A Randomized Trial of the Effects of Dietary n3-PUFAs on Skeletal Muscle Function and Acute Exercise Response in Healthy Older Adults
Abstract
:1. Introduction
2. Materials and Methods
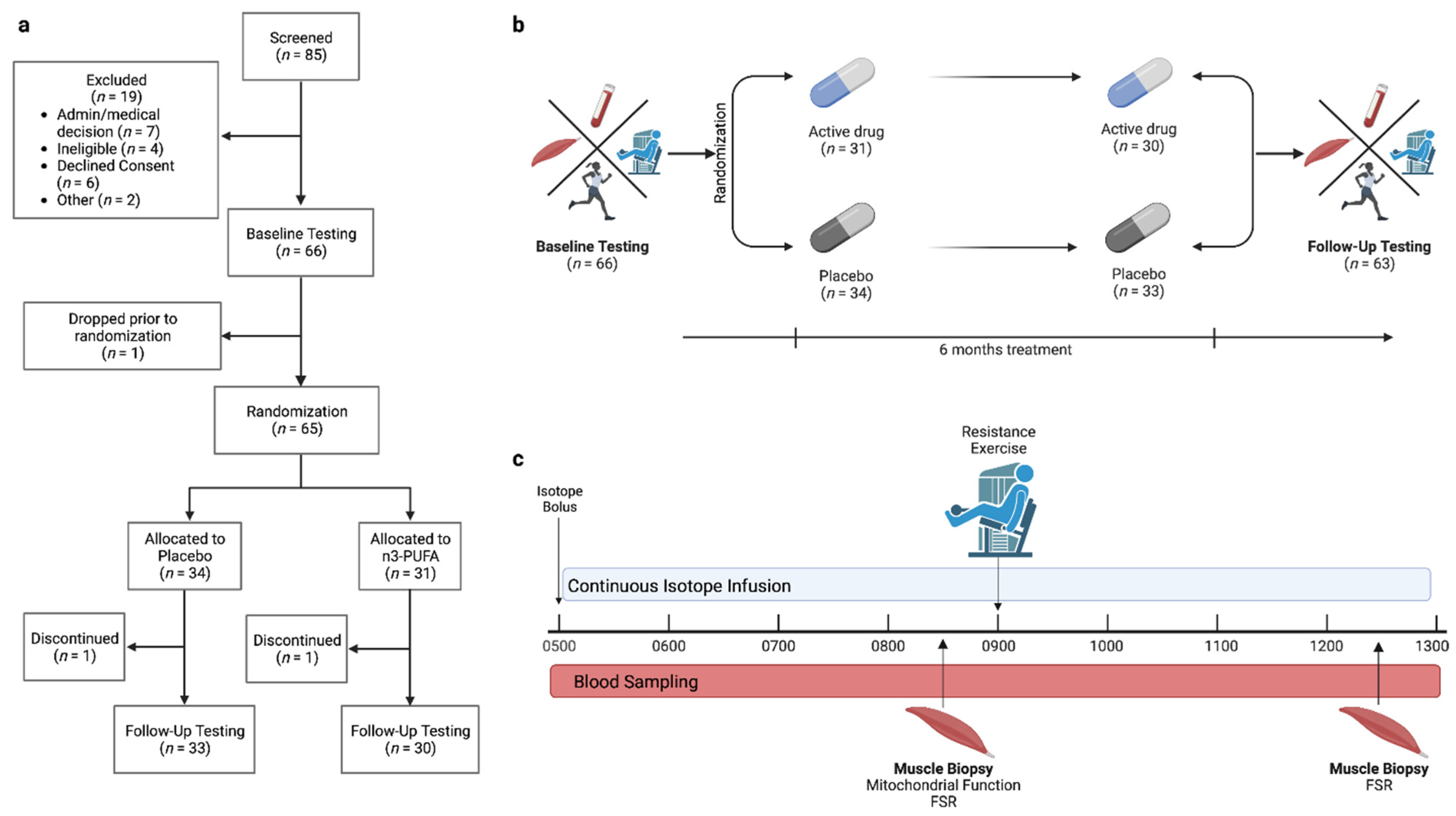
2.1. Participants and Study Design
2.2. Screening Visit
2.3. Outpatient Testing Visit
2.4. Inpatient Study Day
2.5. Intervention
2.6. Mitochondrial Measurements in Permeabilized Muscle Fibers
2.7. Stable Isotope Tracer Administration
2.8. Whole-Body Protein Turnover
2.9. Skeletal Muscle Fractional Synthesis Rates
2.10. Statistical Analysis
3. Results
3.1. Participants and Intervention
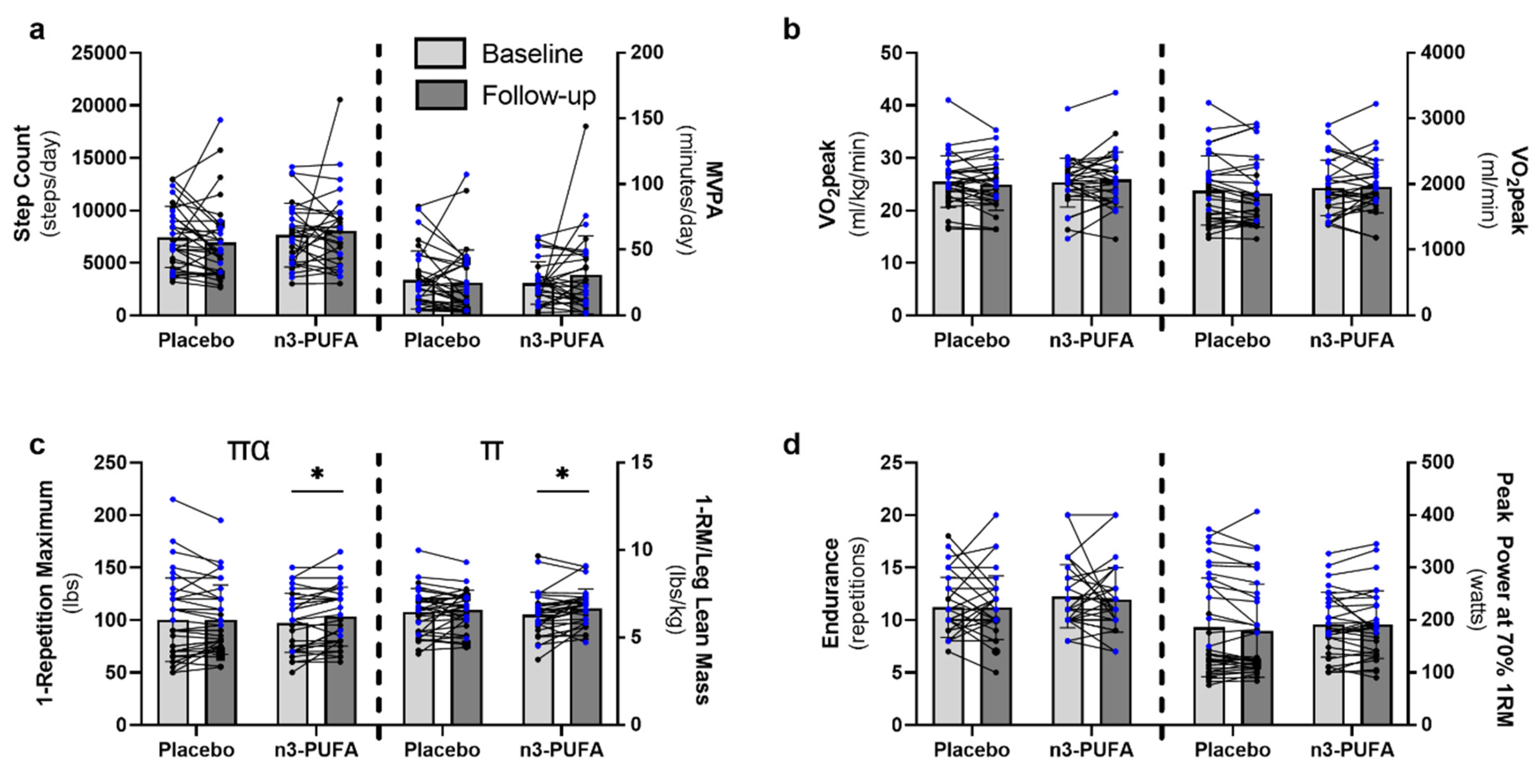
3.2. Physical Activity and Function
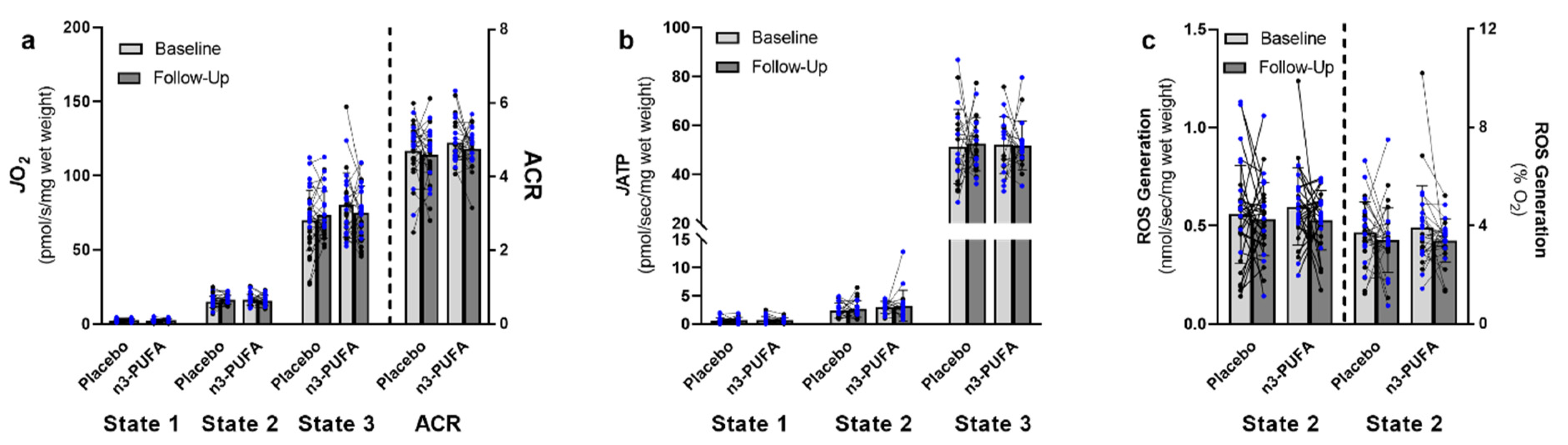
3.3. Mitochondrial Function
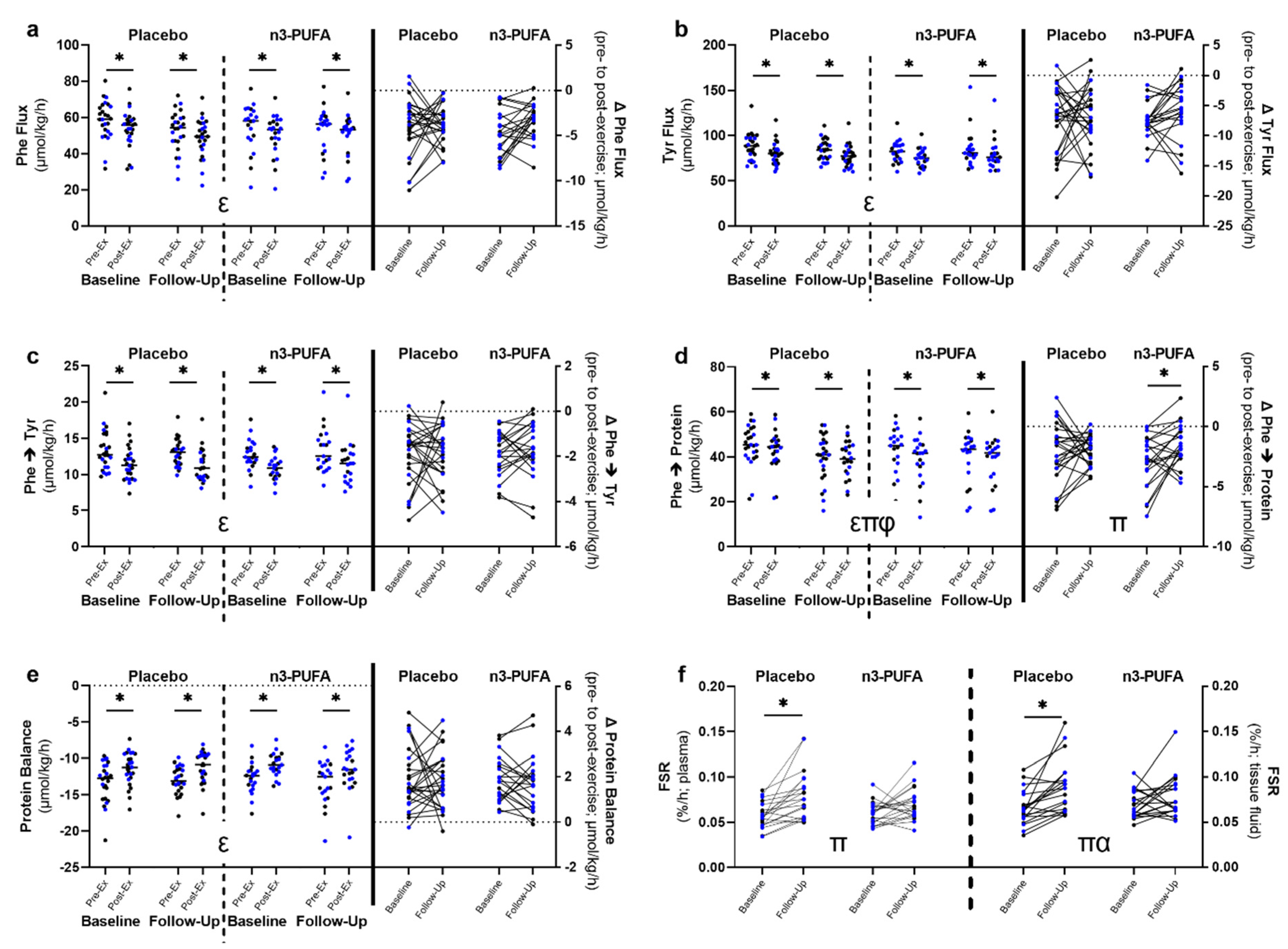
3.4. Whole-Body Amino Acid Kinetics and Muscle Fractional Synthesis Rates
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kallman, D.A.; Plato, C.C.; Tobin, J.D. The Role of Muscle Loss in the Age-Related Decline of Grip Strength: Cross-Sectional and Longitudinal Perspectives. J. Gerontol. 1990, 45, M82–M88. [Google Scholar] [CrossRef] [PubMed]
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Fielding, R.A. Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life. Osteoporos. Int. 2016, 27, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Manini, T.M. Functional consequences of sarcopenia and dynapenia in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Montero-Fernández, N.; Serra-Rexach, J. Role of exercise on sarcopenia in the elderly. Eur. J. Phys. Rehabil. Med. 2013, 49, 131–143. [Google Scholar] [PubMed]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef]
- Fink, R.I.; Kolterman, O.G.; Griffin, J.; Olefsky, J.M. Mechanisms of Insulin Resistance in Aging. J. Clin. Investig. 1983, 71, 1523–1535. [Google Scholar] [CrossRef]
- Hunter, G.R.; McCarthy, J.P.; Bamman, M.M. Effects of Resistance Training on Older Adults. Sports Med. 2004, 34, 329–348. [Google Scholar] [CrossRef]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance Exercise as a Countermeasure for Aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef]
