Effect of Omega-3 Fatty Acids on Telomeres—Are They the Elixir of Youth?
Abstract
:1. Telomeres
2. Omega-3 Fatty Acids
3. Omega-3 Fatty Acids and Telomeres
3.1. Human Studies
3.2. Rodent Model Studies
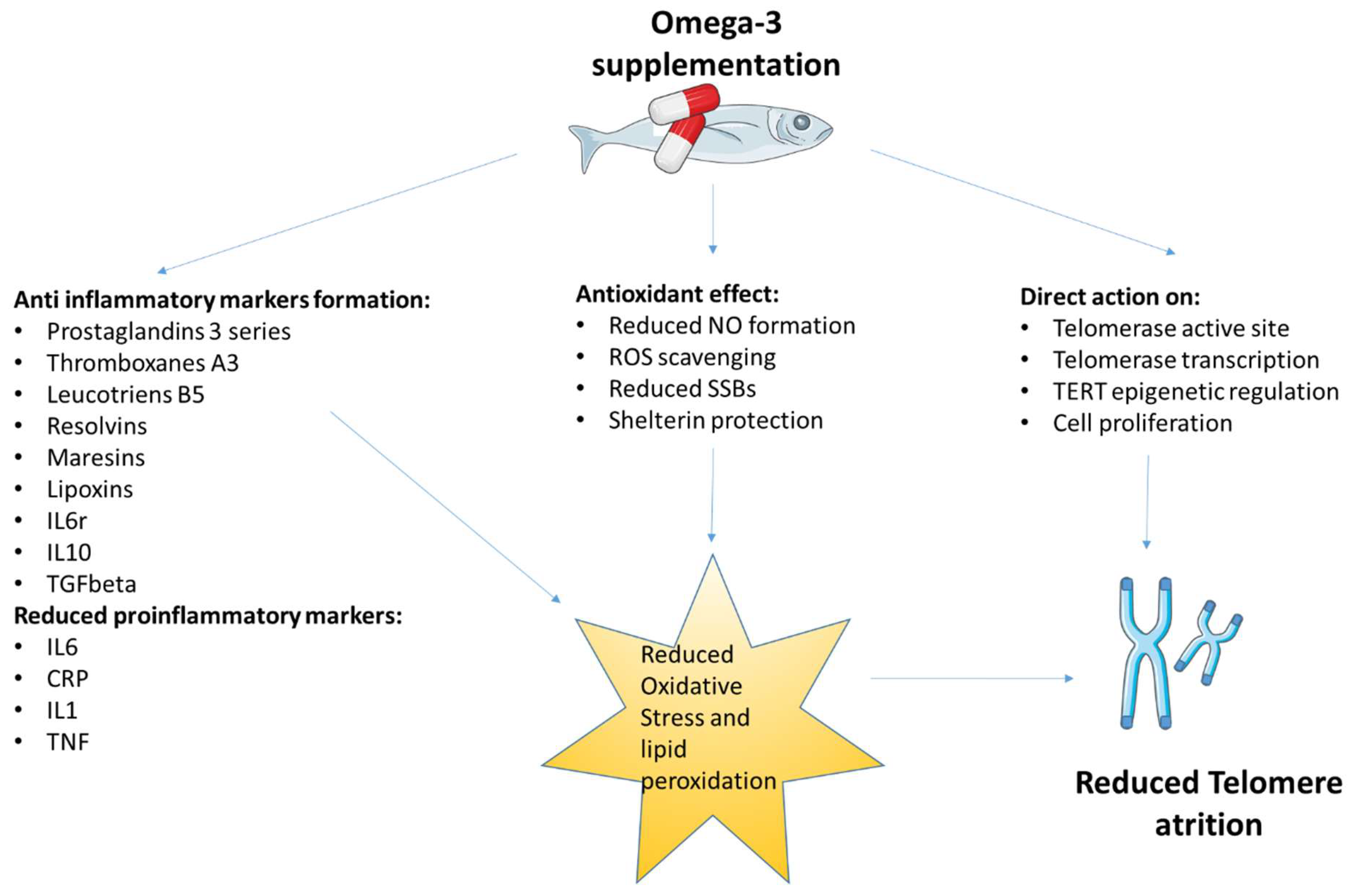
4. The Role of Omega-3 Fatty Acids in the Modulation of Oxidative Stress and Inflammation: Involvement in the Telomere Theory of Aging
Direct Effect of Omega-3 Fatty Acids on Telomere Length
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McClintock, B. The stability of broken ends of chromosomes in zea mays. Genetics 1941, 26, 234–282. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.J. The remaking of chromosomes. Collect. Net. 1938, 13, 181–198. [Google Scholar]
- Wysoczańska, B. Maintaining telomere length. Postep. Hig. Med. Dosw. 2013, 67, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.G.; Donato, A.J.; Walker, A.E. Telomere uncapping and vascular aging. Am. J. Physiol. Circ. Physiol. 2018, 315, H1–H5. [Google Scholar] [CrossRef]
- De Lange, T. How Telomeres Solve the End-Protection Problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef]
- Riethman, H. Human Telomere Structure and Biology. Annu. Rev. Genom. Hum. Genet. 2008, 9, 172017. [Google Scholar] [CrossRef]
- Kipling, D.; Cooke, H.J. Hypervariable ultra-long telomeres in mice. Nature 1990, 347, 400–402. [Google Scholar] [CrossRef]
- Mefford, H.C.; Trask, B.J. The complex structure and dynamic evolution of human subtelomeres. Nat. Rev. Genet. 2002, 3, 91. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef]
- Watson, J.D. Origin of Concatemeric T7DNA. Nat. New Biol. 1972, 239, 197–201. [Google Scholar] [CrossRef]
- Ohki, R.; Tsurimoto, T.; Ishikawa, F. In Vitro Reconstitution of the End Replication Problem. Mol. Cell. Biol. 2001, 21, 5753–5766. [Google Scholar] [CrossRef]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Olovnikov, A. A theory of marginotomy: The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef]
- Olovnikov, A.M. Principle of marginotomy in template synthesis of polynucleotides. Dokl. Akad. Nauk. SSSR 1971, 201, 1496–1499. [Google Scholar]
- Arnoult, N.; Karlseder, J. Complex interactions between the DNA-damage response and mammalian telomeres. Nat. Struct. Mol. Biol. 2015, 22, 859–866. [Google Scholar] [CrossRef]
- Cesare, A.; Karlseder, J. A three-state model of telomere control over human proliferative boundaries. Curr. Opin. Cell Biol. 2012, 24, 731–738. [Google Scholar] [CrossRef]
- Roake, C.M.; Artandi, S.E. Control of Cellular Aging, Tissue Function, and Cancer by p53 Downstream of Telomeres. Cold Spring Harb. Perspect. Med. 2017, 7, a026088. [Google Scholar] [CrossRef]
- Harley, C.B. Telomere loss: Mitotic clock or genetic time bomb? Mutat. Res. 1991, 256, 271–282. [Google Scholar] [CrossRef]
- Turner, K.; Vasu, V.; Griffin, D. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Ding, X.; Wang, F.; Geng, X. Telomere and its role in the aging pathways: Telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 2018, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bernades de Jesus, B.; Schneeberger, K.; Vera, E.; Tejera, A.; Harley, C.B.; Blasco, M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 2011, 10, 604–621. [Google Scholar] [CrossRef] [Green Version]
- Derevyanko, A.; Whittemore, K.; Schneider, R.P.; Jiménez, V.; Bosch, F.; Blasco, M.A. Gene therapy with the TRF 1 telomere gene rescues decreased TRF 1 levels with aging and prolongs mouse health span. Aging Cell 2017, 16, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Steenstrup, T.; Kark, J.D.; Verhulst, S.; Thinggaard, M.; Hjelmborg, J.V.B.; Dalgård, C.; Kyvik, K.O.; Christiansen, L.; Mangino, M.; Spector, T.D.; et al. Telomeres and the natural lifespan limit in humans. Aging 2017, 9, 1130–1142. [Google Scholar] [CrossRef]
- Entringer, S.; Epel, E.S.; Kumsta, R.; Lin, J.; Hellhammer, D.H.; Blackburn, E.H.; Wüst, S.; Wadhwa, P.D. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl. Acad. Sci. USA 2011, 108, E513–E518. [Google Scholar] [CrossRef] [PubMed]
