Nutritional Composition of Infant Cereal Prototypes Can Precisely Predict Their Glycemic Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Infant Cereal Prototypes
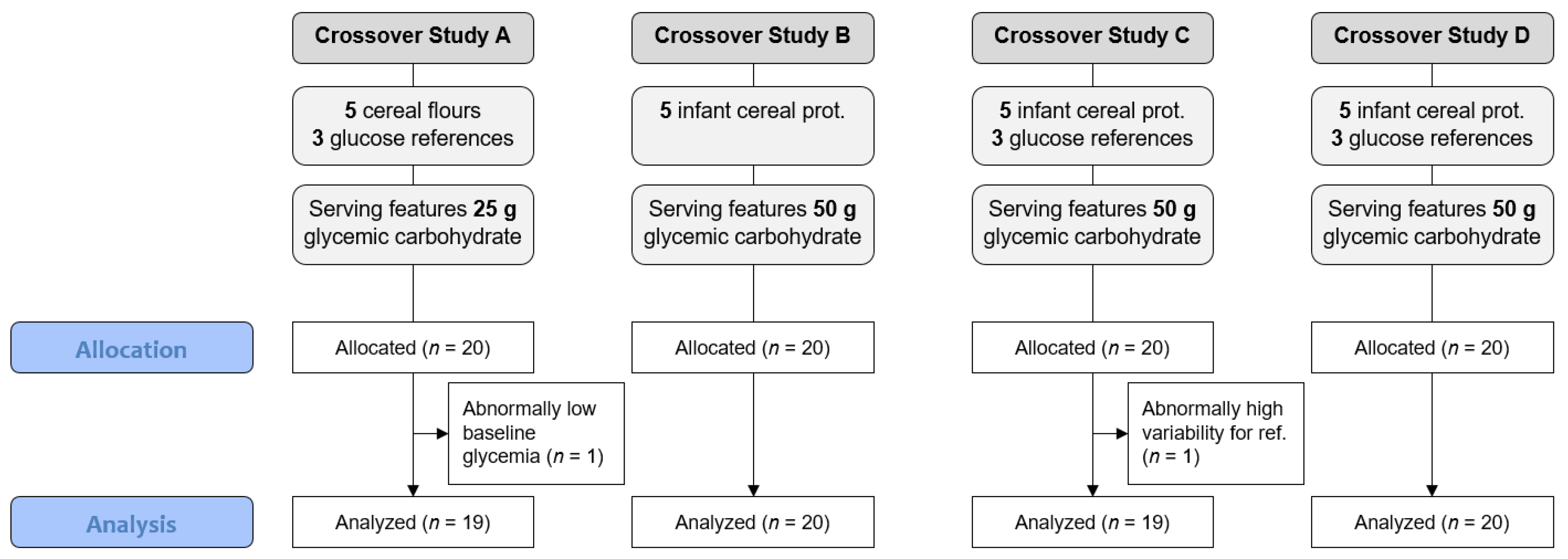
2.2. In Vivo Trials
2.3. Model Predicting GI and GL from Nutrient Composition
2.4. Statistical Analyses
3. Results
3.1. Measured GI vs. Predicted GI
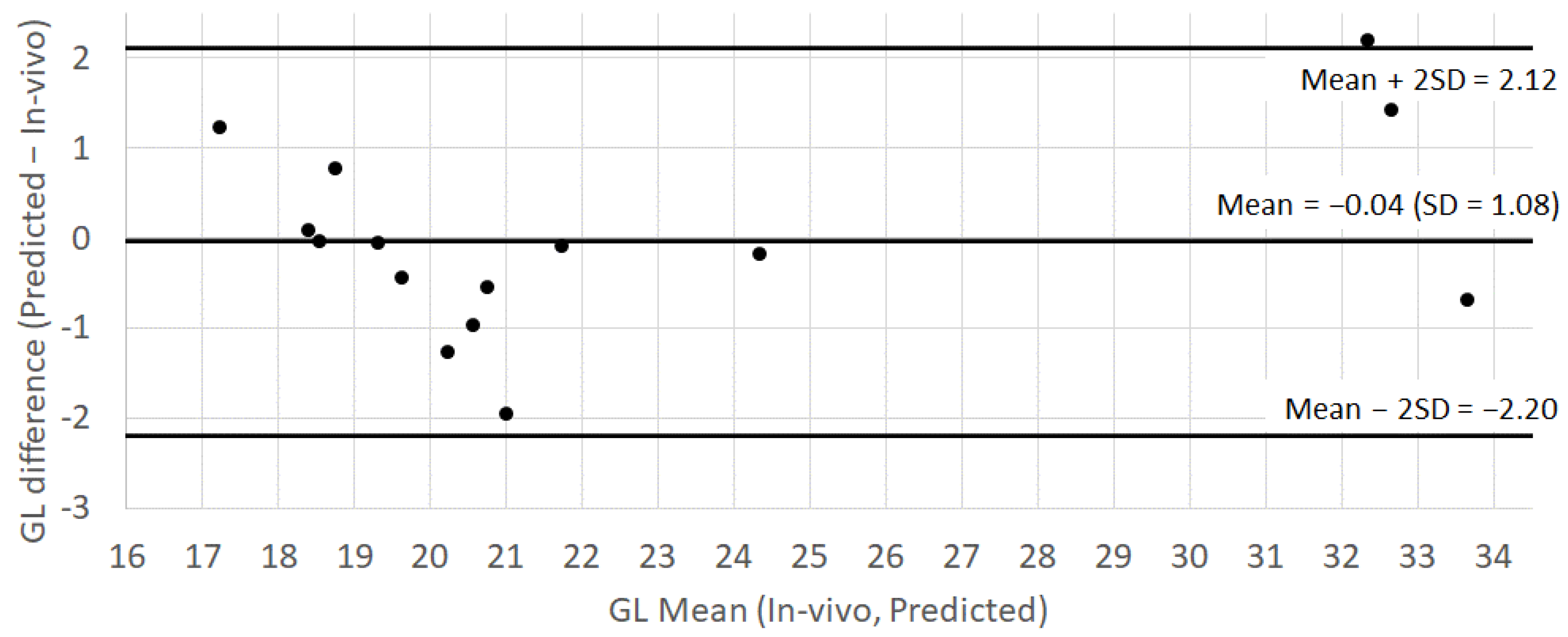
3.2. Measured GL vs. Predicted GL
3.3. Measured PPGR vs. Predicted GL
4. Discussion
4.1. Measured Glycemic Response to Complete Infant Cereal Prototypes
4.2. Validity of GI Predictions
4.3. Relationship between eGL and PPGR
4.4. Application of the Model for Future Product Development
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awika, J.M. Advances in Cereal Science: Implications to Food Processing and Health PromotionMajor. In Cereal Grains Production and Use around the World; ACS Publications: Washington, DC, USA, 2011; pp. 1–13. [Google Scholar]
- US Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; USDA: Washington, DC, USA, 2020.
- World Health Organization. Report of the Expert Consultation on the Optimal Duration of Exclusive Breastfeeding; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Klerks, M.; Bernal, M.J.; Roman, S.; Bodenstab, S.; Gil, A.; Sanchez-Siles, L.M. Infant Cereals: Current Status, Challenges, and Future Opportunities for Whole Grains. Nutrients 2019, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.; Wolever, T.M.S. Effect of Fat on Glycaemic Responses in Normal Subjects: A Dose-Response Study. Nutr. Res. 2003. [Google Scholar] [CrossRef]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic Index, Glycemic Load and Chronic Disease Risk—A Meta-Analysis of Observational Studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic Index, Glycemic Load and Glycemic Response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of Macronutrients and Fiber on Postprandial Glycemic Responses and Meal Glycemic Index and Glycemic Load Value Determinations. Am. J. Clin. Nutr. 2017, 105, 842–853. [Google Scholar] [CrossRef]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of Fat, Protein, and Glycemic Index on Postprandial Glucose Control in Type 1 Diabetes: Implications for Intensive Diabetes Management in the Continuous Glucose Monitoring Era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Jenkins, A.L. Dietary Fiber and the Glycemic Response. Exp. Biol. Med. 1985, 180, 422–431. [Google Scholar] [CrossRef]
- Rytz, A.; Adeline, D.; Lê, K.-A.; Tan, D.; Lamothe, L.; Roger, O.; Macé, K. Predicting Glycemic Index and Glycemic Load from Macronutrients to Accelerate Development of Foods and Beverages with Lower Glucose Responses. Nutrients 2019, 11, 1172. [Google Scholar] [CrossRef]
- Bai, J.; Ren, Y.; Li, Y.; Fan, M.; Qian, H.; Wang, L.; Wu, G.; Zhang, H.; Qi, X.; Xu, M.; et al. Physiological Functionalities and Mechanisms of β-Glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Standard for Processed Cereal-Based Foods for Infants and Young Children; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Gaitherburg, MD, USA, 2011. [Google Scholar]
- ISO 26642:2010; Food Products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. International Organization for Standardization: Geneva, Switzerland, 2010.
