The Burden of Carbohydrates in Health and Disease
Abstract
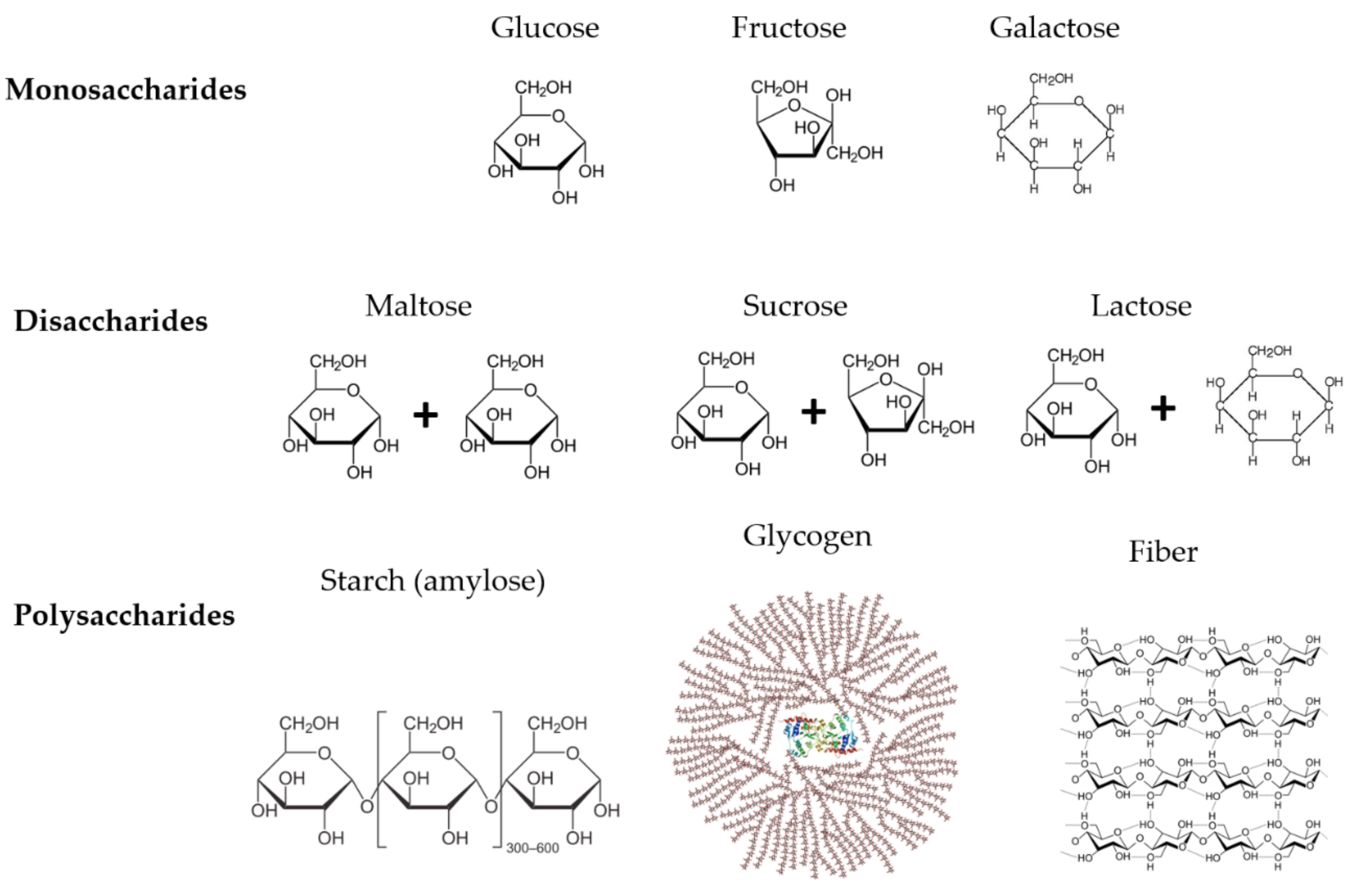
1. Introduction
2. Methods
3. Human Evolution and Carbohydrates Consumption
Nutritional Needs of Today’s Human Beings
4. Gut and Carbohydrates
5. The Role of Carbohydrate Consumption in Behaviors
6. Carbohydrates and Metabolic Disease
7. Carbohydrates and Cardiovascular Disease
8. Carbohydrates and Mental Diseases
9. Carbohydrates and Cancer
10. Carbohydrates and Chronic Kidney Failure
11. Carbohydrates and Allergies and Asthma
12. Carbohydrates: Actual Dietary Recommendations
13. Practical Statements
- Increases in the incidence of obesity and diet-related metabolic diseases are related to profound environmental and behavioral changes, especially dietary behavior.
- The increase in the consumption of cereals and refined sugars, as well as dairy products, refined vegetable oils, and fatty meats, are at the root of the epidemic of nutrition-related chronic diseases.
- Diverse microbiota are synonymous with health, with dietary fiber having a major impact on the composition, diversity, and richness of the microbiome. Thus, accessible carbohydrates are beneficial for the well-being and growth of microorganisms and consequently for the host in this symbiotic relationship.
- Carbohydrates may have mood-enhancing properties, affecting mental and psychological well-being. CHO-rich foods have a negative impact on mood categories, including alertness, tiredness, and higher rates of depression in the long term.
- Carbohydrates, when taken in an adequate amount and in the right balance in the diet, can sustain good mental health.
- Carbohydrate intake can affect the development and prognosis of metabolic disease, as an uncontrolled intake of refined carbohydrates puts individuals at risk of developing metabolic syndrome and subsequently developing metabolic disease.
- The nature of the carbohydrates rather than the amount is the key factor in reducing the risk of cardiovascular disease or in improving the cardiovascular risk markers.
- Complex carbohydrates may modulate insulin-like growth factor binding protein 3 blocking cell proliferation and tumoral growth.
- Fiber intake may modulate gut microbiota and, consequently, their short chain fatty acid production, reducing inflammation and having a positive effect on cancer development.
- Complex carbohydrates and fiber may increase fecal excretion of carcinogens, improving their elimination and reducing their negative effects in organisms.
- Consumption of simple sugars as sweeteners in different sugary beverages is related to asthma due to malabsorption. Formation of pro-inflammatory products between unabsorbed fructose and some dietary proteins, after intestinal absorption, are associated with asthma.
- Nutrition guidelines stablished that carbohydrates make up 40% to 65% of total daily calories to fulfil body energy demands and to reduce the risk of some non-communicable diseases.
- Recommended daily intake of carbohydrates should be based on whole grains.
- Low glycemic index sources and fiber intake should be higher than 25–30 g per day.
