exposed intestine-like Caco-2 monolayer after combinatorial knockdown of TRPV5, TRPV6 and Ca(v)1.3. J. Physiol. Sci. 2010, 60, 9–17. [Google Scholar] [CrossRef]
- Beggs, M.R.; Lee, J.J.; Busch, K.; Raza, A.; Dimke, H.; Weissgerber, P.; Engel, J.; Flockerzi, V.; Alexander, R.T. TRPV6 and Cav1.3 Mediate Distal Small Intestine Calcium Absorption Before Weaning. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Gill, R.; Lee, S.; Li, H. Molecular aspects of the calbindins. J. Nutr. 1992, 122, 678–682. [Google Scholar] [CrossRef]
- Wasserman, R.H.; Taylor, A.N. Vitamin D3-induced calcium-binding proteins in chick intestinal mucosa. Science 1966, 252, 791–793. [Google Scholar] [CrossRef]
- Bronner, F.; Buckley, M. The molecular nature of 1,25-(OH)2-D3-induced calcium-binding protein biosynthesis in the rat. Adv. Exp. Med. Biol. 1982, 151, 355–360. [Google Scholar] [PubMed]
- Pansu, D.; Bellaton, C.; Roche, C.; Bronner, F. Theophylline inhibits active Ca transport in rat intestine by inhibiting Ca binding by CaBP. Prog. Clin. Biol. Res. 1988, 252, 115–120. [Google Scholar]
- Feher, J.J.; Fullmer, C.S.; Wasserman, R.H. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am. J. Physiol. 1992, 262, C517–C526. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Kutuzova, G.D.; Christakos, S.; DeLuca, H.F. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch. Biochem. Biophys. 2007, 460, 227–232. [Google Scholar] [CrossRef]
- Spencer, R.; Charman, M.; Wilson, P.W.; Lawson, D.E.M. The relationship between vitamin D-stimulated calcium transport and intestinal calcium-binding protein in the chicken. Biochem. J. 1978, 170, 93–101. [Google Scholar] [CrossRef]
- Wasserman, R.H.; Smith, C.A.; Brindak, M.E.; Detalamoni, N.; Fullmer, C.S.; Penniston, J.T.; Kumar, R. Vitamin-D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology 1992, 102, 886–894. [Google Scholar] [CrossRef]
- Cai, Q.; Chandler, J.S.; Wasserman, R.H.; Kumar, R.; Penniston, J.T. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc. Natl. Acad. Sci. USA 1993, 90, 1345–1349. [Google Scholar] [CrossRef]
- Liu, C.; Weng, H.; Chen, L.; Yang, S.; Wang, H.; Debnath, G.; Guo, X.; Wu, L.; Mohandas, N.; An, X. Impaired intestinal calcium absorption in protein 4.1R-deficient mice due to altered expression of plasma membrane calcium ATPase 1b (PMCA1b). J. Biol. Chem. 2013, 288, 11407–11415. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Z.C.; Craig, T.A.; Filoteo, A.G.; Westendorf, J.J.; Cartwright, E.J.; Neyses, L.; Strehler, E.E.; Kumar, R. Deletion of the intestinal plasma membrane calcium pump, isoform 1, Atp2b1, in mice is associated with decreased bone mineral density and impaired responsiveness to 1, 25-dihydroxyvitamin D3. Biochem. Biophys. Res. Commun. 2015, 467, 152–156. [Google Scholar] [CrossRef] [PubMed]
- van Corven, E.J.; Roche, C.; Van Os, C.H. Distribution of Ca2+-ATPase, ATP-dependent Ca2+-transport, calmodulin and vitamin D-dependent Ca2+-binding protein along the villus-crypt axis in rat duodenum. Biochim. Biophys. Acta 1985, 820, 274–282. [Google Scholar] [CrossRef]
- Karbach, U. Paracellular Calcium Transport Across the Small Intestine. J. Nutr. 1992, 122, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Tudpor, K.; Teerapornpuntakit, J.; Jantarajit, W.; Krishnamra, N.; Charoenphandhu, N. 1,25-dihydroxyvitamin d(3) rapidly stimulates the solvent drag-induced paracellular calcium transport in the duodenum of female rats. J. Physiol. Sci. 2008, 58, 297–307. [Google Scholar] [CrossRef]