- Price, L.H.; Kao, H.-T.; Burgers, D.E.; Carpenter, L.L.; Tyrka, A.R. Telomeres and Early-Life Stress: An Overview. Biol. Psychiatry 2012, 73, 15–23. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E.; Blackburn, E. Telomeres and lifestyle factors: Roles in cellular aging. Mutat. Res. Mol. Mech. Mutagen. 2012, 730, 85–89. [Google Scholar] [CrossRef]
- Ridout, K.K.; Ridout, S.J.; Price, L.H.; Sen, S.; Tyrka, A.R. Depression and telomere length: A meta-analysis. J. Affect. Disord. 2015, 191, 237–247. [Google Scholar] [CrossRef]
- Huzen, J.; Wong, L.S.M.; Van Veldhuisen, D.J.; Samani, N.J.; Zwinderman, A.H.; Codd, V.; Cawthon, R.M.; Benus, G.F.J.D.; Van Der Horst, I.C.C.; Navis, G.; et al. Telomere length loss due to smoking and metabolic traits. J. Intern. Med. 2013, 275, 155–163. [Google Scholar] [CrossRef]
- García-Calzón, S.; Gea, A.; Razquin, C.; Corella, D.; Lamuela-Raventós, R.M.; Martínez, J.A.; A Martínez-González, M.; Zalba, G.; Marti, A. Longitudinal association of telomere length and obesity indices in an intervention study with a Mediterranean diet: The predimed-navarra trial. Int. J. Obes. 2013, 38, 177–182. [Google Scholar] [CrossRef]
- Strandberg, T.; Strandberg, A.Y.; Saijonmaa, O.; Tilvis, R.S.; Pitkälä, K.; Fyhrquist, F. Association between alcohol consumption in healthy midlife and telomere length in older men. The Helsinki Businessmen Study. Eur. J. Epidemiol. 2012, 27, 815–822. [Google Scholar] [CrossRef]
- Ppendergrass, W.R.; Penn, P.E.; Li, J.; Wolf, N.S. Age-Related Telomere Shortening Occurs in Lens Epithelium from Old Rats and is Slowed by Caloric Restriction. Exp. Eye Res. 2001, 73, 221–228. [Google Scholar] [CrossRef]
- García-Calzón, S.; Moleres, A.; Martínez-González, M.A.; Martínez, J.A.; Zalba, G.; Marti, A. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin. Nutr. 2015, 34, 694–699. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary fats and membrane function: Implications for metabolism and disease. Biol. Rev. 2005, 80, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Jones, P.J. Lipids: Absorption and transport. Present Knowl. Nutr. 2001, 10, 118–131. [Google Scholar]
- Ogłuszka, M.; Lipiński, P.; Starzyński, R.R. Interaction between iron and omega-3 fatty acids metabolisms: Where is the cross-link? Crit. Rev. Food Sci. Nutr. 2020, 62, 3002–3022. [Google Scholar] [CrossRef]
- Vessby, B.; Gustafsson, I.-B.; Tengblad, S.; Boberg, M.; Andersson, A. Desaturation and Elongation of Fatty Acids and Insulin Action. Ann. N. Y. Acad. Sci. 2006, 967, 183–195. [Google Scholar] [CrossRef]
- Deckelbaum, R.J.; Torrejon, C. The Omega-3 Fatty Acid Nutritional Landscape: Health Benefits and Sources. J. Nutr. 2012, 142, 587S–591S. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Omega-3 polyunsaturated fatty acids and human health outcomes. BioFactors 2009, 35, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Edidin, M. Polyunsaturated fatty acids, membrane organization, T cells, and antigen presentation. Am. J. Clin. Nutr. 2006, 84, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Deckelbaum, R.J.; Worgall, T.S.; Seo, T. n-3 Fatty acids and gene expression. Am. J. Clin. Nutr. 2006, 83, 1520S–1525S. [Google Scholar] [CrossRef]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez-Lopez, E.; Martínez, J.A. Fatty acids, epigenetic mechanisms and chronic diseases: A systematic review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef]
- Lands, B. Consequences of Essential Fatty Acids. Nutrients 2012, 4, 1338–1357. [Google Scholar] [CrossRef]
- Farzaneh-Far, R. Association of Marine Omega-3 Fatty Acid Levels with Telomeric Aging in Patients with Coronary Heart Disease. JAMA 2010, 303, 250–257. [Google Scholar] [CrossRef]
- Cassidy, A.; De Vivo, I.; Liu, Y.; Han, J.; Prescott, J.; Hunter, D.J.; Rimm, E.B. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 2010, 91, 1273–1280. [Google Scholar] [CrossRef]