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkins, D.R. Disease Eradication. N. Engl. J. Med. 2013, 368, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet and Lifestyle Drive the Pandemic of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The Global Obesity Pandemic: Shaped by Global Drivers and Local Environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Maccallum, P.R. The Obesity, Metabolic Syndrome, and Type 2 Diabetes Mellitus Pandemic: II. Therapeutic Management of Atherogenic Dyslipidemia. J. Clin. Hypertens. 2009, 11, 520–527. [Google Scholar] [CrossRef]
- Hjelm, K.; Mufunda, E.; Nambozi, G.; Kemp, J. Preparing Nurses to Face the Pandemic of Diabetes Mellitus: A Literature Review. J. Adv. Nurs. 2003, 41, 424–434. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Hormeño-Holgado, A.; Jiménez, M.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Perez-Palencia, N.; Maestre-Serrano, R.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines 2020, 8, 236. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Simón-Sanjurjo, J.A.; Beltran-Velasco, A.I.; Laborde-Cárdenas, C.C.; Benitez-Agudelo, J.C.; Bustamante-Sánchez, Á.; Tornero-Aguilera, J.F. Mis--Dis Information in COVID-19 Health Crisis: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 5321. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Ramos-Campo, D.J.; Mielgo-Ayuso, J.; Dalamitros, A.A.; Nikolaidis, P.A.; Hormeño-Holgado, A.; Tornero-Aguilera, J.F. Nutrition in the Actual COVID-19 Pandemic. A Narrative Review. Nutrients 2021, 13, 1924. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martínez-González, M.B.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Beltran-Velasco, A.I.; Ruisoto, P.; Diaz Arroyo, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Impact of the COVID-19 Pandemic on Mental Disorders. A Critical Review. Int. J. Environ. Res. Public Health 2021, 18, 10041. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Ruisoto, P.; Dalamitros, A.A.; Beltran-Velasco, A.I.; Hormeño-Holgado, A.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Performance of Fuzzy Multi-Criteria Decision Analysis of Emergency System in COVID-19 Pandemic. An Extensive Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 5208. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional and Exercise Interventions in Cancer-Related Cachexia: An Extensive Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 4604. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Navarro-Jiménez, E.; Jimenez, M.; Hormeño-Holgado, A.; Martinez-Gonzalez, M.B.; Benitez-Agudelo, J.C.; Perez-Palencia, N.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Impact of COVID-19 Pandemic in Public Mental Health: An Extensive Narrative Review. Sustainability 2021, 13, 3221. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic Variation and Nutrition. World Rev. Nutr. Diet. 1999, 84, 118–140. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M.; Shostak, M. Stone Agers in the Fast Lane: Chronic Degenerative Diseases in Evolutionary Perspective. Am. J. Med. 1988, 84, 739–749. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Milton, K. The Critical Role Played by Animal Source Foods in Human (Homo) Evolution. J. Nutr. 2003, 133 (Suppl. S2), 3886S–3892S. [Google Scholar] [CrossRef]
- Bramble, D.M.; Lieberman, D.E. Endurance Running and the Evolution of Homo. Nature 2004, 432, 345–352. [Google Scholar] [CrossRef]
- Leonard, W.R.; Robertson, M.L.; Snodgrass, J.J.; Kuzawa, C.W. Metabolic Correlates of Hominid Brain Evolution. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 5–15. [Google Scholar] [CrossRef]
- Leonard, W.R.; Snodgrass, J.J.; Robertson, M.L. Effects of Brain Evolution on Human Nutrition and Metabolism. Annu. Rev. Nutr. 2007, 27, 311–327. [Google Scholar] [CrossRef]
- Wrangham, R.W.; Jones, J.H.; Laden, G.; Pilbeam, D.; Conklin-Brittain, N. The Raw and the Stolen. Cooking and the Ecology of Human Origins. Curr. Anthropol. 1999, 40, 567–594. [Google Scholar] [CrossRef]
- Wrangham, R.; Conklin-Brittain, N. Cooking as a Biological Trait. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 35–46. [Google Scholar] [CrossRef]
- Wrangham, R. Catching Fire: How Cooking Made Us Human; Basic Book: New York, NY, USA, 2009. [Google Scholar]
- Carmody, R.N.; Weintraub, G.S.; Wrangham, R.W. Energetic Consequences of Thermal and Nonthermal Food Processing. Proc. Natl. Acad. Sci. USA 2011, 108, 19199–19203. [Google Scholar] [CrossRef]
- Fonseca-Azevedo, K.; Herculano-Houzel, S. Metabolic Constraint Imposes Tradeoff between Body Size and Number of Brain Neurons in Human Evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 18571–18576. [Google Scholar] [CrossRef] [PubMed]
- Wong, K. New Look at Human Evolution; Rennie, J., Ed.; Scientific American: New York, NY, USA, 2003. [Google Scholar]
- James, W.P.T.; Johnson, R.J.; Speakman, J.R.; Wallace, D.C.; Frühbeck, G.; Iversen, P.O.; Stover, P.J. Nutrition and Its Role in Human Evolution. J. Intern. Med. 2019, 285, 533–549. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Ecological Control of the Gastrointestinal Tract. The Role of Probiotic Flora. Gut 1998, 42, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Natividad, J.M.M.; Verdu, E.F. Modulation of Intestinal Barrier by Intestinal Microbiota: Pathological and Therapeutic Implications. Pharmacol. Res. 2013, 69, 42–51. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Sperandio, V. Interactions between the Microbiota and Pathogenic Bacteria in the Gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How Colonization by Microbiota in Early Life Shapes the Immune System. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The Control and Consequences of Bacterial Fermentation in the Human Colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. Gut Microbiota: Filling up on Fibre for a Healthy Gut. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 67. [Google Scholar] [CrossRef]
- Burkitt, D.P. Related Disease—Related Cause? Lancet 1969, 2, 1229–1231. [Google Scholar] [CrossRef]
- Burkitt, D.P.; Walker, A.R.; Painter, N.S. Effect of Dietary Fibre on Stools and the Transit-Times, and Its Role in the Causation of Disease. Lancet 1972, 2, 1408–1412. [Google Scholar] [CrossRef]
- O’Keefe, S.J. The Association between Dietary Fibre Deficiency and High-Income Lifestyle-Associated Diseases: Burkitt’s Hypothesis Revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- De Menezes, E.W.; Giuntini, E.B.; Dan, M.C.T.; Sardá, F.A.H.; Lajolo, F.M. Codex Dietary Fibre Definition—Justification for Inclusion of Carbohydrates from 3 to 9 Degrees of Polymerisation. Food Chem. 2013, 140, 581–585. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review Article: Dietary Fibre in the Era of Microbiome Science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef]
