High-Dose Nitrate Supplementation Attenuates the Increased Blood Pressure Responses to Isometric Blood Flow Restriction Exercise in Healthy Males
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Supplementation Procedures
2.4. Measurement
2.5. Statistical Analysis
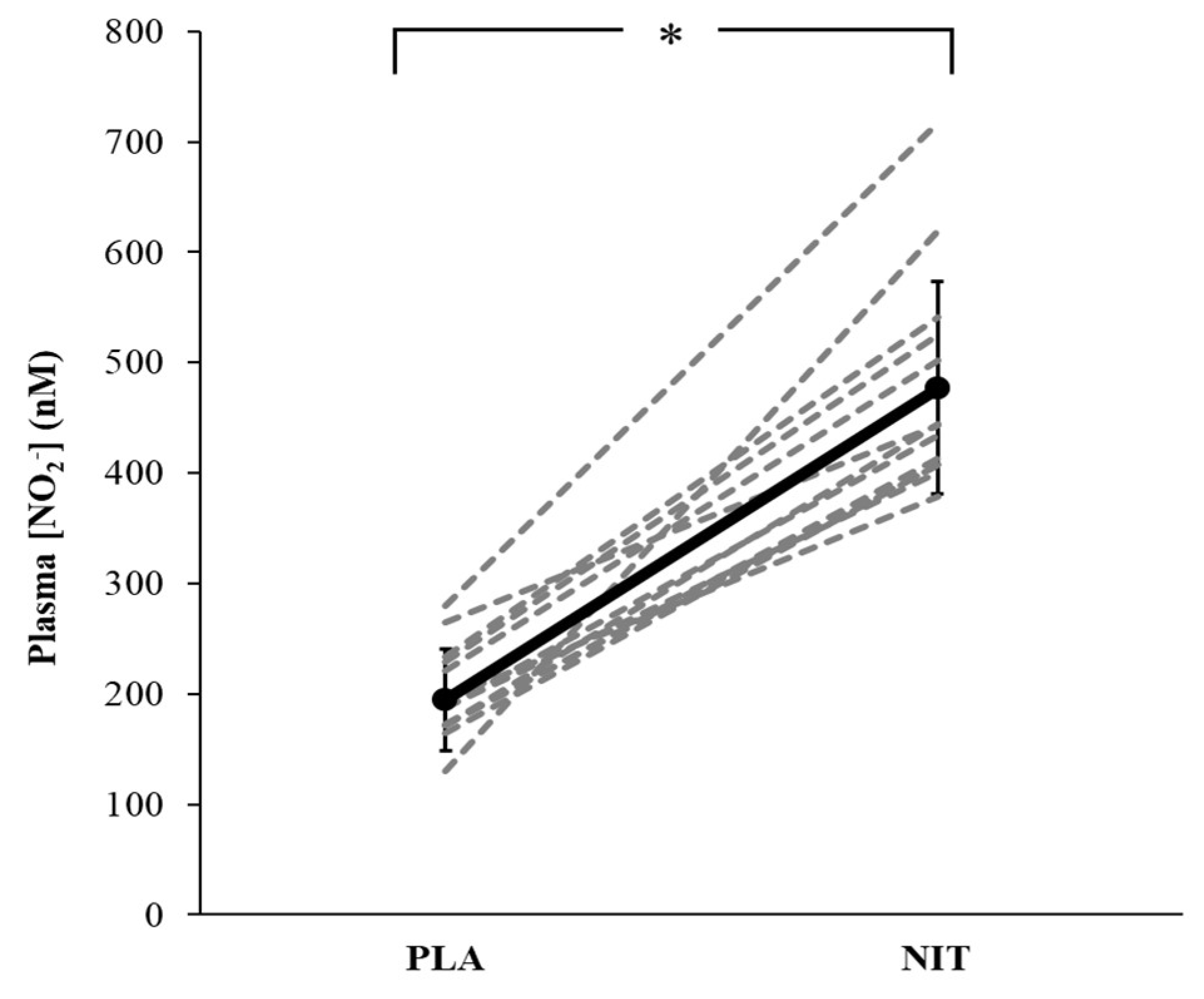
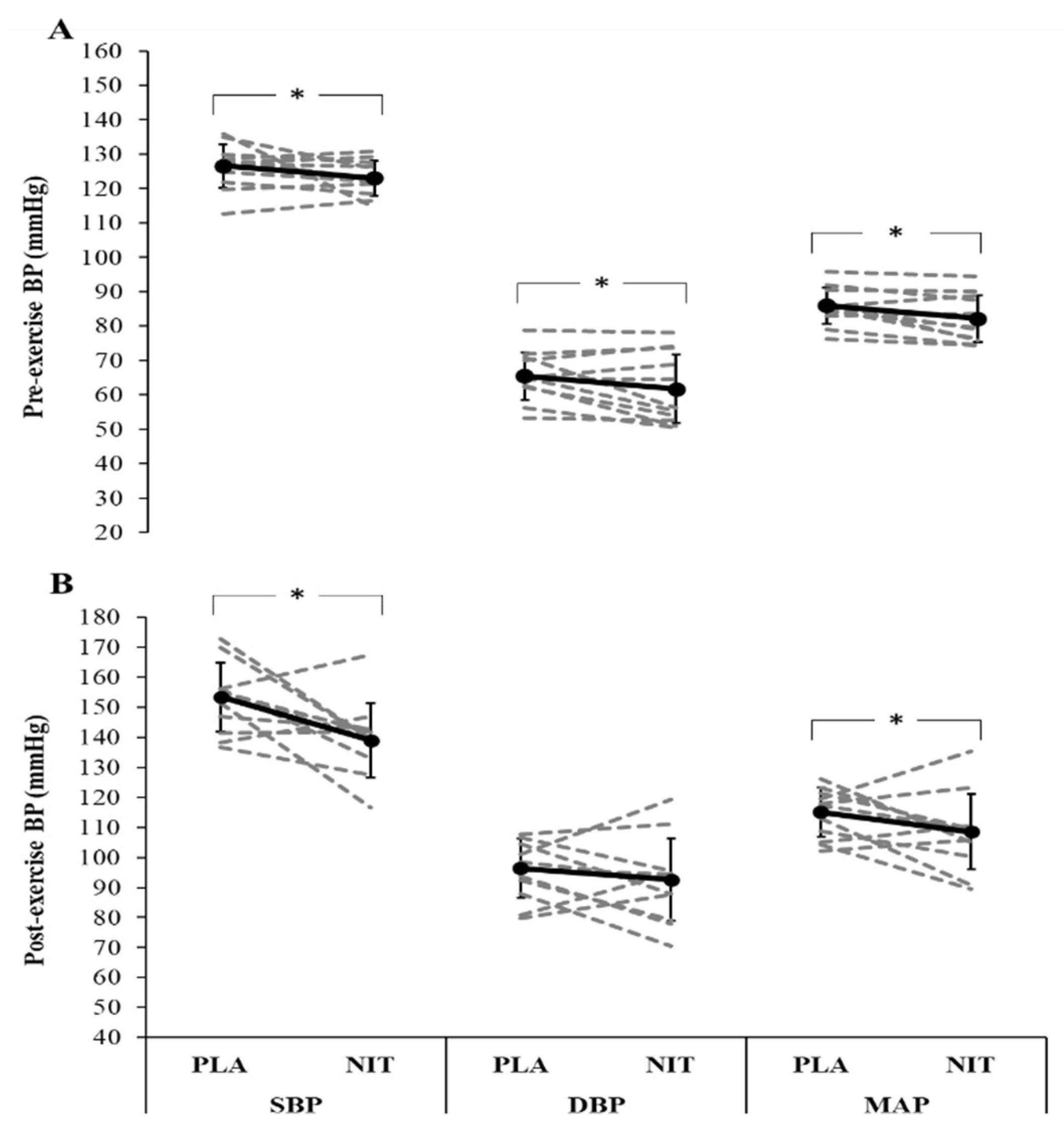
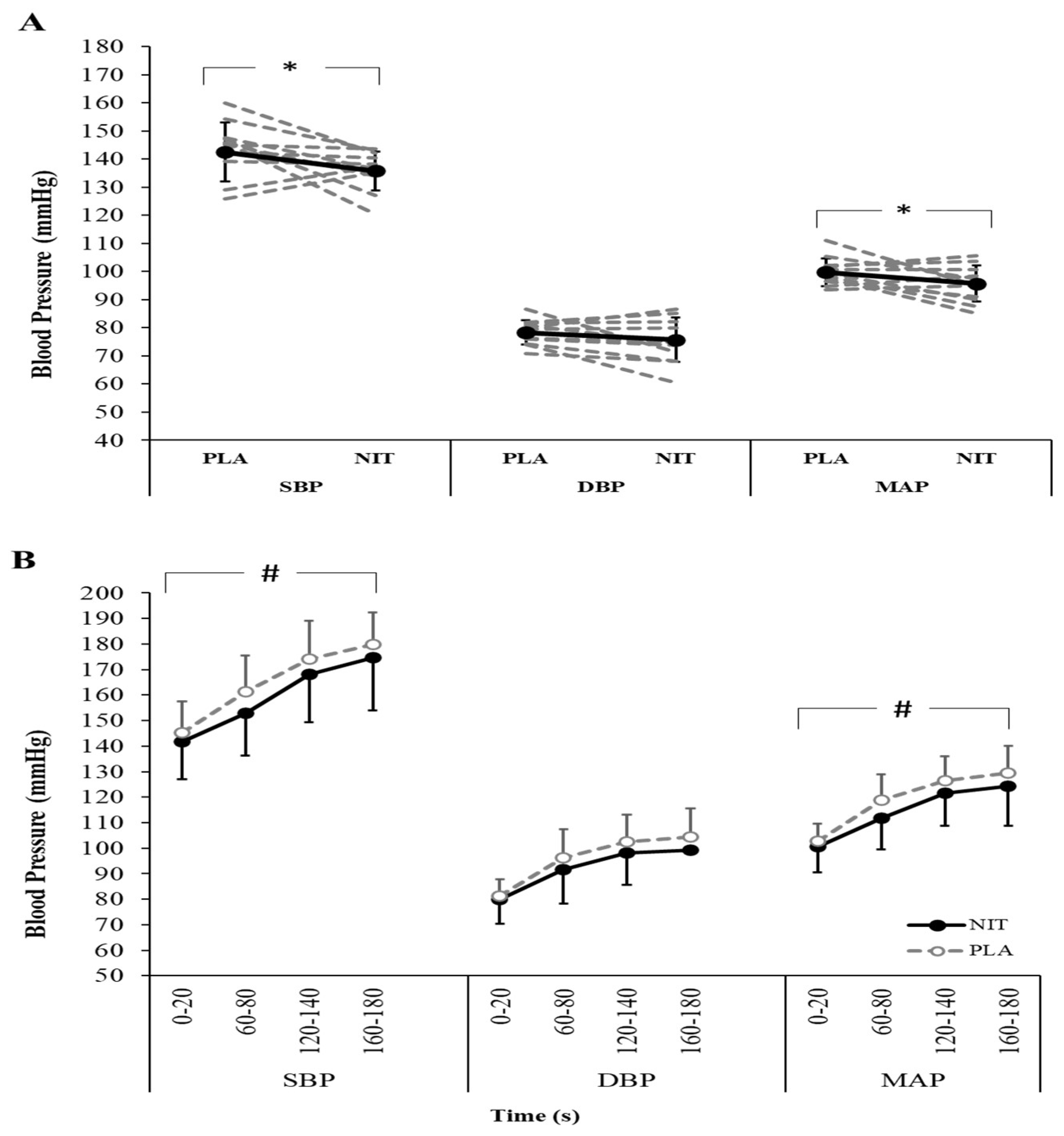
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Esen, O.; Domínguez, R.; Karayigit, R. Acute Beetroot Juice Supplementation Enhances Intermittent Running Performance but Does Not Reduce Oxygen Cost of Exercise among Recreational Adults. Nutrients 2022, 14, 2839. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef]
- Hirst, D.G.; Robson, T. Nitric Oxide Physiology and Pathology; Humana Press: Clifton, NJ, USA, 2011. [Google Scholar]
- Lundberg, J.O.; Carlstrӧm, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef]
- Reutov, V.P. Nitric oxide cycle in mammals and the cyclicity principle. Biochemistry 2002, 67, 293–311. [Google Scholar]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Lundberg, J.O.; Weitzberg, E. NO generation from inorganic nitrate and nitrite: Role in physiology, nutrition and therapeutics. Arch. Pharmacal Res. 2009, 32, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Meyer, C.; Totzeck, M.; Hendgen-Cotta, U.B.; Heinen, Y.; Luedike, P.; Keymel, S.; Ayoub, N.; Lundberg, J.O.; Weitzberg, E.; et al. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radic. Biol. Med. 2012, 52, 1767–1772. [Google Scholar] [CrossRef]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Erm_dis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Loscalzo, J. Vascular nitric oxide: Formation and function. J. Blood Med. 2010, 1, 147. [Google Scholar]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Ahluwalia, A. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef]