- Chang, X.; Dorajoo, R.; Sun, Y.; Wang, L.; Ong, C.N.; Liu, J.; Khor, C.C.; Yuan, J.-M.; Koh, W.P.; Friedlander, Y.; et al. Effect of plasma polyunsaturated fatty acid levels on leukocyte telomere lengths in the Singaporean Chinese population. Nutr. J. 2020, 19, 119. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Shi, Q.; Fan, X.; Qi, K. Association of telomere length and telomerase methylation with n-3 fatty acids in preschool children with obesity. BMC Pediatr. 2021, 21, 24. [Google Scholar] [CrossRef]
- Freitas-Simoes, T.-M.; Cofán, M.; Blasco, M.A.; Soberón, N.; Foronda, M.; Corella, D.; Asensio, E.M.; Serra-Mir, M.; Roth, I.; Calvo, C.; et al. The red blood cell proportion of arachidonic acid relates to shorter leukocyte telomeres in Mediterranean elders: A secondary analysis of a randomized controlled trial. Clin. Nutr. 2018, 38, 958–961. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Epel, E.S.; Belury, M.A.; Andridge, R.; Lin, J.; Glaser, R.; Malarkey, W.B.; Hwang, B.S.; Blackburn, E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav. Immun. 2012, 28, 16–24. [Google Scholar] [CrossRef]
- O’Callaghan, N.; Parletta, N.; Milte, C.M.; Benassi-Evans, B.; Fenech, M.; Howe, P.R. Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with ω-3 fatty acid supplementation: A randomized controlled pilot study. Nutrition 2014, 30, 489–491. [Google Scholar] [CrossRef]
- Barden, A.; O’Callaghan, N.; Burke, V.; Mas, E.; Beilin, L.J.; Fenech, M.; Irish, A.B.; Watts, G.F.; Puddey, I.B.; Huang, R.-C.; et al. n-3 Fatty Acid Supplementation and Leukocyte Telomere Length in Patients with Chronic Kidney Disease. Nutrients 2016, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- See, V.; Mas, E.; Burrows, S.; O’Callaghan, N.; Fenech, M.; Prescott, S.; Beilin, L.; Huang, R.; Mori, T. Prenatal omega-3 fatty acid supplementation does not affect offspring telomere length and F2-isoprostanes at 12 years: A double blind, randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2016, 112, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yeates, A.J.; Thurston, S.W.; Li, H.; Mulhern, M.S.; McSorley, E.M.; Watson, G.E.; Shamlaye, C.F.; Strain, J.J.; Myers, G.J.; Davidson, P.W.; et al. PUFA Status and Methylmercury Exposure Are Not Associated with Leukocyte Telomere Length in Mothers or Their Children in the Seychelles Child Development Study. J. Nutr. 2017, 147, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, Q.; Fan, X.; Chen, H.; Chen, N.; Zhao, Y.; Qi, K. Associations of Maternal Polyunsaturated Fatty Acids with Telomere Length in the Cord Blood and Placenta in Chinese Population. Front. Nutr. 2022, 8, 779306. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Chen, X.; Jiao, J.; Zhang, Y. Polyunsaturated fatty acids ameliorate aging via redox-telomere-antioncogene axis. Oncotarget 2016, 8, 7301–7314. [Google Scholar] [CrossRef]
- Varela-Lopez, A.; Pérez-López, M.P.; Ramirez-Tortosa, C.L.; Battino, M.; Granados-Principal, S.; Ramirez-Tortosa, M.D.C.; Ochoa, J.J.; Vera-Ramirez, L.; Giampieri, F.; Quiles, J.L. Gene pathways associated with mitochondrial function, oxidative stress and telomere length are differentially expressed in the liver of rats fed lifelong on virgin olive, sunflower or fish oils. J. Nutr. Biochem. 2018, 52, 36–44. [Google Scholar] [CrossRef]
- Gao, J.; Xiao, H.; Li, J.; Guo, X.; Cai, W.; Li, D. n-3 Polyunsaturated Fatty Acids Decrease Long-Term Diabetic Risk of Offspring of Gestational Diabetes Rats by Postponing Shortening of Hepatic Telomeres and Modulating Liver Metabolism. Nutrients 2019, 11, 1699. [Google Scholar] [CrossRef]
- Hayflick, L. Mortality and immortality at the cellular level. A review. Biochemistry 1997, 62, 1180–1190. [Google Scholar]