- Bijkerk, C.J.; Muris, J.W.M.; Knottnerus, J.A.; Hoes, A.W.; de Wit, N.J. Systematic Review: The Role of Different Types of Fibre in the Treatment of Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2004, 19, 245–251. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Walter, J. Murine Gut Microbiota-Diet Trumps Genes. Cell Host Microbe 2015, 17, 3–5. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut Microbiome of the Hadza Hunter-Gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Stegen, J.C.; Maldonado-Gómez, M.X.; Eren, A.M.; Siba, P.M.; Greenhill, A.R.; Walter, J. The Gut Microbiota of Rural Papua New Guineans: Composition, Diversity Patterns, and Ecological Processes. Cell Rep. 2015, 11, 527–538. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Chassard, C.; Lacroix, C. Carbohydrates and the Human Gut Microbiota. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Mei, G.-Y.; Carey, C.M.; Tosh, S.; Kostrzynska, M. Utilization of Different Types of Dietary Fibres by Potential Probiotics. Can. J. Microbiol. 2011, 57, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.N.; Chassard, C.; Lacroix, C. Gut Microbial Adaptation to Dietary Consumption of Fructose, Artificial Sweeteners and Sugar Alcohols: Implications for Host-Microbe Interactions Contributing to Obesity. Obes. Rev. Off. J. Int. Assoc. Stud. Obes. 2012, 13, 799–809. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How Glycan Metabolism Shapes the Human Gut Microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, Fibre and Cancer Risk in African Americans and Rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef]
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable Responses of Human Microbiomes to Dietary Supplementation with Resistant Starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef]
- Martínez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant Starches Types 2 and 4 Have Differential Effects on the Composition of the Fecal Microbiota in Human Subjects. PLoS ONE 2010, 5, e15046. [Google Scholar] [CrossRef]
- Sünram-Lea, S.I.; Owen, L. The Impact of Diet-Based Glycaemic Response and Glucose Regulation on Cognition: Evidence across the Lifespan. Proc. Nutr. Soc. 2017, 76, 466–477. [Google Scholar] [CrossRef]
- Smith, M.A.; Riby, L.M.; van Eekelen, J.A.M.; Foster, J.K. Glucose Enhancement of Human Memory: A Comprehensive Research Review of the Glucose Memory Facilitation Effect. Neurosci. Biobehav. Rev. 2011, 35, 770–783. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Wurtman, J.J. Carbohydrates and Depression. Sci. Am. 1989, 260, 68–75. [Google Scholar] [CrossRef]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of Sugar-Sweetened Beverages and Weight Gain: A Systematic Review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of Soft Drink Consumption on Nutrition and Health: A Systematic Review and Meta-Analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef]
- Knüppel, A.; Shipley, M.J.; Llewellyn, C.H.; Brunner, E.J. Sugar Intake from Sweet Food and Beverages, Common Mental Disorder and Depression: Prospective Findings from the Whitehall II Study. Sci. Rep. 2017, 7, 6287. [Google Scholar] [CrossRef]
- Westover, A.N.; Marangell, L.B. A Cross-National Relationship between Sugar Consumption and Major Depression? Depress. Anxiety 2002, 16, 118–120. [Google Scholar] [CrossRef]
- Ooi, C.P.; Loke, S.C.; Yassin, Z.; Hamid, T.-A. Carbohydrates for Improving the Cognitive Performance of Independent-Living Older Adults with Normal Cognition or Mild Cognitive Impairment. Cochrane Database Syst. Rev. 2011, 2011, CD007220. [Google Scholar] [CrossRef]
- Markus, C.R. Dietary Amino Acids and Brain Serotonin Function; Implications for Stress-Related Affective Changes. Neuromol. Med. 2008, 10, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Marek, G.J.; Carpenter, L.L.; McDougle, C.J.; Price, L.H. Synergistic Action of 5-HT2A Antagonists and Selective Serotonin Reuptake Inhibitors in Neuropsychiatric Disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2003, 28, 402–412. [Google Scholar] [CrossRef]
- Markus, C.R.; Panhuysen, G.; Jonkman, L.M.; Bachman, M. Carbohydrate Intake Improves Cognitive Performance of Stress-Prone Individuals under Controllable Laboratory Stress. Br. J. Nutr. 1999, 82, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.R.; Panhuysen, G.; Tuiten, A.; Koppeschaar, H.; Fekkes, D.; Peters, M.L. Does Carbohydrate-Rich, Protein-Poor Food Prevent a Deterioration of Mood and Cognitive Performance of Stress-Prone Subjects When Subjected to a Stressful Task? Appetite 1998, 31, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, H.M.; Rogers, P.J.; Hedderley, D.I.; Walker, A.F. Acute Effects on Mood and Cognitive Performance of Breakfasts Differing in Fat and Carbohydrate Content. Appetite 1996, 27, 151–164. [Google Scholar] [CrossRef]
- Deijen, J.B.; Heemstra, M.L.; Orlebeke, J.F. Dietary Effects on Mood and Performance. J. Psychiatr. Res. 1989, 23, 275–283. [Google Scholar] [CrossRef]
- Anderson, K.E.; Rosner, W.; Khan, M.S.; New, M.I.; Pang, S.Y.; Wissel, P.S.; Kappas, A. Diet-Hormone Interactions: Protein/Carbohydrate Ratio Alters Reciprocally the Plasma Levels of Testosterone and Cortisol and Their Respective Binding Globulins in Man. Life Sci. 1987, 40, 1761–1768. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic Stress and Obesity: A New View of “Comfort Food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef]
- Soh, N.L.; Walter, G.; Baur, L.; Collins, C. Nutrition, Mood and Behaviour: A Review. Acta Neuropsychiatr. 2009, 21, 214–227. [Google Scholar] [CrossRef]
- Wurtman, J.; Wurtman, R. The Trajectory from Mood to Obesity. Curr. Obes. Rep. 2018, 7, 1–5. [Google Scholar] [CrossRef]
- Mantantzis, K.; Schlaghecken, F.; Sünram-Lea, S.I.; Maylor, E.A. Sugar Rush or Sugar Crash? A Meta-Analysis of Carbohydrate Effects on Mood. Neurosci. Biobehav. Rev. 2019, 101, 45–67. [Google Scholar] [CrossRef]
- Benton, D. Carbohydrate Ingestion, Blood Glucose and Mood. Neurosci. Biobehav. Rev. 2002, 26, 293–308. [Google Scholar] [CrossRef]
- Bernard, B.N.; Louise, L.C.; Louise, D. The Effects of Carbohydrates, in Isolation and Combined with Caffeine, on Cognitive Performance and Mood-Current Evidence and Future Directions. Nutrients 2018, 10, 192. [Google Scholar] [CrossRef]
- Van de Rest, O.; van der Zwaluw, N.L.; de Groot, L.C.P.G.M. Effects of Glucose and Sucrose on Mood: A Systematic Review of Interventional Studies. Nutr. Rev. 2018, 76, 108–116. [Google Scholar] [CrossRef]
- Messier, C. Glucose Improvement of Memory: A Review. Eur. J. Pharmacol. 2004, 490, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.; Weinem, M.; Stefanadis, C. Diet, Exercise and the Metabolic Syndrome. Rev. Diabet. Stud. 2006, 3, 118–126. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic Syndrome Update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Li, X.; Zhai, Y.; Zhao, J.; He, H.; Li, Y.; Liu, Y.; Feng, A.; Li, L.; Huang, T.; Xu, A.; et al. Impact of Metabolic Syndrome and It’s Components on Prognosis in Patients With Cardiovascular Diseases: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 704145. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- MedlinePlus. Carbohydrate Metabolism Disorders. Available online: https://medlineplus.gov/carbohydratemetabolismdisorders.html (accessed on 21 May 2022).