- Czyż, S.H.; Toriola, A.L.; Starościak, W.; Lewandowski, M.; Paul, Y.; Oyeyemi, A.L. Physical Fitness, Physical Activity, Sedentary Behavior, or Diet—What Are the Correlates of Obesity in Polish School Children? Int. J. Environ. Res. Public Health 2017, 14, 664. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Gibbons, L.W.; Mitchell, T.L.; Kampert, J.B.; Lee, C.D.; Blair, S.N. The Association between Cardiorespiratory Fitness and Impaired Fasting Glucose and Type 2 Diabetes Mellitus in Men. Ann. Intern. Med. 1999, 130, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.-C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Löllgen, H.; Böckenhoff, A.; Knapp, G. Physical Activity and All-cause Mortality: An Updated Meta-analysis with Different Intensity Categories. Int. J. Sports Med. 2009, 30, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef]
- Burd, N.; Gorissen, S.; van Loon, L.J. Anabolic Resistance of Muscle Protein Synthesis with Aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Joseph, G.A.; Wang, S.X.; Jacobs, C.E.; Zhou, W.; Kimble, G.C.; Tse, H.W.; Eash, J.K.; Shavlakadze, T.; Glass, D.J. Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Mol. Cell. Biol. 2019, 39, e00141-19. [Google Scholar] [CrossRef]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef]
- Dungan, C.M.; Figueiredo, V.C.; Wen, Y.; VonLehmden, G.L.; Zdunek, C.J.; Thomas, N.T.; Mobley, C.B.; Murach, K.A.; Brightwell, C.R.; Long, D.E.; et al. Senolytic treatment rescues blunted muscle hypertrophy in old mice. GeroScience, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Dungan, C.M.; Murach, K.A.; Zdunek, C.J.; Tang, Z.J.; VonLehmden, G.L.; Brightwell, C.R.; Hettinger, Z.; Englund, D.A.; Liu, Z.; Fry, C.S.; et al. Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell 2021, 21, e13528. [Google Scholar] [CrossRef]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Ser. Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-D.; Yeun, Y.-R. Effects of Resistance Training on C-Reactive Protein and Inflammatory Cytokines in Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 3434. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.J.; Matthews, D.E.; Tracy, R.P.; Previs, M.J. Age-related differences in skeletal muscle protein synthesis: Relation to markers of immune activation. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E883–E891. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.H.; Frost, R.A.; Nairn, A.C.; MacLean, D.A.; Vary, T.C. TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E336–E347. [Google Scholar] [CrossRef] [PubMed]