- Von Zglinicki, T. Telomeres and replicative senescence: Is it only length that counts? Cancer Lett. 2001, 168, 111–116. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J.; Andersen, J.K.; Kapahi, P.; Melov, S. Cellular senescence: A link between cancer and age-related degenerative disease? Semin. Cancer Biol. 2011, 21, 354–359. [Google Scholar] [CrossRef]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kronmal, R.A.; Kimura, M.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Hardikar, S.; Aviv, A. Leukocyte Telomere Length and Mortality in the Cardiovascular Health Study. J. Gerontol. Ser. A 2011, 66, 421–429. [Google Scholar] [CrossRef]
- Fragkiadaki, P.; Nikitovic, D.; Kalliantasi, K.; Sarandi, E.; Thanasoula, M.; Stivaktakis, P.D.; Spandidos, D.A.; Theodoros, T.; Tsatsakis, A. Telomere length and telomerase activity in osteoporosis and osteoarthritis (Review). Exp. Ther. Med. 2019, 19, 1626–1632. [Google Scholar] [CrossRef]
- Sadr, M.; Mugahi, S.M.H.N.; Hassanzadeh, G.; Nadji, S.A.; Kiani, A.; Abedini, A.; Javadi, A.; Mohammadi, F.; Masjedi, M.R.; Bahadori, M. Telomere Shortening in Blood Leukocytes of Patients with Chronic Obstructive Pulmonary Disease. Tanaffos 2015, 14, 10–16. [Google Scholar] [PubMed]
- Von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Niveta, J.P.S.; Kumar, M.A.; Parvathi, V.D. Telomere attrition and inflammation: The chicken and the egg story. Egypt. J. Med. Hum. Genet. 2022, 23, 131. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014, 5, 4172. [Google Scholar] [CrossRef]
- Pejenaute, Á.; Cortés, A.; Marqués, J.; Montero, L.; Beloqui, O.; Fortuño, A.; Martí, A.; Orbe, J.; Zalba, G. NADPH Oxidase Overactivity Underlies Telomere Shortening in Human Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 1434. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Reichert, S.; Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.L.; Hsueh, W.-C.; Satterfield, S.; et al. Cumulative Inflammatory Load Is Associated with Short Leukocyte Telomere Length in the Health, Aging and Body Composition Study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef] [PubMed]
- Kruk, P.A.; Rampino, N.J.; Bohr, V.A. DNA damage and repair in telomeres: Relation to aging. Proc. Natl. Acad. Sci. USA 1995, 92, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, A.; Kosiyatrakul, S.T.; Hockemeyer, D.; MacRae, S.L.; Karlseder, J.; Schildkraut, C.L.; de Lange, T. Mammalian Telomeres Resemble Fragile Sites and Require TRF1 for Efficient Replication. Cell 2009, 138, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhang, Z.; Tong, T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int. J. Biochem. Cell Biol. 2005, 37, 1407–1420. [Google Scholar] [CrossRef]
- Brandl, A.; Hartmann, A.; Bechmann, V.; Graf, B.; Nerlich, M.; Angele, P. Oxidative stress induces senescence in chondrocytes. J. Orthop. Res. 2011, 29, 1114–1120. [Google Scholar] [CrossRef]
- Du, G.; Mouithys-Mickalad, A.; Sluse, F.E. Generation of superoxide anion by mitochondria and impairment of their functions during anoxia and reoxygenation in vitro. Free Radic. Biol. Med. 1998, 25, 1066–1074. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S. Mechanism of Telomere Shortening by Oxidative Stress. Ann. N. Y. Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef]
- Chou, T.-C.; Yen, M.-H.; Li, C.-Y.; Ding, Y.-A. Alterations of Nitric Oxide Synthase Expression with Aging and Hypertension in Rats. Hypertension 1998, 31, 643–648. [Google Scholar] [CrossRef]
- Lyons, D.; Roy, S.; Patel, M.; Benjamin, N.; Swift, C.G. Impaired Nitric Oxide-Mediated Vasodilatation and Total Body Nitric Oxide Production in Healthy Old Age. Clin. Sci. 1997, 93, 519–525. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Chang, E.; Glassford, A.J.; Cooke, J.P.; Chiu, C.-P.; Tsao, P.S. eNOS Activity Is Reduced in Senescent Human Endothelial Cells. Circ. Res. 2001, 89, 793–798. [Google Scholar] [CrossRef]
- Xu, D.; Erickson, S.; Szeps, M.; Gruber, A.; Sangfelt, O.; Einhorn, S.; Pisa, P.; Grandér, D. Interferon α down-regulates telomerase reverse transcriptase and telomerase activity in human malignant and nonmalignant hematopoietic cells. Blood 2000, 96, 4313–4318. [Google Scholar] [CrossRef] [PubMed]
