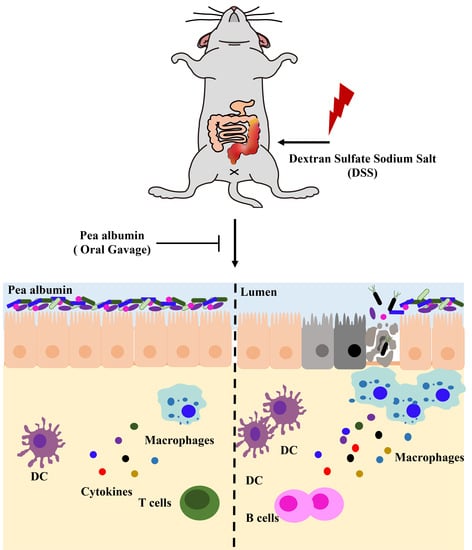
Pea Albumin Attenuates Dextran Sulfate Sodium-Induced Colitis by Regulating NF-κB Signaling and the Intestinal Microbiota in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Animals
2.3. Preparation of Pea Albumin
2.4. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis
2.5. Composition Analysis of Pea Albumin
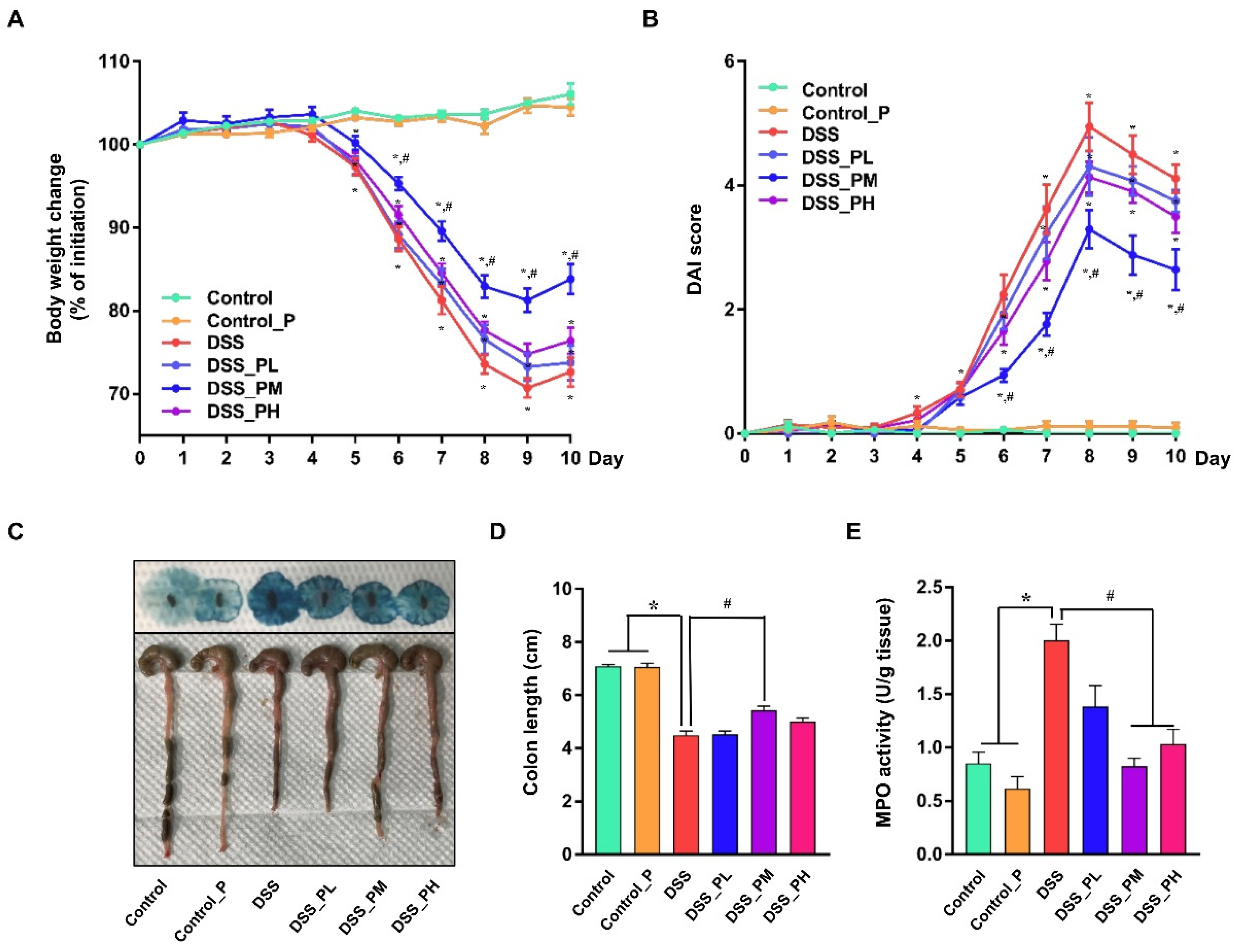
2.6. Disease Activity Index Assessment and Colon Length
2.7. Colonic Myeloperoxidase Activity
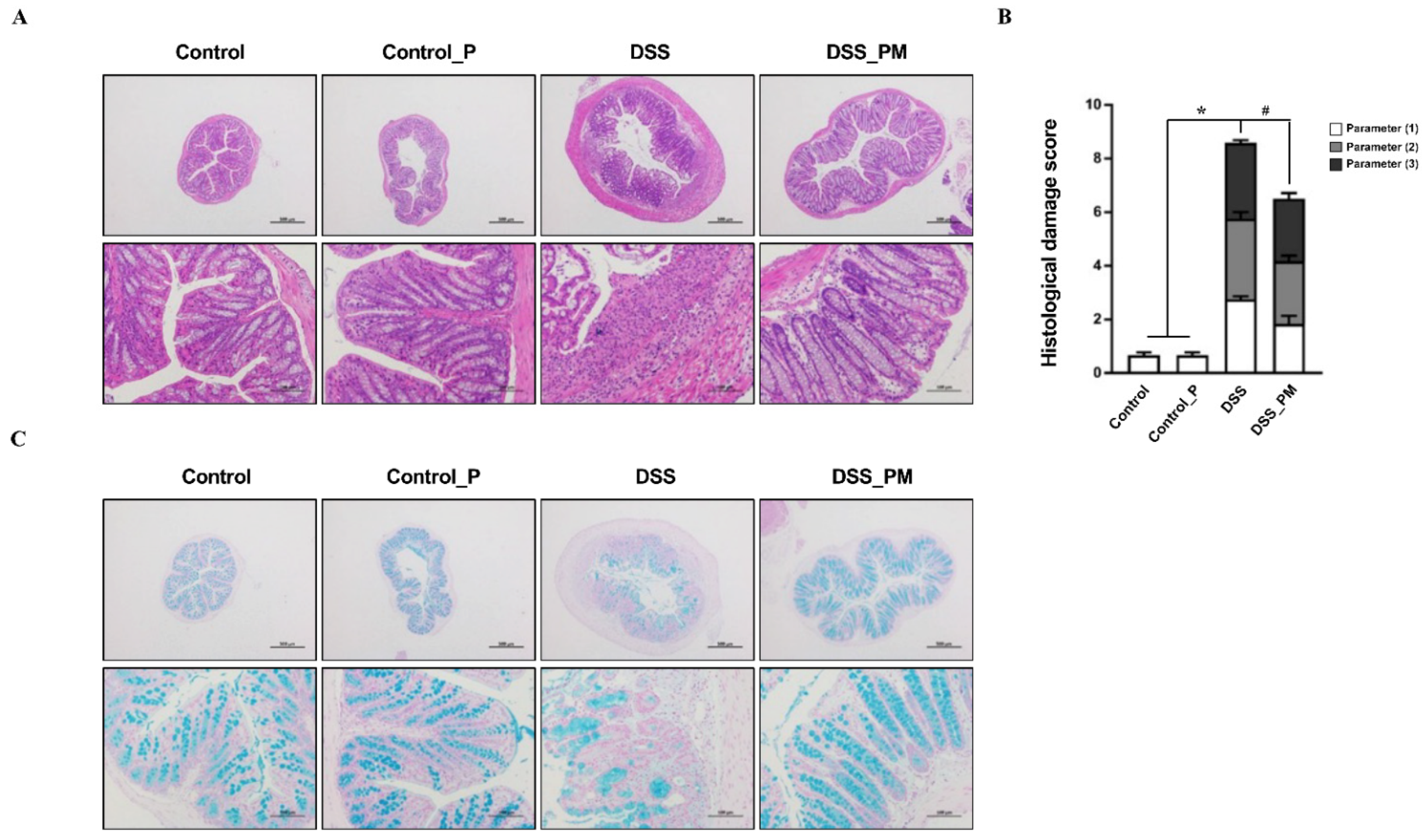
2.8. Histopathological Analyses
2.9. Immunofluorescence Staining
2.10. Detection of IL-6, IL-10, IL-17, and IL-22
2.11. Quantitative Real-Time PCR
2.12. Western Blot Analysis
2.13. Gut Microbial Analysis