- Trappe, T.A.; Carroll, C.C.; Dickinson, J.M.; Lemoine, J.K.; Haus, J.M.; Sullivan, B.E.; Lee, J.D.; Jemiolo, B.; Weinheimer, E.M.; Hollon, C.J. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R655–R662. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia–hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef]
- Moosavi, D.; Vuckovic, I.; Kunz, H.E.; Lanza, I.R. A Randomized Trial of ω-3 Fatty Acid Supplementation and Circulating Lipoprotein Subclasses in Healthy Older Adults. J. Nutr. 2022, 152, 1675–1689. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83 (Suppl. S6), 1505S–1519S. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.D.; Li, T.; Rau, C.M.; LeSuer, W.E.; Wang, P.; Coletta, D.K.; Madura, J.A., 2nd; Jacobsen, E.A.; De Filippis, E. ω-3PUFA supplementation ameliorates adipose tissue inflammation and insulin-stimulated glucose disposal in subjects with obesity: A potential role for apolipoprotein E. Int. J. Obes. 2021, 45, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Finlin, B.S.; Unal, R.; Zhu, B.; Morris, A.J.; Shipp, L.R.; Lee, J.; Walton, R.G.; Adu, A.; Erfani, R.; et al. Omega-3 Fatty Acids Reduce Adipose Tissue Macrophages in Human Subjects With Insulin Resistance. Diabetes 2013, 62, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Lalia, A.Z.; Dasari, S.; Pallauf, M.; Fitch, M.D.; Hellerstein, M.K.; Lanza, I.R. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell 2015, 14, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of Accelerometer Wear and Nonwear Time Classification Algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Bruce, R.A.; Kusumi, F.; Hosmer, D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973, 85, 546–562. [Google Scholar] [CrossRef]
- Hossack, K.; Eldridge, J.; Wolfel, E.; Leddy, C.; Berger, N. Aerobic responses to low level exercise testing following an acute myocardial infarction. Am. Heart J. 1987, 113, 694–699. [Google Scholar] [CrossRef]