- Gizard, F.; Heywood, E.B.; Findeisen, H.M.; Zhao, Y.; Jones, K.L.; Cudejko, C.; Post, G.R.; Staels, B.; Bruemmer, D. Telomerase Activation in Atherosclerosis and Induction of Telomerase Reverse Transcriptase Expression by Inflammatory Stimuli in Macrophages. Arter. Thromb. Vasc. Biol. 2011, 31, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.S.; Wu, Y.; Okobi, Q.; Adekoya, D.; Atefi, M.; Clarke, O.; Dutta, P.; Vadgama, J.V. Proinflammatory Cytokines IL-6 and TNF-α Increased Telomerase Activity through NF-κB/STAT1/STAT3 Activation, and Withaferin A Inhibited the Signaling in Colorectal Cancer Cells. Mediat. Inflamm. 2017, 2017, 5958429. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Hubbard, A.K.; Giardina, C. NF-κB regulates transcription of the mouse telomerase catalytic subunit. J. Biol. Chem. 2000, 275, 36671–36675. [Google Scholar] [CrossRef]
- Nanni, S.; Narducci, M.; Della Pietra, L.; Moretti, F.; Grasselli, A.; De Carli, P.; Sacchi, A.; Pontecorvi, A.; Farsetti, A. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J. Clin. Investig. 2002, 110, 219–227. [Google Scholar] [CrossRef]
- Bayne, S.; Liu, J.-P. Hormones and growth factors regulate telomerase activity in ageing and cancer. Mol. Cell. Endocrinol. 2005, 240, 11–22. [Google Scholar] [CrossRef]
- Holub, A.; Mousa, S.; Abdolahi, A.; Godugu, K.; Tu, X.M.; Brenna, J.T.; Block, R.C. The effects of aspirin and N-3 fatty acids on telomerase activity in adults with diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1795–1799. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Mori, T.A.; Dunstan, D.W.; Burke, V.; Croft, K.D.; Rivera, J.H.; Beilin, L.J.; Puddey, I.B. Effect of dietary fish and exercise training on urinary F2-isoprostane excretion in non-insulin-dependent diabetic patients. Metabolism 1999, 48, 1402–1408. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Rousinou, G.; Toutouza, M.; Stefanadis, C. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin. Chim. Acta 2010, 411, 584–591. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Hwang, B.S.; Glaser, R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav. Immun. 2012, 26, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.X. Differential effects of omega-6 and omega-3 fatty acids on telomere length. Am. J. Clin. Nutr. 2010, 92, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shi, L.; Prescott, J.; Chiuve, S.; Hu, F.B.; De Vivo, I.; Stampfer, M.J.; Franks, P.; Manson, J.E.; Rexrode, K. Healthy Lifestyle and Leukocyte Telomere Length in U.S. Women. PLoS ONE 2012, 7, e38374. [Google Scholar] [CrossRef]
- Freitas-Simoes, T.-M.; Ros, E.; Sala-Vila, A. Nutrients, foods, dietary patterns and telomere length: Update of epidemiological studies and randomized trials. Metabolism 2015, 65, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; You, N.-C.Y.; Song, Y.; Kang, M.K.; Hou, L.; Wallace, R.; Eaton, C.B.; Tinker, L.F.; Liu, S. Intake of small-to-medium-chain saturated fatty acids is associated with peripheral leukocyte telomere length in postmenopausal women. J. Nutr. 2013, 143, 907–914. [Google Scholar] [CrossRef]
- Tiainen, A.-M.; Männistö, S.; Blomstedt, P.A.; Moltchanova, E.; Perälä, M.-M.; Kaartinen, N.E.; Kajantie, E.; Kananen, L.; Hovatta, I.; Eriksson, J. Leukocyte telomere length and its relation to food and nutrient intake in an elderly population. Eur. J. Clin. Nutr. 2012, 66, 1290–1294. [Google Scholar] [CrossRef]
- Paul, L. Diet, nutrition and telomere length. J. Nutr. Biochem. 2011, 22, 895–901. [Google Scholar] [CrossRef]
- Marcon, F.; Siniscalchi, E.; Crebelli, R.; Saieva, C.; Sera, F.; Fortini, P.; Simonelli, V.; Palli, D. Diet-related telomere shortening and chromosome stability. Mutagenesis 2011, 27, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Schwarz, Y.; Wang, C.; Breymeyer, K.; Coronado, G.; Wang, C.-Y.; Noar, K.; Song, X.; Lampe, J.W. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J. Nutr. 2011, 142, 369–374. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.; Hu, F.B.; De Vivo, I. Mediterranean diet and telomere length in Nurses’ Health Study: Population based cohort study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef]