- Kapil, V.; Rathod, K.S.; Khambata, R.S.; Bahra, M.; Velmurugan, S.; Purba, A.; Watson, D.S.; Barnes, M.R.; Wade, W.G.; Ahluwalia, A. Sex differences in the nitrate-nitrite-NO pathway: Role of oral nitrate-reducing bacteria. Free. Radic. Biol. Med. 2018, 126, 113–121. [Google Scholar] [CrossRef]
- Esen, O.; Nicholas, C.; Morris, M.; Bailey, S.J. No effect of beetroot juice supplementation on 100-m and 200-m swimming performance in moderately trained swimmers. Int. J. Sports Physiol. Perform. 2019, 14, 706–710. [Google Scholar] [CrossRef]
- Bond, V.; Curry, B.H.; Adams, R.G.; Asadi, M.S.; Stancil, K.A.; Millis, R.M.; Haddad, G.E. Effects of nitrate supplementation on cardiovascular and autonomic reactivity in African-American females. Int. Sch. Res. Not. 2014, 23, 676235. [Google Scholar] [CrossRef]
- Lee, J.S.; Stebbins, C.L.; Jung, E.; Nho, H.; Kim, J.K.; Chang, M.J.; Choi, H.M. Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. Am. J. Physiol. Regul. Integr Comp. Physiol. 2015, 309, R459–R466. [Google Scholar] [CrossRef] [PubMed]
- Zafeiridis, A.; Triantafyllou, A.; Papadopoulos, S.; Koletsos, N.; Touplikioti, P.; Zafeiridis, A.S.; Gkaliagkousi, E.; Dipla, K.; Douma, S. Dietary nitrate improves muscle microvascular reactivity and lowers blood pressure at rest and during isometric exercise in untreated hypertensives. Microcirculation 2019, 26, 12525. [Google Scholar] [CrossRef] [PubMed]
- Notay, K.; Incognito, A.V.; Millar, P.J. Acute beetroot juice supplementation on sympathetic nerve activity: A randomized, double-blind, placebo-controlled proof-of- concept study. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H59–H65. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.D.; Victor, R.G. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J. Physiol. 1998, 506, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Jendzjowsky, N.G.; DeLorey, D.S. Short-term exercise training augments 2-adrenoreceptor-mediated sympathetic vasoconstriction in resting and contracting skeletal muscle. J. Physiol. 2013, 591, 5221–5233. [Google Scholar] [CrossRef] [PubMed]
- Jendzjowsky, N.G.; DeLorey, D.S. Short-term exercise training enhances functional sympatholysis through a nitric oxide-dependent mechanism. J. Physiol. 2013, 591, 1535–1549. [Google Scholar] [CrossRef]
- Jendzjowsky, N.G.; Just, T.P.; DeLorey, D.S. Exercise training augments neuronal nitric oxide synthase-mediated inhibition of sympathetic vasoconstriction in contracting skeletal muscle of rats. J. Physiol. 2014, 592, 4789–4802. [Google Scholar] [CrossRef]
- Modin, A.; Bjorne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J.O. Nitrite-derived nitric oxide: A possible mediator of ‘acidicmetabolic’ vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar] [CrossRef]
- Castello, P.R.; David, P.S.; McClure, T.; Crook, Z.R.; Poyton, R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006, 3, 277–287. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.G.; Fulford, J.; Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of walking and running: A placebo-controlled study. J. Appl. Physiol. 2011, 110, 591–600. [Google Scholar] [CrossRef]
- Jones, D.A.; Turner, D.L.; McIntyre, D.B.; Newham, D.J. Energy turnover in relation to slowing of contractile properties during fatiguing contractions of the human anterior tibialis muscle. J. Physiol. 2009, 587, 4329–4338. [Google Scholar] [CrossRef]