- Simon, D.B.; Lu, Y.; Choate, K.A.; Velazquez, H.; Al-Sabban, E.; Praga, M.; Casari, G.; Bettinelli, A.; Colussi, G.; Rodriquez-Soriano, J.; et al. Paracellin-1, a renal tight junction protein required for paracellular Mg 2+ resorption. Science 1999, 285, 103–106. [Google Scholar] [CrossRef]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and-12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell. 2008, 19, 1912–1921. [Google Scholar] [CrossRef]
- Beggs, M.R.; Young, K.; Pan, W.; O’Neill, D.D.; Saurette, M.; Plain, A.; Rievaj, J.; Doschak, M.R.; Cordat, E.; Dimke, H.; et al. Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis. Proc. Natl. Acad. Sci. USA 2021, 118, e2111247118. [Google Scholar] [CrossRef]
- Fujita, H.; Chiba, H.; Yokozaki, H.; Sakai, N.; Sugimoto, K.; Wada, T.; Kojima, T.; Yamashita, T.; Sawada, N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J. Histochem. Cytochem. 2006, 54, 933–944. [Google Scholar] [CrossRef]
- Davis, W.L.; Jones, R.G. Lysosomal proliferation in rachitic avian intestinal absorptive cells following 1,25-dihydroxycholecalciferol. Tissue Cell. 1982, 14, 585–595. [Google Scholar] [CrossRef]
- Nemere, I.; Szego, C.M. Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine: 1. Limited lysosomal enzyme release and calcium uptake. Endocrinology 1981, 108, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Norman, A.W. 1,25-Dihydroxyvitamin D3-mediated vesicular transport of calcium in intestine: Time-course studies. Endocrinology 1988, 122, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Leathers, V.; Norman, A.W. 1, 25 dihydroxyvitamin D3-mediated intestinal calcium transport. Biochemical identification of lysozomes containing calcium and calcium-binding protein (calbindin-D 28k ). J. Biol. Chem. 1986, 261, 16106–16114. [Google Scholar] [CrossRef]
- Favus, M.J.; Tembe, V.; Tanklefsky, M.D.; Ambrosic, K.A.; Nellans, H.N. Effects of quinacrine on calcium active transport by rat intestinal epithelium. Am. J. Physiol. 1989, 257, G818–G822. [Google Scholar] [CrossRef]
- Nemere, I.; Yoshimoto, Y.; Norman, A.W. Calcium transport in perfused duodena from normal chicks: Enhancement within fourteen minutes of exposure to 1,25 dihydroxyvitamin D3. Endocrinology 1984, 115, 1476–1483. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Keidel, D.; Bula, C.M.; Bishop, J.E.; Zanello, L.P.; Wurtz, J.M.; Moras, D.; Norman, A.W. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 12876–12881. [Google Scholar] [CrossRef]
- Norman, A.W.; Bishop, J.E.; Bula, C.M.; Olivera, C.J.; Mizwicki, M.T.; Zanello, L.P.; Ishida, H.; Okamura, W.H. Molecular tools for study of genomic and rapid signal transduction responses initiated by 1 alpha,25(OH)(2)-vitamin D(3). Steroids 2002, 67, 457–466. [Google Scholar] [CrossRef]
- Huhtakangas, J.A.; Olivera, C.J.; Bishop, J.E.; Zanello, L.P.; Norman, A.W. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)(2)-vitamin D-3 in vivo and in vitro. Mol. Endocrinol. 2004, 18, 2660–2671. [Google Scholar] [CrossRef]
- Nemere, I.; Garbi, N.; Hammerling, G.J.; Khanal, R.C. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. J. Biol. Chem. 2010, 285, 31859–31866. [Google Scholar] [CrossRef]