- Sahyoun, N.R.; Jacques, P.F.; Zhang, X.L.; Juan, W.; McKeown, N.M. Whole-Grain Intake Is Inversely Associated with the Metabolic Syndrome and Mortality in Older Adults. Am. J. Clin. Nutr. 2006, 83, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes, Cardiovascular Disease, and Weight Gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic Index, Glycemic Load, Carbohydrates, and Type 2 Diabetes: Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Zhang, Y.-H.; Wang, P.; Qin, L.-Q. Meta-Analysis of Dietary Glycemic Load and Glycemic Index in Relation to Risk of Coronary Heart Disease. Am. J. Cardiol. 2012, 109, 1608–1613. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut Hormone PYY(3-36) Physiologically Inhibits Food Intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- D’Alessio, D.A.; Kahn, S.E.; Leusner, C.R.; Ensinck, J.W. Glucagon-like Peptide 1 Enhances Glucose Tolerance Both by Stimulation of Insulin Release and by Increasing Insulin-Independent Glucose Disposal. J. Clin. Investig. 1994, 93, 2263–2266. [Google Scholar] [CrossRef]
- Dube, S.; Errazuriz, I.; Cobelli, C.; Basu, R.; Basu, A. Assessment of Insulin Action on Carbohydrate Metabolism: Physiological and Non-Physiological Methods. Diabet. Med. 2013, 30, 664–670. [Google Scholar] [CrossRef]
- Hu, F.B. Globalization of Diabetes: The Role of Diet, Lifestyle, and Genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- King, B.M. The Modern Obesity Epidemic, Ancestral Hunter-Gatherers, and the Sensory/Reward Control of Food Intake. Am. Psychol. 2013, 68, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Erlanson-Albertsson, C. How Palatable Food Disrupts Appetite Regulation. Basic Clin. Pharmacol. Toxicol. 2005, 97, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Meguid, M.M.; Fetissov, S.O.; Varma, M.; Sato, T.; Zhang, L.; Laviano, A.; Rossi-Fanelli, F. Hypothalamic Dopamine and Serotonin in the Regulation of Food Intake. Nutrition 2000, 16, 843–857. [Google Scholar] [CrossRef]
- Lam, D.D.; Garfield, A.S.; Marston, O.J.; Shaw, J.; Heisler, L.K. Brain Serotonin System in the Coordination of Food Intake and Body Weight. Pharmacol. Biochem. Behav. 2010, 97, 84–91. [Google Scholar] [CrossRef]
- Spadaro, P.A.; Naug, H.L.; Du Toit, E.F.; Donner, D.; Colson, N.J. A Refined High Carbohydrate Diet Is Associated with Changes in the Serotonin Pathway and Visceral Obesity. Genet. Res. 2015, 97, e23. [Google Scholar] [CrossRef]
- Lim, S.; Eckel, R.H. Pharmacological Treatment and Therapeutic Perspectives of Metabolic Syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 329–341. [Google Scholar] [CrossRef]
- Lakka, T.A.; Laaksonen, D.E. Physical Activity in Prevention and Treatment of the Metabolic Syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef]
- Feldeisen, S.E.; Tucker, K.L. Nutritional Strategies in the Prevention and Treatment of Metabolic Syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 46–60. [Google Scholar] [CrossRef] [PubMed]
- De la Iglesia, R.; Loria-Kohen, V.; Zulet, M.A.; Martinez, J.A.; Reglero, G.; Ramirez de Molina, A. Dietary Strategies Implicated in the Prevention and Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 1877. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-Analyses. J. Chiropr. Med. 2017, 16, 289–299. [Google Scholar] [CrossRef]
- Chanmuang, S.; Nguyen, Q.-A.; Kim, H.-J. Current Research on the Effects of Non-Digestible Carbohydrates on Metabolic Disease. Appl. Sci. 2022, 12, 3768. [Google Scholar] [CrossRef]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Correction: Low-Carbohydrate Diets and All-Cause Mortality: A Systematic Review and Meta-Analysis of Observational Studies. PLoS ONE 2019, 8, e0212203. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Sattar, N.; Banach, M. Lower Carbohydrate Diets and All-Cause and Cause-Specific Mortality: A Population-Based Cohort Study and Pooling of Prospective Studies. Eur. Heart J. 2019, 40, 2870–2879. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.-E.; Maki, K.C. Review of Current Evidence and Clinical Recommendations on the Effects of Low-Carbohydrate and Very-Low-Carbohydrate (Including Ketogenic) Diets for the Management of Body Weight and Other Cardiometabolic Risk Factors: A Scientific Statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711.e1. [Google Scholar] [CrossRef]
- Mann, J. Dietary Carbohydrate: Relationship to Cardiovascular Disease and Disorders of Carbohydrate Metabolism. Eur. J. Clin. Nutr. 2007, 61 (Suppl. S1), S100–S111. [Google Scholar] [CrossRef]
- Baghurst, K.I.; Baghurst, P.A.; Record, S.J. Demographic and Dietary Profiles of High and Low Fat Consumers in Australia. J. Epidemiol. Community Health 1994, 48, 26–32. [Google Scholar] [CrossRef][Green Version]
- Slavin, J. Why Whole Grains Are Protective: Biological Mechanisms. Proc. Nutr. Soc. 2003, 62, 129–134. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole Grain Consumption and Risk of Cardiovascular Disease, Cancer, and All Cause and Cause Specific Mortality: Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole Grain Intake and Cardiovascular Disease: A Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef]
- Steffen, L.M.; Jacobs, D.R.J.; Stevens, J.; Shahar, E.; Carithers, T.; Folsom, A.R. Associations of Whole-Grain, Refined-Grain, and Fruit and Vegetable Consumption with Risks of All-Cause Mortality and Incident Coronary Artery Disease and Ischemic Stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003, 78, 383–390. [Google Scholar] [CrossRef]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Wilson, P.W.F.; Jacques, P.F. Whole-Grain Intake Is Favorably Associated with Metabolic Risk Factors for Type 2 Diabetes and Cardiovascular Disease in the Framingham Offspring Study. Am. J. Clin. Nutr. 2002, 76, 390–398. [Google Scholar] [CrossRef]
- Kelly, S.A.; Hartley, L.; Loveman, E.; Colquitt, J.L.; Jones, H.M.; Al-Khudairy, L.; Clar, C.; Germanò, R.; Lunn, H.R.; Frost, G.; et al. Whole Grain Cereals for the Primary or Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2017, 8, CD005051. [Google Scholar] [CrossRef]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.Y.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do Ketogenic Diets Really Suppress Appetite? A Systematic Review and Meta-Analysis. Obes. Rev. Off. J. Int. Assoc. Stud. Obes. 2015, 16, 64–76. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129 (Suppl. S2), S102–S138. [Google Scholar] [CrossRef]
- Naude, C.E.; Schoonees, A.; Senekal, M.; Young, T.; Garner, P.; Volmink, J. Low Carbohydrate versus Isoenergetic Balanced Diets for Reducing Weight and Cardiovascular Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e100652. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.V.; de Oliveira, S.L.; da Rocha Ataide, T. Very-Low-Carbohydrate Ketogenic Diet v. Low-Fat Diet for Long-Term Weight Loss: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Adam-Perrot, A.; Clifton, P.; Brouns, F. Low-Carbohydrate Diets: Nutritional and Physiological Aspects. Obes. Rev. Off. J. Int. Assoc. Stud. Obes. 2006, 7, 49–58. [Google Scholar] [CrossRef]