- Esen, O.; Faisal, A.; Zambolin, F.; Bailey, S.J.; Callaghan, M.J. Effect of nitrate supplementation on skeletal muscle motor unit activity during isometric blood flow restriction exercise. Eur. J. Appl. Physiol. 2022, 122, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef]
- Senefeld, J.W.; Wiggins, C.C.; Regimbal, R.J.; Dominelli, P.B.; Baker, S.E.; Joyner, M.J. Ergogenic effect of nitrate supplementation: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2020, 52, 2250. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Baranauskas, M.N.; Hinrichs, R.J.; Liu, Z.; Carter, S.J. Effect of dietary nitrate on human muscle power: A systematic review and individual participant data meta-analysis. J. Int. Soc. Sports Nutr. 2021, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J. IOC Medical and Scientific Commission reviews its position on the use of dietary supplements by elite athletes. Br. J. Sports Med. 2018, 52, 418–419. [Google Scholar] [CrossRef]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef]
- Faisal, A.; Dyson, K.S.; Hughson, R.L. Prolonged ischaemia impairs muscle blood flow and oxygen uptake dynamics during subsequent heavy exercise. J. Physiol. 2010, 588, 3785–3797. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates Hillsdale: Routledge, NJ, USA, 1988. [Google Scholar]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Carlström, M.; Lundberg, J.O.; Weitzberg, E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol. 2018, 224, e13080. [Google Scholar] [CrossRef]
- de Vries, C.J.; DeLorey, D.S. Effect of acute dietary nitrate supplementation on sympathetic vasoconstriction at rest and during exercise. J. Appl. Physiol. 2019, 127, 81–88. [Google Scholar] [CrossRef]