- Zhang, X.; Kunz, H.E.; Gries, K.; Hart, C.R.; Polley, E.C.; Lanza, I.R. Preserved skeletal muscle oxidative capacity in older adults despite decreased cardiorespiratory fitness with ageing. J. Physiol. 2021, 599, 3581–3592. [Google Scholar] [CrossRef]
- Reid, K.F.; Callahan, D.M.; Carabello, R.J.; Phillips, E.M.; Frontera, W.R.; Fielding, R.A. Lower extremity power training in elderly subjects with mobility limitations: A randomized controlled trial. Aging Clin. Exp. Res. 2008, 20, 337–343. [Google Scholar] [CrossRef]
- Reid, K.F.; Martin, K.I.; Doros, G.; Clark, D.J.; Hau, C.; Patten, C.; Phillips, E.M.; Frontera, W.R.; Fielding, R.A. Comparative Effects of Light or Heavy Resistance Power Training for Improving Lower Extremity Power and Physical Performance in Mobility-Limited Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 70, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hanson, E.D.; Sheaff, A.K.; Sood, S.; Ma, L.; Francis, J.D.; Goldberg, A.P.; Hurley, B.F. Strength Training Induces Muscle Hypertrophy and Functional Gains in Black Prostate Cancer Patients Despite Androgen Deprivation Therapy. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 68, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Johnson, M.L.; Jensen, M.D.; Hames, K.C.; Port, J.D.; Lanza, I.R. Effects of Dietary n-3 Fatty Acids on Hepatic and Peripheral Insulin Sensitivity in Insulin-Resistant Humans. Diabetes Care 2015, 38, 1228–1237. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]
- Lanza, I.R.; Zabielski, P.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.J.; Bergen, H.R., 3rd; Dasari, S.; Walrand, S.; Short, K.R.; Johnson, M.L.; et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012, 16, 777–788. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Kunz, H.E.; Dorschner, J.M.; Berent, T.E.; Meyer, T.; Wang, X.; Jatoi, A.; Kumar, R.; Lanza, I.R. Methylarginine metabolites are associated with attenuated muscle protein synthesis in cancer-associated muscle wasting. J. Biol. Chem. 2020, 295, 17441–17459. [Google Scholar] [CrossRef]
- Lanza, I.R.; Nair, K.S. Chapter 20: Functional Assessment of Isolated Mitochondria In Vitro. Methods Enzymol. 2009, 457, 349–372. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Kane, D.A.; Lin, C.-T.; Kozy, R.; Cathey, B.L.; Lark, D.S.; Kane, C.L.; Brophy, P.M.; Gavin, T.P.; Anderson, E.J.; et al. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem. J. 2011, 437, 215–222. [Google Scholar] [CrossRef]