- Willoughby, D.; Hewlings, S.; Kalman, D. Body Composition Changes in Weight Loss: Strategies and Supplementation for Maintaining Lean Body Mass, a Brief Review. Nutrients 2018, 10, 1876. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Comparison of Effects of Long-Term Low-Fat vs High-Fat Diets on Blood Lipid Levels in Overweight or Obese Patients: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2013, 113, 1640–1661. [Google Scholar] [CrossRef]
- Gjuladin-Hellon, T.; Davies, I.G.; Penson, P.; Amiri Baghbadorani, R. Effects of Carbohydrate-Restricted Diets on Low-Density Lipoprotein Cholesterol Levels in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 161–180. [Google Scholar] [CrossRef]
- Sackner-Bernstein, J.; Kanter, D.; Kaul, S. Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PLoS ONE 2015, 10, e0139817. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, N.; Vinknes, K.J.; Veierød, M.B.; Retterstøl, K. Effects of Low-Carbohydrate Diets v. Low-Fat Diets on Body Weight and Cardiovascular Risk Factors: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Bai, H.; Wang, S.; Li, Z.; Wang, Q.; Chen, L. Efficacy of Low Carbohydrate Diet for Type 2 Diabetes Mellitus Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Res. Clin. Pract. 2017, 131, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Korsmo-Haugen, H.-K.; Brurberg, K.G.; Mann, J.; Aas, A.-M. Carbohydrate Quantity in the Dietary Management of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 2019, 21, 15–27. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H.; Lennon, L.; Sattar, N. Impact of Diabetes on Cardiovascular Disease Risk and All-Cause Mortality in Older Men: Influence of Age at Onset, Diabetes Duration, and Established and Novel Risk Factors. Arch. Intern. Med. 2011, 171, 404–410. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jönsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A Consideration of Biomarkers to Be Used for Evaluation of Inflammation in Human Nutritional Studies. Br. J. Nutr. 2013, 109 (Suppl. S1), S1–S34. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Dickinson, S.; Hancock, D.P.; Petocz, P.; Ceriello, A.; Brand-Miller, J. High-Glycemic Index Carbohydrate Increases Nuclear Factor-KappaB Activation in Mononuclear Cells of Young, Lean Healthy Subjects. Am. J. Clin. Nutr. 2008, 87, 1188–1193. [Google Scholar] [CrossRef]
- Qi, L.; Hu, F.B. Dietary Glycemic Load, Whole Grains, and Systemic Inflammation in Diabetes: The Epidemiological Evidence. Curr. Opin. Lipidol. 2007, 18, 3–8. [Google Scholar] [CrossRef]
- King, D.E.; Egan, B.M.; Woolson, R.F.; Mainous, A.G., 3rd; Al-Solaiman, Y.; Jesri, A. Effect of a High-Fiber Diet vs a Fiber-Supplemented Diet on C-Reactive Protein Level. Arch. Intern. Med. 2007, 167, 502–506. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Amjad, A.; Nisar, T.; Javaid, N.; Khan, M.; Munir, A.; Tariq, M.; Tauqeer, R.; e Nayab, D.; Fatima, A. Comparison of Effects of Simple and Complex Carbohydrates on Mental Health, a Systematic. Innov. Med. Health Sci. 2021, 1, 63–68. [Google Scholar]
- Barba, F.; Saraiva, J.M.A.; Cravotto, G.; Lorenzo, J.M. Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Woodhead Publishing: Swaston, UK, 2019. [Google Scholar]
- Howlett, J.; Ashwell, M. Glycemic Response and Health: Summary of a Workshop. Am. J. Clin. Nutr. 2008, 87, 212S–216S. [Google Scholar] [CrossRef]
- Stephen, A.; Alles, M.; de Graaf, C.; Fleith, M.; Hadjilucas, E.; Isaacs, E.; Maffeis, C.; Zeinstra, G.; Matthys, C.; Gil, A. The Role and Requirements of Digestible Dietary Carbohydrates in Infants and Toddlers. Eur. J. Clin. Nutr. 2012, 66, 765–779. [Google Scholar] [CrossRef]
- Chugani, H.T. A Critical Period of Brain Development: Studies of Cerebral Glucose Utilization with PET. Prev. Med. 1998, 27, 184–188. [Google Scholar] [CrossRef]
- Hassevoort, K.M.; Lin, A.S.; Khan, N.A.; Hillman, C.H.; Cohen, N.J. Added Sugar and Dietary Fiber Consumption Are Associated with Creativity in Preadolescent Children. Nutr. Neurosci. 2020, 23, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.E.; Hale, L.; Garcia, L.; Malaspina, D.; Opler, M.G.; Payne, M.E.; Rossom, R.C.; Lane, D. High Glycemic Index Diet as a Risk Factor for Depression: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2015, 102, 454–463. [Google Scholar] [CrossRef]
- Aparicio, A.; Robles, F.; López-Sobaler, A.M.; Ortega, R.M. Dietary Glycaemic Load and Odds of Depression in a Group of Institutionalized Elderly People without Antidepressant Treatment. Eur. J. Nutr. 2013, 52, 1059–1066. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Azadbakht, L.; Keshteli, A.H.; Feinle-Bisset, C.; Daghaghzadeh, H.; Afshar, H.; Feizi, A.; Esmaillzadeh, A.; Adibi, P. Glycemic Index, Glycemic Load, and Common Psychological Disorders. Am. J. Clin. Nutr. 2016, 103, 201–209. [Google Scholar] [CrossRef]
- Breymeyer, K.L.; Lampe, J.W.; McGregor, B.A.; Neuhouser, M.L. Subjective Mood and Energy Levels of Healthy Weight and Overweight/Obese Healthy Adults on High-and Low-Glycemic Load Experimental Diets. Appetite 2016, 107, 253–259. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and Behavioral Health Disorders: Depression and Anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Salari-Moghaddam, A.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Glycemic Index, Glycemic Load, and Depression: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2019, 73, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The Gut Microbiota in Anxiety and Depression—A Systematic Review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The Type and Quantity of Dietary Fat and Carbohydrate Alter Faecal Microbiome and Short-Chain Fatty Acid Excretion in a Metabolic Syndrome “at-Risk” Population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Zheng, M.; Hao, S.; Lum, J.S.; Chen, X.; Huang, X.-F.; Yu, Y.; Zheng, K. Supplement of Microbiota-Accessible Carbohydrates Prevents Neuroinflammation and Cognitive Decline by Improving the Gut Microbiota-Brain Axis in Diet-Induced Obese Mice. J. Neuroinflamm. 2020, 17, 77. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother. Psychosom. 2017, 86, 31–46. [Google Scholar] [CrossRef]
- Lee, J.E.; Walton, D.; O’Connor, C.P.; Wammes, M.; Burton, J.P.; Osuch, E.A. Drugs, Guts, Brains, but Not Rock and Roll: The Need to Consider the Role of Gut Microbiota in Contemporary Mental Health and Wellness of Emerging Adults. Int. J. Mol. Sci. 2022, 23, 6643. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Kenna, H.A.; Nguyen, H.T.; Law, C.W.Y.; Sultan, F.; Woldeyohannes, H.O.; Adams, A.K.; Cheng, J.S.H.; Lourenco, M.; Kennedy, S.H.; et al. Brain Volume Abnormalities and Neurocognitive Deficits in Diabetes Mellitus: Points of Pathophysiological Commonality with Mood Disorders? Adv. Ther. 2010, 27, 63–80. [Google Scholar] [CrossRef]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 564413. [Google Scholar] [CrossRef]
- Leigh, S.-J.; Morris, M.J. The Role of Reward Circuitry and Food Addiction in the Obesity Epidemic: An Update. Biol. Psychol. 2018, 131, 31–42. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Wurtman, J.J. Brain Serotonin, Carbohydrate-Craving, Obesity and Depression. In Recent Advances in Tryptophan Research; Springer: Berlin/Heidelberg, Germany, 1996; Volume 38, pp. 35–42. [Google Scholar]