- Ghosh, S.M.; Kapil, V.; Fuentes-Calvo, I.; Bubb, K.J.; Pearl, V.; Milsom, A.B.; Ahluwalia, A. Enhanced vasodilator activity of nitrite in hypertension: Critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension 2013, 61, 1091–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahadoran, Z.; Mirmiran, P.; Kabir, A.; Azizi, F.; Ghasemi, A. The nitrate-independent blood pressure-lowering effect of beetroot juice: A systematic review and meta-analysis. Adv. Nutr. 2017, 8, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Polito, M.D.; Farinatti, P.T. The effects of muscle mass and number of sets during resistance exercise on postexercise hypotension. J. Strength Cond. Res. 2009, 23, 2351–2357. [Google Scholar] [CrossRef]
- Le Roux-Mallouf, T.; Vibert, F.; Doutreleau, S.; Verges, S. Effect of acute nitrate and citrulline supplementation on muscle microvascular response to ischemia-reperfusion in healthy humans. Appl. Physiol. Nutr. Metab. 2017, 42, 901–908. [Google Scholar] [CrossRef]
- Le Roux-Mallouf, T.; Laurent, J.; Besset, D.; Marillier, M.; Larribaut, J.; Belaidi, E.; Verges, S. Effects of acute nitric oxide precursor intake on peripheral and central fatigue during knee extensions in healthy men. Exp. Physiol. 2019, 104, 1100–1114. [Google Scholar] [CrossRef]
- Porcelli, S.; Ramaglia, M.; Bellistri, G.; Pavei, G.; Pugliese, L.; Montorsi, M.; Marzorati, M. Aerobic fitness affects the exercise performance responses to nitrate supplementation. Med. Sci. Sports Exerc. 2015, 47, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, S.M.; Vaandrager, A.B.; Smolenski, A.; Walter, U.; De Jonge, H.R. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem. Sci. 1997, 22, 307–312. [Google Scholar] [CrossRef]
- Hijmering, M.L.; Stroes, E.S.; Olijhoek, J.; Hutten, B.A.; Blankestijn, P.J.; Rabelink, T.J. Sympathetic activation markedly reduces endotheliumdependent, flow-mediated vasodilation. J. Am. Coll. Cardiol. 2002, 39, 683–688. [Google Scholar] [CrossRef]