- Abid, H.; Ryan, Z.C.; Delmotte, P.; Sieck, G.C.; Lanza, I.R. Extramyocellular interleukin-6 influences skeletal muscle mitochondrial physiology through canonical JAK/STAT signaling pathways. FASEB J. 2020, 34, 14458–14472. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Port, J.D.; Kaufman, K.R.; Jatoi, A.; Hart, C.R.; Gries, K.J.; Lanza, I.R.; Kumar, R. Skeletal muscle mitochondrial dysfunction and muscle and whole body functional deficits in cancer patients with weight loss. J. Appl. Physiol. 2022, 132, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Lark, D.S.; Torres, M.J.; Lin, C.-T.; Ryan, T.E.; Anderson, E.J.; Neufer, P.D. Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers. Am. J. Physiol. Physiol. 2016, 311, C239–C245. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Rouland, R.; Calmettes, G.; Deschodt-Arsac, V.; Franconi, J.-M.; Bourdel-Marchasson, I.; Diolez, P. Accurate Determination of the Oxidative Phosphorylation Affinity for ADP in Isolated Mitochondria. PLoS ONE 2011, 6, e20709. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Meek, S.E.; Moller, N.; Ekberg, K.; Nair, K.S. Whole body protein kinetics using Phe and Tyr tracers: An evaluation of the accuracy of approximated flux values. Am. J. Physiol. Content 1999, 276, E1194–E1200. [Google Scholar] [CrossRef]
- Rooyackers, O.; Kouchek-Zadeh, R.; Tjäder, I.; Norberg; Klaude, M.; Wernerman, J. Whole body protein turnover in critically ill patients with multiple organ failure. Clin. Nutr. 2015, 34, 95–100. [Google Scholar] [CrossRef]
- Nair, K.S.; Ford, G.C.; Ekberg, K.; Fernqvist-Forbes, E.; Wahren, J. Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J. Clin. Investig. 1995, 95, 2926–2937. [Google Scholar] [CrossRef]
- Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. USA 1996, 93, 15364–15369. [Google Scholar] [CrossRef]
- Zabielski, P.; Ford, G.C.; Persson, X.M.; Jaleel, A.; Dewey, J.D.; Nair, K.S. Comparison of different mass spectrometry techniques in the measurement of L-[ring-13C6]phenylalanine incorporation into mixed muscle proteins. Biol. Mass Spectrom. 2013, 48, 269–275. [Google Scholar] [CrossRef]