- Nemere, I.; Garcia-Garbi, N.; Hammerling, G.J.; Winger, Q. Intestinal cell phosphate uptake and the targeted knockout of the 1,25D(3)-MARRS receptor/PDIA3/ERp57. Endocrinology 2012, 153, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Garbi, N.; Hammerling, G.; Hintze, K.J. Role of the 1,25D(3)-MARRS receptor in the 1,25(OH)(2)D(3)-stimulated uptake of calcium and phosphate in intestinal cells. Steroids 2012, 77, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Aldea, D.; Chen, L.; Christakos, S.; Verzi, M. Regulatory domains controlling high intestinal vitamin D receptor gene expression are conserved in mouse and human. J. Biol. Chem. 2022, 298, 101616. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; DeLuca, H.F. Regulation of the intestinal 1,25-dihydroxyvitamin D3 receptor during neonatal development in the rat. Arch. Biochem. Biophys. 1988, 261, 241–249. [Google Scholar] [CrossRef]
- Bronner, F.; Salle, B.L.; Putet, G.; Rigo, J.; Senterre, J. Net calcium absorption in premature infants: Results of 103 metabolic balance studies. Am. J. Clin. Nutr. 1992, 56, 1037–1044. [Google Scholar] [CrossRef]
- Zoidis, E.; Gosteli-Peter, M.; Ghirlanda-Keller, C.; Meinel, L.; Zapf, J.; Schmid, C. IGF-I and GH stimulate Phex mRNA expression in lungs and bones and 1,25-dihydroxyvitamin D(3) production in hypophysectomized rats. Eur. J. Endocrinol. 2002, 146, 97–105. [Google Scholar] [CrossRef][Green Version]
- Fleet, J.C.; Bruns, M.E.; Hock, J.M.; Wood, R.J. Growth hormone and parathyroid hormone stimulate intestinal calcium absorption in aged female rats. Endocrinology 1994, 134, 1755–1760. [Google Scholar] [CrossRef]
- Chen, C.; Noland, K.A.; Kalu, D.N. Modulation of intestinal vitamin D receptor by ovariectomy, estrogen and growth hormone. Mech. Ageing Dev. 1997, 99, 109–122. [Google Scholar] [CrossRef]
- Fatayerji, D.; Mawer, E.B.; Eastell, R. The role of insulin-like growth factor I in age-related changes in calcium homeostasis in men. J. Clin. Endocrinol. Metab. 2000, 85, 4657–4662. [Google Scholar] [CrossRef]
- Quan-Sheng, D.; Miller, S.C. Calciotrophic hormone levels and calcium absorption during pregnancy in rats. Am. J. Physiol. 1989, 257, E118–E123. [Google Scholar] [CrossRef]
- Halloran, B.P.; DeLuca, H.F. Calcium-Transport in Small-Intestine During Pregnancy and Lactation. Am. J. Physiol. 1980, 239, E64–E68. [Google Scholar] [CrossRef] [PubMed]
- Brommage, R.; Baxter, D.C.; Gierke, L.W. Vitamin D-independent intestinal calcium and phosphorus absorption during reproduction. Am. J. Physiol. 1990, 259, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Fudge, N.J.; Kovacs, C.S. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 2010, 151, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.D.; Fung, E.B.; Halloran, B.P.; Turnlund, J.R.; Van Loan, M.D.; Cann, C.E.; King, J.C. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am. J. Clin. Nutr. 1998, 67, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N.A.; Zerwekh, J.E. Relationship of estrogen and pregnancy to calcium homeostasis in pseudohypoparathyroidism. J. Clin. Endocrinol. Metab. 1986, 62, 45–51. [Google Scholar] [CrossRef]