- Boccardi, V.; Esposito, A.; Rizzo, M.R.; Marfella, R.; Barbieri, M.; Paolisso, G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS ONE 2013, 8, e62781. [Google Scholar] [CrossRef]
- Roberts, L.; Morrow, J.D. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000, 28, 505–513. [Google Scholar] [CrossRef]
- Mori, T.A.; Woodman, R.; Burke, V.; Puddey, I.B.; Croft, K.; Beilin, L.J. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic. Biol. Med. 2003, 35, 772–781. [Google Scholar] [CrossRef]
- Mas, E.; Woodman, R.J.; Burke, V.; Puddey, I.B.; Beilin, L.J.; Durand, T.; Mori, T.A. The omega-3 fatty acids EPA and DHA decrease plasma F2-isoprostanes: Results from two placebo-controlled interventions. Free Radic. Res. 2010, 44, 983–990. [Google Scholar] [CrossRef]
- See, V.H.L.; Mas, E.; Prescott, S.L.; Beilin, L.J.; Burrows, S.; Barden, A.E.; Huang, R.-C.; Mori, T.A. Effects of prenatal n-3 fatty acid supplementation on offspring resolvins at birth and 12 years of age: A double-blind, randomised controlled clinical trial. Br. J. Nutr. 2017, 118, 971–980. [Google Scholar] [CrossRef]
- Barden, A.E.; Mori, T.A.; Dunstan, J.A.; Taylor, A.L.; Thornton, C.A.; Croft, K.; Beilin, L.J.; Prescott, S.L. Fish oil supplementation in pregnancy lowers F2-isoprostanes in neonates at high risk of atopy. Free Radic. Res. 2004, 38, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Wu, M.; Bondy, S.C. Telomere shortening during aging: Attenuation by antioxidants and anti-inflammatory agents. Mech. Ageing Dev. 2017, 164, 61–66. [Google Scholar] [CrossRef]
- Das, U.N. Telomere length and polyunsaturated fatty acids. Nutrition 2014, 30, 1218–1221. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Kang, J.X. Rethinking lipid mediators. Lancet 2005, 366, 618–620. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef]
- Iwama, H.; Ohyashiki, K.; Ohyashiki, J.H.; Hayashi, S.; Yahata, N.; Ando, K.; Toyama, K.; Hoshika, A.; Takasaki, M.; Mori, M.; et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Qual. Life Res. 1998, 102, 397–402. [Google Scholar] [CrossRef]
- Saretzki, G. Telomeres, Telomerase and Ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science; Springer: Berlin/Heidelberg, Germany, 2018; Volume 90, pp. 221–308. [Google Scholar] [CrossRef]
- Eitsuka, T.; Nakagawa, K.; Suzuki, T.; Miyazawa, T. Polyunsaturated fatty acids inhibit telomerase activity in DLD-1 human colorectal adenocarcinoma cells: A dual mechanism approach. Biochim. Biophys. Acta 2005, 1737, 1–10. [Google Scholar] [CrossRef]
- Eitsuka, T.; Nakagawa, K.; Kato, S.; Ito, J.; Otoki, Y.; Takasu, S.; Shimizu, N.; Takahashi, T.; Miyazawa, T. Modulation of Telomerase Activity in Cancer Cells by Dietary Compounds: A Review. Int. J. Mol. Sci. 2018, 19, 478. [Google Scholar] [CrossRef]
- Lee, D.D.; Leão, R.; Komosa, M.; Gallo, M.; Zhang, C.H.; Lipman, T.; Remke, M.; Heidari, A.; Nunes, N.M.; Apolónio, J.D.; et al. DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J. Clin. Investig. 2019, 129, 1801. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Alderson, N.L.; Hama, H.; Bielawski, J.; Jiang, J.C.; Bhandari, R.; Snyder, S.H.; Jazwinski, S.M.; Ogretmen, B. Regulation of telomere length by fatty acid elongase 3 in yeast. J. Biol. Chem. 2008, 283, 27514–27524. [Google Scholar] [CrossRef]
- Li, B.; Reddy, S.; Comai, L. Depletion of Ku70/80 reduces the levels of extrachromosomal telomeric circles and inhibits proliferation of ALT cells. Aging 2011, 3, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Askree, S.H.; Yehuda, T.; Smolikov, S.; Gurevich, R.; Hawk, J.; Coker, C.; Krauskopf, A.; Kupiec, M.; McEachern, M.J. A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc. Natl. Acad. Sci. USA 2004, 101, 8658–8663. [Google Scholar] [CrossRef] [PubMed]