- Hryhorczuk, C.; Sharma, S.; Fulton, S.E. Metabolic Disturbances Connecting Obesity and Depression. Front. Neurosci. 2013, 7, 177. [Google Scholar] [CrossRef]
- Oddy, W.H.; Robinson, M.; Ambrosini, G.L.; O’Sullivan, T.A.; de Klerk, N.H.; Beilin, L.J.; Silburn, S.R.; Zubrick, S.R.; Stanley, F.J. The Association between Dietary Patterns and Mental Health in Early Adolescence. Prev. Med. 2009, 49, 39–44. [Google Scholar] [CrossRef]
- Kim, T.-H.; Choi, J.; Lee, H.-H.; Park, Y. Associations between Dietary Pattern and Depression in Korean Adolescent Girls. J. Pediatr. Adolesc. Gynecol. 2015, 28, 533–537. [Google Scholar] [CrossRef]
- Tomé, D.; Benoit, S.; Azzout-Marniche, D. Protein Metabolism and Related Body Function: Mechanistic Approaches and Health Consequences. Proc. Nutr. Soc. 2021, 80, 243–251. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Holt, S.H.A.; Pawlak, D.B.; McMillan, J. Glycemic Index and Obesity. Am. J. Clin. Nutr. 2002, 76, 281S–285S. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef]
- Järbrink-Sehgal, E.; Andreasson, A. The Gut Microbiota and Mental Health in Adults. Curr. Opin. Neurobiol. 2020, 62, 102–114. [Google Scholar] [CrossRef]
- Halverson, T.; Alagiakrishnan, K. Gut Microbes in Neurocognitive and Mental Health Disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg Effect: Essential Part of Metabolic Reprogramming and Central Contributor to Cancer Progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Szypowska, A.; Regulska-Ilow, B. Significance of Low-Carbohydrate Diets and Fasting in Patients with Cancer. Rocz. Panstw. Zakl. Hig. 2019, 70, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef]
- Ghergurovich, J.M.; Lang, J.D.; Levin, M.K.; Briones, N.; Facista, S.J.; Mueller, C.; Cowan, A.J.; McBride, M.J.; Rodriguez, E.S.R.; Killian, A.; et al. Local Production of Lactate, Ribose Phosphate, and Amino Acids within Human Triple-Negative Breast Cancer. Med 2021, 2, 736–754. [Google Scholar] [CrossRef] [PubMed]
- Krall, A.S.; Mullen, P.J.; Surjono, F.; Momcilovic, M.; Schmid, E.W.; Halbrook, C.J.; Thambundit, A.; Mittelman, S.D.; Lyssiotis, C.A.; Shackelford, D.B.; et al. Asparagine Couples Mitochondrial Respiration to ATF4 Activity and Tumor Growth. Cell Metab. 2021, 33, 1013–1026.e6. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Cardona, L.R.; Kong, H.; Vasan, K.; McElroy, G.S.; Werner, M.; Kihshen, H.; Reczek, C.R.; Weinberg, S.E.; Gao, P.; et al. Mitochondrial Ubiquinol Oxidation Is Necessary for Tumour Growth. Nature 2020, 585, 288–292. [Google Scholar] [CrossRef]
- Sellers, K.; Fox, M.P.; Bousamra, M., 2nd; Slone, S.P.; Higashi, R.M.; Miller, D.M.; Wang, Y.; Yan, J.; Yuneva, M.O.; Deshpande, R.; et al. Pyruvate Carboxylase Is Critical for Non-Small-Cell Lung Cancer Proliferation. J. Clin. Investig. 2015, 125, 687–698. [Google Scholar] [CrossRef]
- Christen, S.; Lorendeau, D.; Schmieder, R.; Broekaert, D.; Metzger, K.; Veys, K.; Elia, I.; Buescher, J.M.; Orth, M.F.; Davidson, S.M.; et al. Breast Cancer-Derived Lung Metastases Show Increased Pyruvate Carboxylase-Dependent Anaplerosis. Cell Rep. 2016, 17, 837–848. [Google Scholar] [CrossRef]
- Davidson, S.M.; Papagiannakopoulos, T.; Olenchock, B.A.; Heyman, J.E.; Keibler, M.A.; Luengo, A.; Bauer, M.R.; Jha, A.K.; O’Brien, J.P.; Pierce, K.A.; et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab. 2016, 23, 517–528. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We Need to Talk about the Warburg Effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Czekajło, A.; Różańska, D.; Mandecka, A.; Konikowska, K.; Madalińska, M.; Szuba, A.; Regulska-Ilow, B. Glycemic Load and Carbohydrates Content in the Diets of Cancer Patients. Rocz. Panstw. Zakl. Hig. 2017, 68, 261–268. [Google Scholar]
- Dang, C.V. Rethinking the Warburg Effect with Myc Micromanaging Glutamine Metabolism. Cancer Res. 2010, 70, 859–862. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The Diverse Functions of Glutamine in Metabolism, Cell Biology and Cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- De Groot, S.; Vreeswijk, M.P.G.; Welters, M.J.P.; Gravesteijn, G.; Boei, J.J.W.A.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.M.; Nortier, J.W.R.; et al. The Effects of Short-Term Fasting on Tolerance to (Neo) Adjuvant Chemotherapy in HER2-Negative Breast Cancer Patients: A Randomized Pilot Study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association between Hyperglycemia and Survival in Patients with Newly Diagnosed Glioblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1082–1086. [Google Scholar] [CrossRef]
- Supabphol, S.; Seubwai, W.; Wongkham, S.; Saengboonmee, C. High Glucose: An Emerging Association between Diabetes Mellitus and Cancer Progression. J. Mol. Med. 2021, 99, 1175–1193. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in Cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Sun, P.; Wang, H.; He, Z.; Chen, X.; Wu, Q.; Chen, W.; Sun, Z.; Weng, M.; Zhu, M.; Ma, D.; et al. Fasting Inhibits Colorectal Cancer Growth by Reducing M2 Polarization of Tumor-Associated Macrophages. Oncotarget 2017, 8, 74649–74660. [Google Scholar] [CrossRef]
- Wu, W.K.K.; Coffelt, S.B.; Cho, C.H.; Wang, X.J.; Lee, C.W.; Chan, F.K.L.; Yu, J.; Sung, J.J.Y. The Autophagic Paradox in Cancer Therapy. Oncogene 2012, 31, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Hursting, S.D.; Smith, S.M.; Lashinger, L.M.; Harvey, A.E.; Perkins, S.N. Calories and Carcinogenesis: Lessons Learned from 30 Years of Calorie Restriction Research. Carcinogenesis 2010, 31, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet That Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of Caloric Restriction on Health and Survival in Rhesus Monkeys from the NIA Study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Bergamini, E.; Brunk, U.T.; Dröge, W.; Ffrench, M.; Terman, A. Autophagy and Aging: The Importance of Maintaining “Clean” Cells. Autophagy 2005, 1, 131–140. [Google Scholar] [CrossRef]
- Rieger, J.; Bähr, O.; Maurer, G.D.; Hattingen, E.; Franz, K.; Brucker, D.; Walenta, S.; Kämmerer, U.; Coy, J.F.; Weller, M.; et al. ERGO: A Pilot Study of Ketogenic Diet in Recurrent Glioblastoma. Int. J. Oncol. 2014, 44, 1843–1852. [Google Scholar] [CrossRef]
- Fung, T.T.; Hu, F.B.; Hankinson, S.E.; Willett, W.C.; Holmes, M.D. Low-Carbohydrate Diets, Dietary Approaches to Stop Hypertension-Style Diets, and the Risk of Postmenopausal Breast Cancer. Am. J. Epidemiol. 2011, 174, 652–660. [Google Scholar] [CrossRef]
- Tóth, C.; Clemens, Z. Treatment of Rectal Cancer with the Paleolithic Ketogenic Diet: A 24-Months Follow-Up. Am. J. Med. Case Rep. 2017, 5, 205–216. [Google Scholar] [CrossRef]
- Silvera, S.A.N.; Jain, M.; Howe, G.R.; Miller, A.B.; Rohan, T.E. Dietary Carbohydrates and Breast Cancer Risk: A Prospective Study of the Roles of Overall Glycemic Index and Glycemic Load. Int. J. Cancer 2005, 114, 653–658. [Google Scholar] [CrossRef]