- Robinson, C.J.; Spanos, E.; James, M.F.; Pike, J.W.; Haussler, M.R.; Makeen, A.M.; Hillyard, C.J.; MacIntyre, I. Role of Prolactin in Vitamin-D Metabolism and Calcium-Absorption During Lactation in the Rat. J. Endocrinol. 1982, 94, 443–453. [Google Scholar] [CrossRef]
- Pahuja, D.N.; DeLuca, H.F. Stimulation of Intestinal Calcium-Transport and Bone Calcium Mobilization by Prolactin in Vitamin-D-Deficient Rats. Science 1981, 214, 1038–1039. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Meyer, M.B.; Pike, J.W.; Christakos, S. Evidence for a role of prolactin in calcium homeostasis: Regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin. Endocrinology 2010, 151, 2974–2984. [Google Scholar] [CrossRef]
- Avioli, L.V.; McDonald, J.E.; Lee, S.W. The influence of age on the intestinal absorption of 47Ca in women and its relation to 47Ca absorption in postmenopausal osteoporosis. J. Clin. Investig. 1965, 44, 1960–1967. [Google Scholar] [CrossRef]
- Bullamore, J.R.; Gallagher, J.C.; Wilkinson, R.; Nordin, B.E.C.; Marshall, D.H. Effect of age on calcium absorption. Lancet 1970, 2, 535–537. [Google Scholar] [CrossRef]
- Armbrecht, H.J.; Zenser, T.V.; Bruns, M.E.; Davis, B.B. Effect of age on intestinal calcium absorption and adaptation to dietary calcium. Am. J. Physiol. 1979, 236, E769–E774. [Google Scholar] [CrossRef] [PubMed]
- Ireland, P.; Fordtran, J.S. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J. Clin. Investig. 1973, 52, 2672–2681. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Fleet, J.C.; Cashman, K.; Bruns, M.E.; DeLuca, H.F. Intestinal calcium absorption in the aged rat: Evidence of intestinal resistance to 1,25(OH)2 vitamin D. Endocrinology 1998, 139, 3843–3848. [Google Scholar] [CrossRef] [PubMed]
- Pattanaungkul, S.; Riggs, B.L.; Yergey, A.L.; Vieira, N.E.; O’Fallon, W.M.; Khosla, S. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: Evidence for age- related intestinal resistance to 1,25(OH)2D action. J. Clin. Endocrinol. Metab. 2000, 85, 4023–4027. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Riggs, B.L.; Eisman, J.; Hamstra, A.; Arnaud, S.B.; DeLuca, H.F. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: Effect of age and dietary calcium. J. Clin. Investig. 1979, 64, 729–736. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Kida, K.; Matsuda, H. Role of change in vitamin D metabolism with age in calcium and phosphorus metabolism in normal human subjects. J. Clin. Endocrinol. Metab. 1984, 59, 719–726. [Google Scholar] [CrossRef]
- Scopacasa, F.; Wishart, J.M.; Horowitz, M.; Morris, H.A.; Need, A.G. Relation between calcium absorption and serum calcitriol in normal men: Evidence for age-related intestinal resistance to calcitriol. Eur. J. Clin. Nutr. 2004, 58, 264–269. [Google Scholar] [CrossRef]
- Eastell, R.; Yergey, A.L.; Vieira, N.E.; Cedel, S.L.; Kumar, R.; Riggs, B.L. Interrelationship among vitamin-D metabolism, true calcium absorption, parathyroid function, and age in women—Evidence of an age-related intestinal resistance to 1,25-dihydroxyvitamin-D action. J. Bone Min. Res. 1991, 6, 125–132. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Sandgren, M.E.; Dimagno, E.P.; Lane, A.W.; DeLuca, H.F.; Riggs, B.L. Evidence of an age-related decrease in intestinal responsiveness to vitamin-D—Relationship between serum 1,25-dihydroxyvitamin-D3 and intestinal vitamin-D receptor concentrations in normal women. J. Clin. Endocrinol. Metab. 1992, 75, 176–182. [Google Scholar]