- Bartram, H.-P.; Gostner, A.; Reddy, B.S.; Rao, C.V.; Scheppach, W.; Dusel, G.; Richter, A.; Richter, F.; Kasper, H. Missing anti-proliferative effect of fish oil on rectal epithelium in healthy volunteers consuming a high-fat diet. Eur. J. Cancer Prev. 1995, 4, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bartram, H.-P.; Gostner, A.; Scheppach, W.; Reddy, B.S.; Rao, C.V.; Dusel, G.; Richter, F.; Richter, A.; Kasper, H. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology 1993, 105, 1317–1322. [Google Scholar] [CrossRef]
- Pell, J.D.; Brown, J.C.; Johnson, I. Polyunsaturated fatty acids of the n—3 series influence intestinal crypt cell production in rats. Carcinogenesis 1994, 15, 1115–1119. [Google Scholar] [CrossRef]
| Author, Year [Ref] | Study Design | Examined Group | Omega-3 Fatty Acids Adjustment | Reported Effect on Telomere Length * |
|---|---|---|---|---|
| Farzaneh-Far et al. [47] | Prospective cohort study | 608 U.S. men and women with coronary artery disease, follow-up of 5 years | Quartiles of baseline sum of omega-3 fatty acids (EPA and DHA) in whole blood estimated by gas chromatography (GC) | Inverse associations between levels of omega-3 fatty acids and leukocyte telomere length |
| Cassidy et al. [48] | Cross-sectional study | 2284 U.S. women, 43–69 years old | Marine omega-3 fatty acids and ALA intake per day, estimated based on questionnaire | No association between marine omega-3 fatty acids, ALA, and leukocyte telomere length |
| Chang et al. [49] | Prospective cohort study | 711 Chinese patients with nested coronary artery disease and 638 healthy controls | Plasma levels of ALA, EPA, and DHA quantified using mass spectrometry | Positive correlation between EPA and DHA levels and leukocyte telomere length |
| Liu et al. [50] | Cross-sectional study | 64 children with obesity and equal numbers of normal weight children, 3–4 years old | Erythrocyte levels of ALA, EPA, eicosatetraenoic acid, and DHA measured with GC | Significant association between DHA levels and leukocyte telomere length |
| Freitas-Simoes et al. [51] | Cross-sectional study | 344 Mediterranean people (63–79 years old, 68.6% women) | Erythrocyte levels of ALA, EPA, and DHA measured with GC | No association between level of ALA, sum of EPA and DHA, and leukocyte telomere length, measured by quantitative fluorescence in situ hybridization (FISH) |
| Kiecolt-Glaser et al. [52] | Randomized controlled trial | 106 U.S. men and women, 40–85 years old, receiving 2.5 g omega-3 PUFAs, 1.25 g omega-3 PUFAs, or placebo capsules mirroring the proportions of fatty acids in an average American diet per day for 4 months | Change in the level of omega-6 and omega-3 fatty acids in blood plasma | No significant changes in telomere length between groups. Telomere length increased with decreasing omega-6:omega-3 fatty acid ratio |
| O’Callaghan et al. [53] | Randomized parallel-group pilot trial | 33 Australian men and women, >65 years old, suffering from mild cognitive impairment, receiving a supplement rich in EPA (1.67 g + 0.16 g of DHA; n = 12), a supplement rich in DHA (1.55 g + 0.40 g of EPA; n = 12), or LA (2.2 g; n = 9) per day for 6 months | Erythrocyte DHA level | Telomere shortening was greatest in the LA group (d = 0.21) compared to the DHA (d = 0.12) and EPA (d = 0.06) groups, but results were statistically underpowered. Increased erythrocyte DHA levels were associated with reduced telomere shortening (r = −0.67; p = 0.02) in the DHA group. |
| Barden et al. [54] | Randomized parallel-group pilot trial Double-blind placebo-controlled trial | 85 men and women, 25–75 years old, suffering from chronic renal impairment, receiving omega-3 fatty acids (4 g), or CoQ (200 mg), or both supplements, or control (4 g of olive oil) per day for 8 weeks | None | Telomere length corrected for neutrophil count was increased after omega-3 fatty acids |