- Mettler, S.; Steiner, K.; Colombani, P.C. Influence of Test Interval Length on the Variability of Glycemic Response Tests. Eur. J. Clin. Nutr. 2009, 63, 1452–1454. [Google Scholar] [CrossRef][Green Version]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic Index of Foods: A Physiological Basis for Carbohydrate Exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring Agreement in Method Comparison Studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Stenberg, M.; Frid, A.H.; Holst, J.J.; Björck, I.M.E. Glycemia and Insulinemia in Healthy Subjects after Lactose-Equivalent Meals of Milk and Other Food Proteins: The Role of Plasma Amino Acids and Incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.; Hagander, B.; Björck, I. Metabolic Responses to Starch in Oat and Wheat Products. On the Importance of Food Structure, Incomplete Gelatinization or Presence of Viscous Dietary Fibre. Eur. J. Clin. Nutr. 1995, 49, 189–199. [Google Scholar]
- Liljeberg, H.G.; Granfeldt, Y.E.; Björck, I.M. Products Based on a High Fiber Barley Genotype, but Not on Common Barley or Oats, Lower Postprandial Glucose and Insulin Responses in Healthy Humans. J. Nutr. 1996, 126, 458–466. [Google Scholar] [CrossRef]
- Liljeberg, H.; Björck, I. Bioavailability of Starch in Bread Products. Postprandial Glucose and Insulin Responses in Healthy Subjects and In Vitro Resistant Starch Content. Eur. J. Clin. Nutr. 1994, 48, 151–163. [Google Scholar]
- Granfeldt, Y.; Eliasson, A.-C.; Björck, I. An Examination of the Possibility of Lowering the Glycemic Index of Oat and Barley Flakes by Minimal Processing. J. Nutr. 2000, 130, 2207–2214. [Google Scholar] [CrossRef]
- Liljeberg, H.G.; Lönner, C.H.; Björck, I.M. Sourdough Fermentation or Addition of Organic Acids or Corresponding Salts to Bread Improves Nutritional Properties of Starch in Healthy Humans. J. Nutr. 1995, 125, 1503–1511. [Google Scholar] [CrossRef]
- Liljeberg, H.; Björck, I. Delayed Gastric Emptying Rate May Explain Improved Glycaemia in Healthy Subjects to a Starchy Meal with Added Vinegar. Eur. J. Clin. Nutr. 1998, 52, 368–371. [Google Scholar] [CrossRef]
- Östman, E.M.; Nilsson, M.; Liljeberg Elmståhl, H.G.M.; Molin, G.; Björck, I.M.E. On the Effect of Lactic Acid on Blood Glucose and Insulin Responses to Cereal Products: Mechanistic Studies in Healthy Subjects and In Vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Galgani, J.; Aguirre, C.; Díaz, E. Acute Effect of Meal Glycemic Index and Glycemic Load on Blood Glucose and Insulin Responses in Humans. Nutr. J. 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Nelson, M. Glycemic Index and Glycemic Load Used in Combination to Characterize Metabolic Responses of Mixed Meals in Healthy Lean Young Adults. J. Am. Coll. Nutr. 2011, 30, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Fabricatore, A.N.; Ebbeling, C.B.; Wadden, T.A.; Ludwig, D.S. Continuous Glucose Monitoring to Assess the Ecologic Validity of Dietary Glycemic Index and Glycemic Load. Am. J. Clin. Nutr. 2011, 94, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Sünram-Lea, S.I.; Gentile-Rapinett, G.; Macé, K.; Rytz, A. Assessment of Glycemic Response to Model Breakfasts Varying in Glycemic Index (GI) in 5–7-Year-Old School Children. Nutrients 2021, 13, 4246. [Google Scholar] [CrossRef]