- Takamoto, S.; Seino, Y.; Sacktor, B.; Liang, C.T. Effect of age on duodenal 1,25-dihydroxyvitamin D-3 receptors in Wistar rats. Biochim. Biophys. Acta 1990, 1034, 22–28. [Google Scholar] [CrossRef]
- Horst, R.L.; Goff, J.P.; Reinhardt, T.A. Advancing age results in reduction of intestinal and bone 1,25 dihydroxyvitamin D receptor. Endocrinology 1990, 126, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Recker, R.R.; Saville, P.D. Menopausal changes in calcium balance performance. J. Lab. Clin. Med. 1978, 92, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Ten Bolscher, M.; Netelenbos, J.C.; Barto, R.; Van Buuren, L.M.; Van der vijgh, W.J. Estrogen regulation of intestinal calcium absorption in the intact and ovariectomized adult rat. J. Bone Miner. Res. 1999, 14, 1197–1202. [Google Scholar] [CrossRef]
- Van Abel, M.; Hoenderop, J.G.; van der Kemp, A.W.; van Leeuwen, J.P.; Bindels, R.J. Regulation of the Epithelial Ca2+ Channels in Small Intestine as Studied by Quantitative mRNA Detection. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G78–G85. [Google Scholar] [CrossRef]
- Van Cromphaut, S.J.; Rummens, K.; Stockmans, I.; Van Herck, E.; Dijcks, F.A.; Ederveen, A.; Carmeliet, P.; Verhaeghe, J.; Bouillon, R.; Carmeliet, G. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J. Bone Miner. Res. 2003, 18, 1725–1736. [Google Scholar] [CrossRef]
- Gennari, C.; Agnusdei, D.; Nardi, P.; Civitelli, R. Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J. Clin. Endocrinol. Metab. 1990, 71, 1288–1293. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Holis, B.W.; Kalu, D.N. In vivo effect of 17 b-estradiol on intestinal calcium absorption in rats. Bone Miner. 1994, 26, 181–189. [Google Scholar] [CrossRef]
- Liel, Y.; Shany, S.; Smirnoff, P.; Schwartz, B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology 1999, 140, 280–285. [Google Scholar] [CrossRef]
- Colin, E.M.; Van Den Bemd, G.J.; Van Aken, M.; Christakos, S.; De Jonge, H.R.; Deluca, H.F.; Prahl, J.M.; Birkenhager, J.C.; Buurman, C.J.; Pols, H.A.; et al. Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the Rat. J. Bone Miner. Res. 1999, 14, 57–64. [Google Scholar] [CrossRef]
- Mauras, N.; Haymond, M.W.; Darmaun, D.; Vieira, N.E.; Abrams, S.A.; Yergey, A.L. Calcium and protein kinetics in prepubertal boys. Positive effects of testosterone. J. Clin. Investig. 1994, 93, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Y.; Nordin, B.E.; Need, A.G.; Scopacasa, F.; Wishart, J.; Morris, H.A.; Horowitz, M. Relationship between calcium absorption and plasma dehydroepiandrosterone sulphate (DHEAS) in healthy males. Clin. Endocrinol. 2008, 69, 864–869. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleet, J.C. Vitamin D-Mediated Regulation of Intestinal Calcium Absorption. Nutrients 2022, 14, 3351. https://doi.org/10.3390/nu14163351
Fleet JC. Vitamin D-Mediated Regulation of Intestinal Calcium Absorption. Nutrients. 2022; 14(16):3351. https://doi.org/10.3390/nu14163351
Chicago/Turabian StyleFleet, James C. 2022. "Vitamin D-Mediated Regulation of Intestinal Calcium Absorption" Nutrients 14, no. 16: 3351. https://doi.org/10.3390/nu14163351
APA StyleFleet, J. C. (2022). Vitamin D-Mediated Regulation of Intestinal Calcium Absorption. Nutrients, 14(16), 3351. https://doi.org/10.3390/nu14163351