- Burd, N.A.; Groen, B.B.; Beelen, M.; Senden, J.M.; Gijsen, A.P.; van Loon, L.J. The reliability of using the single-biopsy approach to assess basal muscle protein synthesis rates in vivo in humans. Metabolism 2012, 61, 931–936. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Chiu, W.-C.; Hsu, Y.-P.; Lo, Y.-L.; Wang, Y.-H. Effects of Omega-3 Fatty Acids on Muscle Mass, Muscle Strength and Muscle Performance among the Elderly: A Meta-Analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef] [PubMed]
- Ritti-Dias, R.M.; Avelar, A.; Salvador, E.P.; Cyrino, E. Influence of Previous Experience on Resistance Training on Reliability of One-Repetition Maximum Test. J. Strength Cond. Res. 2011, 25, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil–derived n−3 PUFA therapy increases muscle mass and function in healthy older adults1. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Alkhedhairi, S.A.; Alkhayl, F.F.A.; Ismail, A.D.; Rozendaal, A.; German, M.; MacLean, B.; Johnston, L.; Miller, A.; Hunter, A.; Macgregor, L.; et al. The effect of krill oil supplementation on skeletal muscle function and size in older adults: A randomised controlled trial. Clin. Nutr. 2022, 41, 1228–1235. [Google Scholar] [CrossRef]
- Krzymińska-Siemaszko, R.; Czepulis, N.; Lewandowicz, M.; Zasadzka, E.; Suwalska, A.; Witowski, J.; Wieczorowska-Tobis, K. The Effect of a 12-Week Omega-3 Supplementation on Body Composition, Muscle Strength and Physical Performance in Elderly Individuals with Decreased Muscle Mass. Int. J. Environ. Res. Public Health 2015, 12, 10558–10574. [Google Scholar] [CrossRef]
- Logan, S.L.; Spriet, L.L. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS ONE 2015, 10, e0144828. [Google Scholar] [CrossRef]
- Strandberg, E.; Edholm, P.; Ponsot, E.; Wåhlin-Larsson, B.; Hellmén, E.; Nilsson, A.; Engfeldt, P.; Cederholm, T.; Risérus, U.; Kadi, F. Influence of combined resistance training and healthy diet on muscle mass in healthy elderly women: A randomized controlled trial. J. Appl. Physiol. 2015, 119, 918–925. [Google Scholar] [CrossRef]
- Edholm, P.; Strandberg, E.; Kadi, F. Lower limb explosive strength capacity in elderly women: Effects of resistance training and healthy diet. J. Appl. Physiol. 2017, 123, 190–196. [Google Scholar] [CrossRef]