- Grasgruber, P.; Hrazdira, E.; Sebera, M.; Kalina, T. Cancer Incidence in Europe: An Ecological Analysis of Nutritional and Other Environmental Factors. Front. Oncol. 2018, 8, 151. [Google Scholar] [CrossRef]
- Higginbotham, S.; Zhang, Z.-F.; Lee, I.-M.; Cook, N.R.; Giovannucci, E.; Buring, J.E.; Liu, S. Dietary Glycemic Load and Risk of Colorectal Cancer in the Women’s Health Study. J. Natl. Cancer Inst. 2004, 96, 229–233. [Google Scholar] [CrossRef]
- Kaaks, R.; Lukanova, A. Energy Balance and Cancer: The Role of Insulin and Insulin-like Growth Factor-I. Proc. Nutr. Soc. 2001, 60, 91–106. [Google Scholar] [CrossRef]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like Growth Factor (IGF)-I, IGF Binding Protein-3, and Cancer Risk: Systematic Review and Meta-Regression Analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Murakami, K.; McCaffrey, T.A.; Livingstone, M.B.E. Associations of Dietary Glycaemic Index and Glycaemic Load with Food and Nutrient Intake and General and Central Obesity in British Adults. Br. J. Nutr. 2013, 110, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.A.; Covas, M.I.; Marrugat, J.; Vila, J.; Schröder, H. Glycemic Load, Glycemic Index, and Body Mass Index in Spanish Adults. Am. J. Clin. Nutr. 2009, 89, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, M.F.; Van Der Linden, B.W.A.; Breedveld-Peters, J.J.L.; Koole, J.L.; Van Roekel, E.H.; Breukink, S.O.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Associations of the dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations with patient-reported outcomes in colorectal cancer survivors 2–10 years post-diagnosis: A cross-sectional analysis. Br. J. Nutr. 2021, 125, 1188–1200. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Ma, S.; Zheng, R.; Zhao, P.; Zhang, L.; Liu, Y.; Yu, Q.; Deng, Q.; Zhang, K. Dietary Fibre Intake and Risk of Breast Cancer: A Systematic Review and Meta-Analysis of Epidemiological Studies. Oncotarget 2016, 7, 80980–80989. [Google Scholar] [CrossRef] [PubMed]
- Maino Vieytes, C.A.; Taha, H.M.; Burton-Obanla, A.A.; Douglas, K.G.; Arthur, A.E. Carbohydrate Nutrition and the Risk of Cancer. Curr. Nutr. Rep. 2019, 8, 230–239. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 39 (Suppl. S1), S1–S266. [Google Scholar]
- Tervaert, T.W.C.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic Classification of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef]
- Anders, H.-J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in Diabetes: Diabetic Kidney Disease versus Nondiabetic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Nam, K.H.; An, S.Y.; Joo, Y.S.; Lee, S.; Yun, H.-R.; Jhee, J.H.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Carbohydrate-Rich Diet Is Associated with Increased Risk of Incident Chronic Kidney Disease in Non-Diabetic Subjects. J. Clin. Med. 2019, 8, 793. [Google Scholar] [CrossRef]
- Farhadnejad, H.; Asghari, G.; Emamat, H.; Mirmiran, P.; Azizi, F. Low-Carbohydrate High-Protein Diet Is Associated With Increased Risk of Incident Chronic Kidney Diseases among Tehranian Adults. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2019, 29, 343–349. [Google Scholar] [CrossRef]
- Asghari, G.; Momenan, M.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. Dietary Pattern and Incidence of Chronic Kidney Disease among Adults: A Population-Based Study. Nutr. Metab. 2018, 15, 88. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Asghari, G.; Mirmiran, P.; Hosseini, F.-S.; Azizi, F. Associations of Dietary Macronutrients with Glomerular Filtration Rate and Kidney Dysfunction: Tehran Lipid and Glucose Study. J. Nephrol. 2015, 28, 173–180. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Kwon, S.H.; Jeon, J.S.; Noh, H.; Han, D.C.; Kim, H. Relationship between Carbohydrate-to-Fat Intake Ratio and the Development of Chronic Kidney Disease: A Community-Based Prospective Cohort Study. Clin. Nutr. 2021, 40, 5346–5354. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Meuwese, M.C.; Vink, H.; Hoekstra, J.B.L.; Kastelein, J.J.P.; Stroes, E.S.G. The Endothelial Glycocalyx: A Potential Barrier between Health and Vascular Disease. Curr. Opin. Lipidol. 2005, 16, 507–511. [Google Scholar] [CrossRef]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients With Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary Protein Intake and Chronic Kidney Disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef]
- Mirmiran, P.; Yuzbashian, E.; Asghari, G.; Sarverzadeh, S.; Azizi, F. Dietary Fibre Intake in Relation to the Risk of Incident Chronic Kidney Disease. Br. J. Nutr. 2018, 119, 479–485. [Google Scholar] [CrossRef]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-Based Diets to Manage the Risks and Complications of Chronic Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of Inflammation, Oxidative Stress, and Vascular Dysfunction in Hypertension. Biomed. Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef]
- Judd, E.; Calhoun, D.A. Management of Hypertension in CKD: Beyond the Guidelines. Adv. Chronic Kidney Dis. 2015, 22, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta Med. Indones. 2017, 49, 158–165. [Google Scholar] [PubMed]
- Chrissobolis, S.; Miller, A.A.; Drummond, G.R.; Kemp-Harper, B.K.; Sobey, C.G. Oxidative Stress and Endothelial Dysfunction in Cerebrovascular Disease. Front. Biosci. 2011, 16, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Mele, M.C.; Merendino, N.; Cintoni, M.; Anselmi, G.; Caporossi, A.; Gasbarrini, A.; Minnella, A.M. The Role of Diet, Micronutrients and the Gut Microbiota in Age-Related Macular Degeneration: New Perspectives from the Gut–Retina Axis. Nutrients 2018, 10, 1677. [Google Scholar] [CrossRef]
- Rosas-Villegas, A.; Sánchez-Tapia, M.; Avila-Nava, A.; Ramírez, V.; Tovar, A.R.; Torres, N. Differential Effect of Sucrose and Fructose in Combination with a High Fat Diet on Intestinal Microbiota and Kidney Oxidative Stress. Nutrients 2017, 9, 393. [Google Scholar] [CrossRef]
- Bourdin, A.; Gras, D.; Vachier, I.; Chanez, P. Upper Airway x 1: Allergic Rhinitis and Asthma: United Disease through Epithelial Cells. Thorax 2009, 64, 999–1004. [Google Scholar] [CrossRef]
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The Role of Nutrition in Asthma Prevention and Treatment. Nutr. Rev. 2020, 78, 928–938. [Google Scholar] [CrossRef]
- Holsey, C.N.; Collins, P.; Zahran, H. Disparities in Asthma Care, Management, and Education among Children with Asthma. Clin. Pulm. Med. 2013, 20, 172–177. [Google Scholar] [CrossRef]
- Calatayud-Sáez, F.M.; Calatayud Moscoso Del Prado, B.; Gallego Fernández-Pacheco, J.G.; González-Martín, C.; Alguacil Merino, L.F. Mediterranean Diet and Childhood Asthma. Allergol. Immunopathol. 2016, 44, 99–105. [Google Scholar] [CrossRef]
- Calatayud, F.M.; Calatayud, B.; Gallego, J.G.; González-Martín, C.; Alguacil, L.F. Effects of Mediterranean Diet in Patients with Recurring Colds and Frequent Complications. Allergol. Immunopathol. 2017, 45, 417–424. [Google Scholar] [CrossRef]