- Joyner, M.J.; Charkoudian, N.; Wallin, B.G. Sympathetic nervous system and blood pressure in humans: Individualized patterns of regulation and their implications. Hypertension 2010, 56, 10–16. [Google Scholar] [CrossRef]
- Owlya, R.; Vollenweider, L.; Trueb, L.; Sartori, C.; Lepori, M.; Nicod, P.; Scherrer, U. Cardiovascular and sympathetic effects of nitric oxide inhibition at rest and during static exercise in humans. Circulation 1997, 96, 3897–3903. [Google Scholar] [CrossRef] [PubMed]
- Young, C.N.; Fisher, J.P.; Gallagher, K.M.; Whaley-Connell, A.; Chaudhary, K.; Victor, R.G.; Thomas, G.D.; Fadel, P.J. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J. Physiol. 2009, 587, 4977–4986. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esen, O.; Cepicka, L.; Gabrys, T.; Karayigit, R. High-Dose Nitrate Supplementation Attenuates the Increased Blood Pressure Responses to Isometric Blood Flow Restriction Exercise in Healthy Males. Nutrients 2022, 14, 3645. https://doi.org/10.3390/nu14173645
Esen O, Cepicka L, Gabrys T, Karayigit R. High-Dose Nitrate Supplementation Attenuates the Increased Blood Pressure Responses to Isometric Blood Flow Restriction Exercise in Healthy Males. Nutrients. 2022; 14(17):3645. https://doi.org/10.3390/nu14173645
Chicago/Turabian StyleEsen, Ozcan, Ladislav Cepicka, Tomasz Gabrys, and Raci Karayigit. 2022. "High-Dose Nitrate Supplementation Attenuates the Increased Blood Pressure Responses to Isometric Blood Flow Restriction Exercise in Healthy Males" Nutrients 14, no. 17: 3645. https://doi.org/10.3390/nu14173645
APA StyleEsen, O., Cepicka, L., Gabrys, T., & Karayigit, R. (2022). High-Dose Nitrate Supplementation Attenuates the Increased Blood Pressure Responses to Isometric Blood Flow Restriction Exercise in Healthy Males. Nutrients, 14(17), 3645. https://doi.org/10.3390/nu14173645