| See et al. [55] | Randomized parallel-group study | 98 pregnant atopic women receiving 4 g of omega-3 PUFAs or an equal amount of olive oil from 20 weeks’ gestation until delivery; offspring of examined women | Erythrocyte omega-3 fatty acids level measured with GC | Maternal supplementation with omega-3 fatty acids did not affect offspring’s telomere length at birth or at 12 years, with no changes over time |
| Yeates et al. [56] | Cross-sectional study | 229 mothers (mean age: 27.2 years, range: 15–42 years) and their children | Level of omega-3 fatty acids in blood of mothers (at 28 weeks of gestation and at delivery) and children (at 5 years of age) determined using Gas chromatography/mass spectrometry (GC/MS) | Omega-3 fatty acid level was not associated with telomere length of the mother or child or with telomere length attrition rate |
| Liu et al. [57] | Cross-sectional study | 274 mothers (mean age: 31.96 ± 3.70) and their children | Level of omega-3 fatty acids in maternal erythrocytes and cord blood, determined using GC | Low concentrations of DPA and total omega-3 fatty acids in maternal erythrocytes and low concentrations of cord blood DHA were associated with shortened telomere length in cord blood cells. High concentrations of ALA, eicosatrienoic acid (EA), and DHA in maternal erythrocytes were associated with shortened telomere length in the placenta. |
| Author, Year [Ref] | Study Design | Examined Group | Omega-3 Fatty Acids Adjustment | Reported Effect on Telomere Length * |
|---|---|---|---|---|
| Chen et al. [59] | Animal study | 8-week-old male mice (n = 5) were assigned to groups receiving D-galactose to induce aging; this included a positive D-galactose group and groups receiving 400, 200, and 100 mg of fish oil per kg of body weight per day or 120, 60, and 30 mg of DHA per kg of body weight per day for 2 months. | None | Omega-3 fatty acids protected the liver and testes against telomere shortening within the range of 13–25% and 25–27%, respectively. |
| Varela-Lopez et al. [60] | Animal study | 72 male rats were assigned to three groups and fed—from weaning until 24 months of life—fodder differing in fat source (virgin olive oil, sunflower oil, or fish oil rich in DHA and EPA); the amount of fat fulfilled the standard requirement of a rat’s diet. | Liver fatty acid profile determined with GC | 24-month-old rats receiving a diet rich in fish-based omega-3 fatty acids exhibited the longest liver telomeres compared to 6-month-old animals receiving the same diet and to animals fed virgin olive oil and sunflower oil |
| Gao et al. [61] | Animal study | 10 healthy rats and 34 suffering from gestational diabetes mellitus (GDM) were divided into three groups: offspring fed soybean oil, adequate offspring fed fish oil (rich in omega-3 fatty acids), and omega-3 PUFA-deficient offspring fed safflower oil until 11 months old. Rats belonged to both sexes. | The level of ALA in liver was determined with high-performance liquid chromatography–quadrupole-time of flight mass spectrometry (HPLC-QTOF-MS) | The liver telomere length of rats suffering from GDM was nearly improved (with a nonsignificant tendency) by supplementation with omega-3 fatty acids, compared with non-supplemented GDM rats. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogłuszka, M.; Lipiński, P.; Starzyński, R.R. Effect of Omega-3 Fatty Acids on Telomeres—Are They the Elixir of Youth? Nutrients 2022, 14, 3723. https://doi.org/10.3390/nu14183723
Ogłuszka M, Lipiński P, Starzyński RR. Effect of Omega-3 Fatty Acids on Telomeres—Are They the Elixir of Youth? Nutrients. 2022; 14(18):3723. https://doi.org/10.3390/nu14183723
Chicago/Turabian StyleOgłuszka, Magdalena, Paweł Lipiński, and Rafał R. Starzyński. 2022. "Effect of Omega-3 Fatty Acids on Telomeres—Are They the Elixir of Youth?" Nutrients 14, no. 18: 3723. https://doi.org/10.3390/nu14183723
APA StyleOgłuszka, M., Lipiński, P., & Starzyński, R. R. (2022). Effect of Omega-3 Fatty Acids on Telomeres—Are They the Elixir of Youth? Nutrients, 14(18), 3723. https://doi.org/10.3390/nu14183723