- Brigham, E.P.; Kolahdooz, F.; Hansel, N.; Breysse, P.N.; Davis, M.; Sharma, S.; Matsui, E.C.; Diette, G.; McCormack, M.C. Association between Western Diet Pattern and Adult Asthma: A Focused Review. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Barros, R.; Moreira, A.; Padrão, P.; Teixeira, V.H.; Carvalho, P.; Delgado, L.; Lopes, C.; Severo, M.; Moreira, P. Dietary Patterns and Asthma Prevalence, Incidence and Control. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 1673–1680. [Google Scholar] [CrossRef]
- Lee, S.-C.; Yang, Y.-H.; Chuang, S.-Y.; Liu, S.-C.; Yang, H.-C.; Pan, W.-H. Risk of Asthma Associated with Energy-Dense but Nutrient-Poor Dietary Pattern in Taiwanese Children. Asia Pac. J. Clin. Nutr. 2012, 21, 73–81. [Google Scholar]
- Wang, C.S.; Wang, J.; Zhang, X.; Zhang, L.; Zhang, H.P.; Wang, L.; Wood, L.G.; Wang, G. Is the Consumption of Fast Foods Associated with Asthma or Other Allergic Diseases? Respirology 2018, 23, 901–913. [Google Scholar] [CrossRef]
- Hong, S.-J.; Lee, M.-S.; Lee, S.-Y.; Ahn, K.-M.; Oh, J.-W.; Kim, K.-E.; Lee, J.-S.; Lee, H.-B. High Body Mass Index and Dietary Pattern Are Associated with Childhood Asthma. Pediatr. Pulmonol. 2006, 41, 1118–1124. [Google Scholar] [CrossRef]
- Farshchi, M.K.; Azad, F.J.; Salari, R.; Mirsadraee, M.; Anushiravani, M. A Viewpoint on the Leaky Gut Syndrome to Treat Allergic Asthma: A Novel Opinion. J. Evid. Based. Complement. Altern. Med. 2017, 22, 378–380. [Google Scholar] [CrossRef]
- Binienda, A.; Twardowska, A.; Makaro, A.; Salaga, M. Dietary Carbohydrates and Lipids in the Pathogenesis of Leaky Gut Syndrome: An Overview. Int. J. Mol. Sci. 2020, 21, 8368. [Google Scholar] [CrossRef]
- Frontela-Saseta, C.; González-Bermúdez, C.A.; García-Marcos, L. Diet: A Specific Part of the Western Lifestyle Pack in the Asthma Epidemic. J. Clin. Med. 2020, 9, 2063. [Google Scholar] [CrossRef]
- Pereira, M.T.; Malik, M.; Nostro, J.A.; Mahler, G.J.; Musselman, L.P. Effect of Dietary Additives on Intestinal Permeability in Both Drosophila and a Human Cell Co-Culture. Dis. Model. Mech. 2018, 11, dmm034520. [Google Scholar] [CrossRef] [PubMed]
- DeChristopher, L.R.; Tucker, K.L. Excess Free Fructose, High-Fructose Corn Syrup and Adult Asthma: The Framingham Offspring Cohort. Br. J. Nutr. 2018, 119, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Sim, S.; Park, B.; Kim, J.-H.; Choi, H.G. High-Fat and Low-Carbohydrate Diets Are Associated with Allergic Rhinitis But Not Asthma or Atopic Dermatitis in Children. PLoS ONE 2016, 11, e0150202. [Google Scholar] [CrossRef] [PubMed]
- Tamay, Z.; Akcay, A.; Ergin, A.; Güler, N. Dietary Habits and Prevalence of Allergic Rhinitis in 6 to 7-Year-Old Schoolchildren in Turkey. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2014, 63, 553–562. [Google Scholar] [CrossRef]
- Koumpagioti, D.; Boutopoulou, B.; Moriki, D.; Priftis, K.N.; Douros, K. Does Adherence to the Mediterranean Diet Have a Protective Effect against Asthma and Allergies in Children? A Systematic Review. Nutrients 2022, 14, 1618. [Google Scholar] [CrossRef]
- Saadeh, D.; Salameh, P.; Caillaud, D.; Charpin, D.; De Blay, F.; Kopferschmitt, C.; Lavaud, F.; Annesi-Maesano, I.; Baldi, I.; Raherison, C. Prevalence and Association of Asthma and Allergic Sensitization with Dietary Factors in Schoolchildren: Data from the French Six Cities Study. BMC Public Health 2015, 15, 993. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.B.; Salvi, S.; Chhatwal, J.; Jain, K.C.; Kumar, L.; Joshi, M.K.; Pandramajal, S.B.; Awasthi, S.; Bhave, S.; et al. Allergic Rhinitis, Rhinoconjunctivitis, and Eczema: Prevalence and Associated Factors in Children. Clin. Respir. J. 2018, 12, 547–556. [Google Scholar] [CrossRef]
- Arnold, M.J.; Harding, M.C.; Conley, A.T. Dietary Guidelines for Americans 2020-2025: Recommendations from the US Departments of Agriculture and Health and Human Services. Am. Fam. Physician 2021, 104, 533–536. [Google Scholar]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Hauner, H.; Bechthold, A.; Boeing, H.; Brönstrup, A.; Buyken, A.; Leschik-Bonnet, E.; Linseisen, J.; Schulze, M.; Strohm, D.; Wolfram, G. Evidence-Based Guideline of the German Nutrition Society: Carbohydrate Intake and Prevention of Nutrition-Related Diseases. Ann. Nutr. Metab. 2012, 60 (Suppl. S1), 1–58. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Aranceta, J. Nutritional Objectives for the Spanish Population. Consensus from the Spanish Society of Community Nutrition. Public Health Nutr. 2001, 4, 1409–1413. [Google Scholar] [CrossRef]
- Lawrence, A.S. The Australian Dietary Guidelines Review: Time to Plan for Wider Dissemination via General Practitioners. Aust. J. Gen. Pract. 2021, 50, 252–253. [Google Scholar] [CrossRef]
- Van den Brandt, P.A. Dietary Reference Intakes: Energy, Proteins, Fats and Digestible Carbohydrates; Health Council of the Netherlands: The Hague, The Netherlands, 2001. [Google Scholar]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- European Food Safety Authority Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol; EFSA: Parma, Italy, 2010. [Google Scholar]
- Ministerråd, N. Nordic Nutrition Recommendations 2012. Part 1: Summary, Principles and Use; Nordic Council of Ministers: Copenhague, Danemark, 2013. [Google Scholar]
- Christensen, J.J.; Arnesen, E.K.; Andersen, R.; Eneroth, H.; Erkkola, M.; Høyer, A.; Lemming, E.W.; Meltzer, H.M.; Halldórsson, Þ.I.; Þórsdóttir, I. The Nordic Nutrition Recommendations 2022—Principles and Methodologies. Food Nutr. Res. 2020, 64, 70. [Google Scholar] [CrossRef]
- Buyken, A.E.; Mela, D.J.; Dussort, P.; Johnson, I.T.; Macdonald, I.A.; Stowell, J.D.; Brouns, F.J.P.H. Dietary Carbohydrates: A Review of International Recommendations and the Methods Used to Derive Them. Eur. J. Clin. Nutr. 2018, 72, 1625–1643. [Google Scholar] [CrossRef]
- Food Safety Authority of Ireland. Scientific Recommendations for Healthy Eating Guidelines in Ireland; Food Safety Authority of Ireland: Dublin, Ireland, 2011. [Google Scholar]
- Scarborough, P.; Kaur, A.; Cobiac, L.; Owens, P.; Parlesak, A.; Sweeney, K.; Rayner, M. Eatwell Guide: Modelling the Dietary and Cost Implications of Incorporating New Sugar and Fibre Guidelines. BMJ Open 2016, 6, e013182. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2015. [Google Scholar]
- McGuire, S. US Department of Agriculture and US Department of Health and Human Services, Dietary Guidelines for Americans, 2010. Washington, DC: US Government Printing Office, January 2011. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. https://doi.org/10.3390/nu14183809
Clemente-Suárez VJ, Mielgo-Ayuso J, Martín-Rodríguez A, Ramos-Campo DJ, Redondo-Flórez L, Tornero-Aguilera JF. The Burden of Carbohydrates in Health and Disease. Nutrients. 2022; 14(18):3809. https://doi.org/10.3390/nu14183809
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Juan Mielgo-Ayuso, Alexandra Martín-Rodríguez, Domingo Jesús Ramos-Campo, Laura Redondo-Flórez, and Jose Francisco Tornero-Aguilera. 2022. "The Burden of Carbohydrates in Health and Disease" Nutrients 14, no. 18: 3809. https://doi.org/10.3390/nu14183809
APA StyleClemente-Suárez, V. J., Mielgo-Ayuso, J., Martín-Rodríguez, A., Ramos-Campo, D. J., Redondo-Flórez, L., & Tornero-Aguilera, J. F. (2022). The Burden of Carbohydrates in Health and Disease. Nutrients, 14(18), 3809. https://doi.org/10.3390/nu14183809