- Trappe, T.A.; Ratchford, S.M.; Brower, B.E.; Liu, S.Z.; Lavin, K.M.; Carroll, C.C.; Jemiolo, B.; Trappe, S.W. COX Inhibitor Influence on Skeletal Muscle Fiber Size and Metabolic Adaptations to Resistance Exercise in Older Adults. J. Gerontol. Ser. A 2016, 71, 1289–1294. [Google Scholar] [CrossRef]
- Ye, J.; Ghosh, S. Omega-3 PUFA vs. NSAIDs for Preventing Cardiac Inflammation. Front. Cardiovasc. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Akima, H.; Takahashi, H.; Kuno, S.-Y.; Masuda, K.; Masuda, T.; Shimojo, H.; Anno, I.; Itai, Y.; Katsuta, S. Early phase adaptations of muscle use and strength to isokinetic training. Med. Sci. Sports Exerc. 1999, 31, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Drummond, M.J.; Glynn, E.L.; Dickinson, J.M.; Gundermann, D.M.; Timmerman, K.L.; Walker, D.K.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet. Muscle 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Amino acid and protein metabolism during exercise and recovery. Med. Sci. Sports Exerc. 1987, 19 (Suppl. S5), S150–S156. [Google Scholar] [CrossRef]
- Lilja, M.; Mandić, M.; Apró, W.; Melin, M.; Olsson, K.; Rosenborg, S.; Gustafsson, T.; Lundberg, T.R. High doses of anti-inflammatory drugs compromise muscle strength and hypertrophic adaptations to resistance training in young adults. Acta Physiol. 2017, 222, e12948. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The Cytokine Response to Physical Activity and Training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the Regulation of Inflammatory Responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar] [CrossRef]
- Johnson, M.L.; Robinson, M.M.; Nair, K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013, 24, 247–256. [Google Scholar] [CrossRef] [Green Version]
| Placebo (n = 33) Mean (SD) | n3-PUFA (n = 30) Mean (SD) | Group Effect Statistic (p-Value) | Time Effect Statistic (p-Value) | Interaction Statistic (p-Value) | |||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | ||||
| Sex | 20F/13M | 14F/16M | |||||
| Age (years) | 71.5 (4.8) | 71.4 (4.5) | 0.100 (0.920) | ||||
| Height (cm) | 168.1 ± 10.1 | 168.6 ± 8.9 | −0.208 (0.836) | ||||
| Weight (kg) | 74.5 (14.5) | 74.5 (14.5) | 77.3 (13.1) | 77.1 (13.6) | 0.592 (0.445) | 0.148 (0.702) | 0.145 (0.705) |