- Bao, J.; Atkinson, F.; Petocz, P.; Willett, W.C.; Brand-Miller, J.C. Prediction of Postprandial Glycemia and Insulinemia in Lean, Young, Healthy Adults: Glycemic Load Compared with Carbohydrate Content Alone. Am. J. Clin. Nutr. 2011, 93, 984–996. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Thomas, M.; Swan, V.; Ahmad, Z.I.; Petocz, P.; Colagiuri, S. Physiological Validation of the Concept of Glycemic Load in Lean Young Adults. J. Nutr. 2003, 133, 2728–2732. [Google Scholar] [CrossRef]
- Kongkachuichai, R.; Charoensiri, R.; Meekhruerod, A.; Kettawan, A. Effect of Processing Conditions on Bioactive Compounds and Glycemic Index of the Selected Landrace Rice Variety in Pre-Diabetes. J. Cereal Sci. 2020, 94, 102994. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Roundtable on Research and Development of Drugs, Biologics, and Medical Devices. Similarities and Dissimilarities in Physiology, Metabolism, and Disease States and Responses to Therapy in Children and Adults. In Rational Therapeutics for Infants and Children: Workshop Summary; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]


| Glucose | Fructose | Maltose | Sucrose | Lactose | Isomaltu. | Starch | CHO | Fib.Sol. | Fib.Ins. | Fat | Protein | Ash | Moisture | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 | 0.0 | 49.0 | 51.1 | 9.4 | 6.1 | 0.6 | 27.0 | 3.3 | 2.5 |
| A2 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | 50.0 | 52.0 | 8.9 | 6.1 | 0.6 | 25.6 | 3.1 | 3.7 |
| A3 | 0.0 | 0.0 | 3.7 | 0.5 | 0.0 | 0.0 | 70.9 | 75.1 | 4.2 | 3.6 | 0.9 | 10.8 | 1.3 | 4.1 |
| A4 | 0.0 | 0.0 | 4.2 | 0.8 | 0.0 | 0.0 | 70.5 | 75.5 | 4.7 | 2.0 | 0.8 | 11.2 | 1.3 | 4.6 |
| A5 | 0.0 | 0.0 | 4.1 | 0.9 | 0.0 | 0.0 | 70.6 | 75.6 | 4.5 | 2.0 | 0.8 | 11.2 | 1.3 | 4.7 |
| B1 | 12.7 | 2.6 | 6.7 | 0.0 | 12.7 | 0.0 | 33.5 | 68.1 | 3.1 | 2.0 | 7.0 | 15.5 | 2.1 | 2.2 |
| B2 | 1.6 | 0.0 | 1.3 | 10.5 | 13.1 | 0.0 | 40.0 | 66.5 | 3.0 | 2.0 | 9.4 | 15.1 | 2.1 | 1.8 |
| B3 | 1.2 | 3.4 | 6.6 | 1.9 | 6.8 | 5.1 | 36.4 | 61.3 | 5.4 | 3.6 | 14.7 | 11.1 | 2.1 | 1.9 |
| B4 | 0.7 | 0.0 | 7.3 | 7.9 | 7.3 | 4.8 | 34.1 | 62.1 | 5.0 | 3.4 | 14.1 | 11.6 | 2.1 | 1.7 |
| B5 | 0.8 | 0.0 | 6.8 | 10.4 | 8.5 | 0.0 | 37.2 | 63.6 | 5.5 | 3.7 | 11.1 | 12.1 | 2.1 | 1.9 |
| C1 | 11.7 | 0.0 | 8.9 | 0.0 | 13.2 | 0.0 | 33.1 | 66.9 | 1.7 | 1.2 | 8.7 | 16.3 | 3.1 | 2.1 |
| C2 | 0.0 | 0.0 | 1.1 | 12.1 | 12.9 | 0.0 | 39.2 | 65.3 | 2.2 | 1.5 | 9.2 | 14.6 | 2.9 | 4.5 |
| C3 | 0.0 | 0.0 | 1.3 | 11.0 | 9.2 | 0.0 | 39.3 | 60.8 | 3.6 | 2.4 | 11.5 | 14.6 | 2.9 | 4.3 |
| C4 | 0.0 | 0.0 | 1.2 | 6.3 | 9.1 | 0.0 | 44.1 | 60.7 | 3.5 | 2.3 | 12.2 | 15.3 | 2.8 | 3.2 |
| C5 | 0.0 | 0.0 | 1.7 | 0.7 | 8.4 | 0.0 | 47.5 | 58.3 | 4.4 | 2.9 | 12.4 | 16.1 | 2.8 | 3.2 |