| BMI (kg/m2) | 26.3 (3.9) | 26.3 (3.8) | 27.1 (4.0) | 27.2 (4.3) | 0.718 (0.400) | 0.178 (0.675) | 0.040 (0.841) |
| SMI (kg/m2) | 7.0 (1.3) | 7.0 (1.3) | 7.2 (1.0) | 7.2 (1.1) | 0.241 (0.625) | 0.747 (0.391) | 0.020 (0.889) |
| Body Fat (%) | 35.8 (8.9) | 35.8 (9.1) | 36.9 (7.3) | 36.7 (7.2) | 0.228 (0.635) | 0.075 (0.785) | 0.176 (0.677) |
| Lean Mass (kg) | 45.5 (10.6) | 45.5 (10.5) | 46.0 (8.0) | 46.1 (8.1) | 0.058 (0.811) | 0.008 (0.929) | 0.002 (0.957) |
| Leg Lean Mass (kg) | 15.2 (3.9) | 15.2 (3.8) | 15.3 (3.0) | 15.4 (3.1) | 0.057 (0.813) | 0.225 (0.637) | 0.302 (0.585) |
| RBC EPA (µM) | 7.8 (4.2) | 5.1 (1.5) * | 6.2 (4.9) † | 37.1 (12.4) †* | 94.845 (<0.001) | 145.908 (<0.001) | 302.957 (<0.001) |
| RBC DHA (µM) | 69.4 (23.3) | 57.0 (13.0) * | 58.0 (25.5) † | 88.7 (17.4) †* | 3.958 (0.051) | 15.171 (<0.001) | 69.690 (<0.001) |
| ESR (mm/h) | 8.8 (6.5) | 10.1 (9.3) | 7.8 (6.2) | 7.5 (6.2) | 0.753 (0.389) | 0.053 (0.818) | 0.504 (0.480) |
| CRP (mg/L) | 1.6 (1.3) | 2.2 (2.5) | 1.7 (1.8) | 1.5 (1.9) | 0.373 (0.543) | 0.028 (0.867) | 0.940 (0.336) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunz, H.E.; Michie, K.L.; Gries, K.J.; Zhang, X.; Ryan, Z.C.; Lanza, I.R. A Randomized Trial of the Effects of Dietary n3-PUFAs on Skeletal Muscle Function and Acute Exercise Response in Healthy Older Adults. Nutrients 2022, 14, 3537. https://doi.org/10.3390/nu14173537
Kunz HE, Michie KL, Gries KJ, Zhang X, Ryan ZC, Lanza IR. A Randomized Trial of the Effects of Dietary n3-PUFAs on Skeletal Muscle Function and Acute Exercise Response in Healthy Older Adults. Nutrients. 2022; 14(17):3537. https://doi.org/10.3390/nu14173537
Chicago/Turabian StyleKunz, Hawley E., Kelly L. Michie, Kevin J. Gries, Xiaoyan Zhang, Zachary C. Ryan, and Ian R. Lanza. 2022. "A Randomized Trial of the Effects of Dietary n3-PUFAs on Skeletal Muscle Function and Acute Exercise Response in Healthy Older Adults" Nutrients 14, no. 17: 3537. https://doi.org/10.3390/nu14173537
APA StyleKunz, H. E., Michie, K. L., Gries, K. J., Zhang, X., Ryan, Z. C., & Lanza, I. R. (2022). A Randomized Trial of the Effects of Dietary n3-PUFAs on Skeletal Muscle Function and Acute Exercise Response in Healthy Older Adults. Nutrients, 14(17), 3537. https://doi.org/10.3390/nu14173537