| D1 | 0.6 | 0.0 | 1.2 | 10.2 | 0.6 | 0.0 | 44.4 | 57.0 | 8.0 | 4.8 | 12.6 | 14.1 | 2.0 | 1.5 |
| D2 | 0.7 | 0.0 | 1.4 | 9.9 | 7.0 | 0.0 | 39.7 | 58.7 | 6.0 | 4.6 | 12.4 | 14.0 | 2.6 | 1.7 |
| D3 | 0.7 | 0.0 | 1.6 | 9.2 | 7.8 | 0.0 | 39.5 | 58.6 | 6.1 | 4.2 | 12.5 | 14.1 | 2.6 | 1.9 |
| D4 | 0.7 | 0.0 | 1.3 | 9.5 | 9.6 | 0.0 | 38.2 | 59.3 | 4.7 | 3.5 | 13.8 | 13.8 | 2.8 | 2.1 |
| D5 | 0.7 | 0.0 | 1.5 | 9.7 | 12.8 | 0.0 | 34.2 | 59.0 | 6.1 | 3.5 | 12.3 | 13.8 | 3.0 | 2.3 |
| In Vivo Estimates (Mean ± SE) | Model Predictions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2h-iAUC (mmol/L × min) | Measured GI | Measured GL (g/50 g) | eGI | eGL (g/50 g) | |||||
| A1 | 111 ± 9.5 | 65 ± 4.9 | 16.6 ± 1.2 | 70 | 17.8 | ||||
| A2 | 125 ± 10.2 | 71 ± 5.0 | 18.6 ± 1.3 | 71 | 18.5 | ||||
| A3 | 171 ± 14.8 | 91 ± 4.3 | 34.0 ± 1.6 | 89 | 33.3 | ||||
| A4 | 154 ± 13.3 | 85 ± 5.4 | 32.0 ± 2.0 | 88 | 33.4 | ||||
| A5 | 152 ± 10.3 | 83 ± 4.1 | 31.2 ± 1.6 | 89 | 33.4 | ||||
| B1 | 177 ± 14.5 | 72 | 24.4 | ||||||
| B2 | 148 ± 12.1 | 67 | 22.2 | ||||||
| B3 | 130 ± 10.5 | 64 | 19.7 | ||||||
| B4 | 132 ± 15.7 | 65 | 20.3 | ||||||
| B5 | 151 ± 13.2 | 69 | 22.0 | ||||||
| C1 | 210 ± 20.2 | 73 ± 4.2 | 24.4 ± 1.4 | 72 | 24.2 | ||||
| C2 | 177 ± 12.5 | 67 ± 4.9 | 21.8 ± 1.6 | 66 | 21.7 | ||||
| C3 | 185 ± 17.1 | 72 ± 5.3 | 22.0 ± 1.6 | 66 | 20.0 | ||||
| C4 | 176 ± 12.5 | 69 ± 3.4 | 21.0 ± 1.0 | 67 | 20.5 | ||||
| C5 | 174 ± 12.0 | 72 ± 4.3 | 21.1 ± 1.3 | 69 | 20.1 | ||||
| D1 | 193 ± 18.0 | 73 ± 5.1 | 20.9 ± 1.4 | 69 | 19.6 | ||||
| D2 | 177 ± 12.8 | 68 ± 4.3 | 19.8 ± 1.3 | 66 | 19.4 | ||||
| D3 | 176 ± 14.1 | 66 ± 4.7 | 19.4 ± 1.4 | 66 | 19.3 | ||||
| D4 | 164 ± 12.5 | 62 ± 4.7 | 18.4 ± 1.4 | 65 | 19.1 | ||||
| D5 | 167 ± 17.0 | 62 ± 4.3 | 18.4 ± 1.3 | 63 | 18.4 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monnard, C.; Rytz, A.; Tudorica, C.M.; Fiore, G.L.; Do, T.A.L.; Bhaskaran, K.; Macé, K.; Shahkhalili, Y. Nutritional Composition of Infant Cereal Prototypes Can Precisely Predict Their Glycemic Index. Nutrients 2022, 14, 3702. https://doi.org/10.3390/nu14183702
Monnard C, Rytz A, Tudorica CM, Fiore GL, Do TAL, Bhaskaran K, Macé K, Shahkhalili Y. Nutritional Composition of Infant Cereal Prototypes Can Precisely Predict Their Glycemic Index. Nutrients. 2022; 14(18):3702. https://doi.org/10.3390/nu14183702
Chicago/Turabian StyleMonnard, Cathriona, Andreas Rytz, Carmen Mirela Tudorica, Gina L. Fiore, Tram Anh Line Do, Kalpana Bhaskaran, Katherine Macé, and Yasaman Shahkhalili. 2022. "Nutritional Composition of Infant Cereal Prototypes Can Precisely Predict Their Glycemic Index" Nutrients 14, no. 18: 3702. https://doi.org/10.3390/nu14183702
APA StyleMonnard, C., Rytz, A., Tudorica, C. M., Fiore, G. L., Do, T. A. L., Bhaskaran, K., Macé, K., & Shahkhalili, Y. (2022). Nutritional Composition of Infant Cereal Prototypes Can Precisely Predict Their Glycemic Index. Nutrients, 14(18), 3702. https://doi.org/10.3390/nu14183702