2.14. Statistical Analysis
3. Results
3.1. Composition of Pea Albumin
3.2. Pea Albumin Alleviated DSS-Induced Colitis
3.3. Pea Albumin Attenuated Inflammatory Cell Infiltration in Colon Tissue
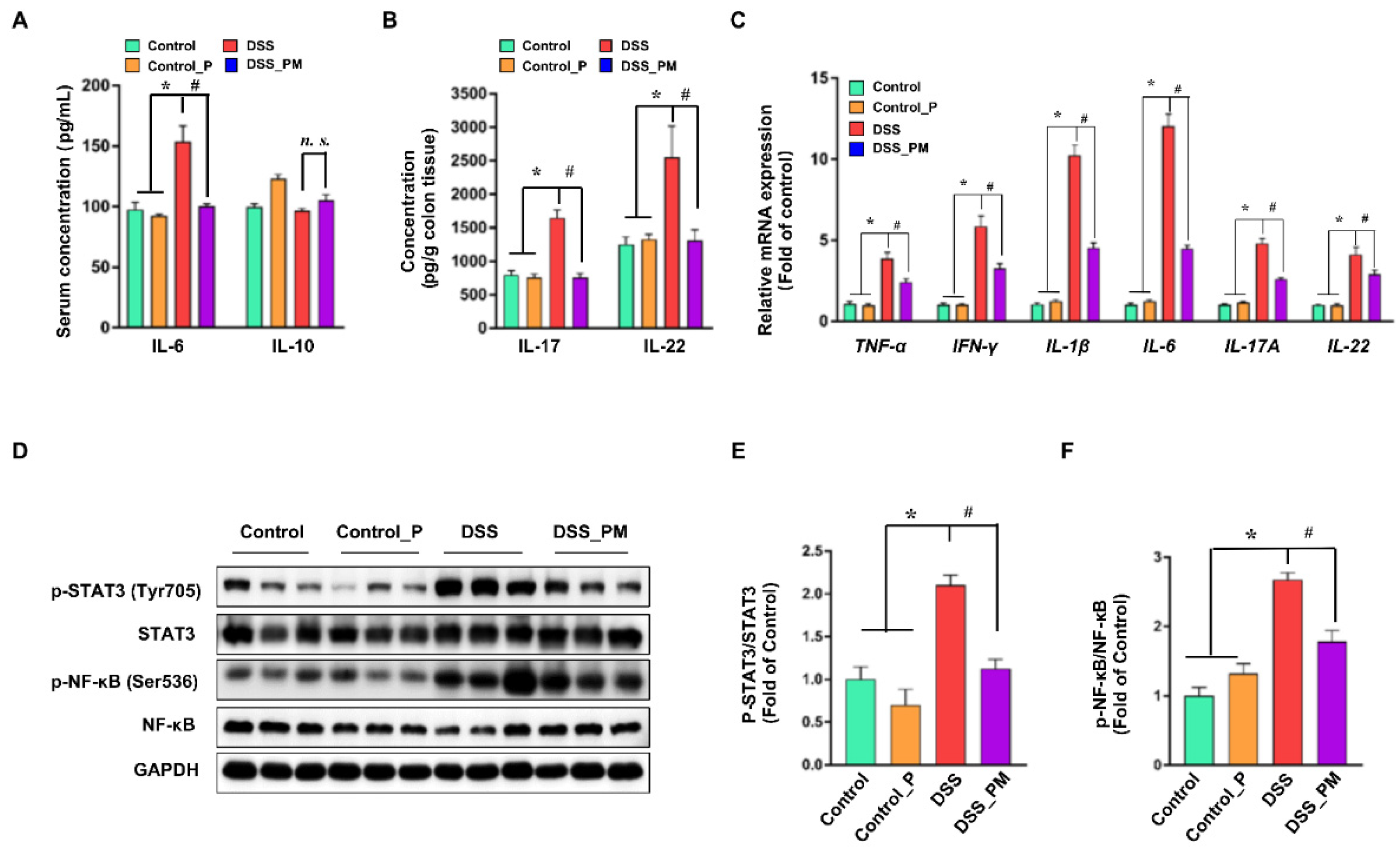
3.4. Pea Albumin Ameliorated DSS-Induced Secretion of Inflammatory Cytokines and Overactivation of STAT3 and NF-κB
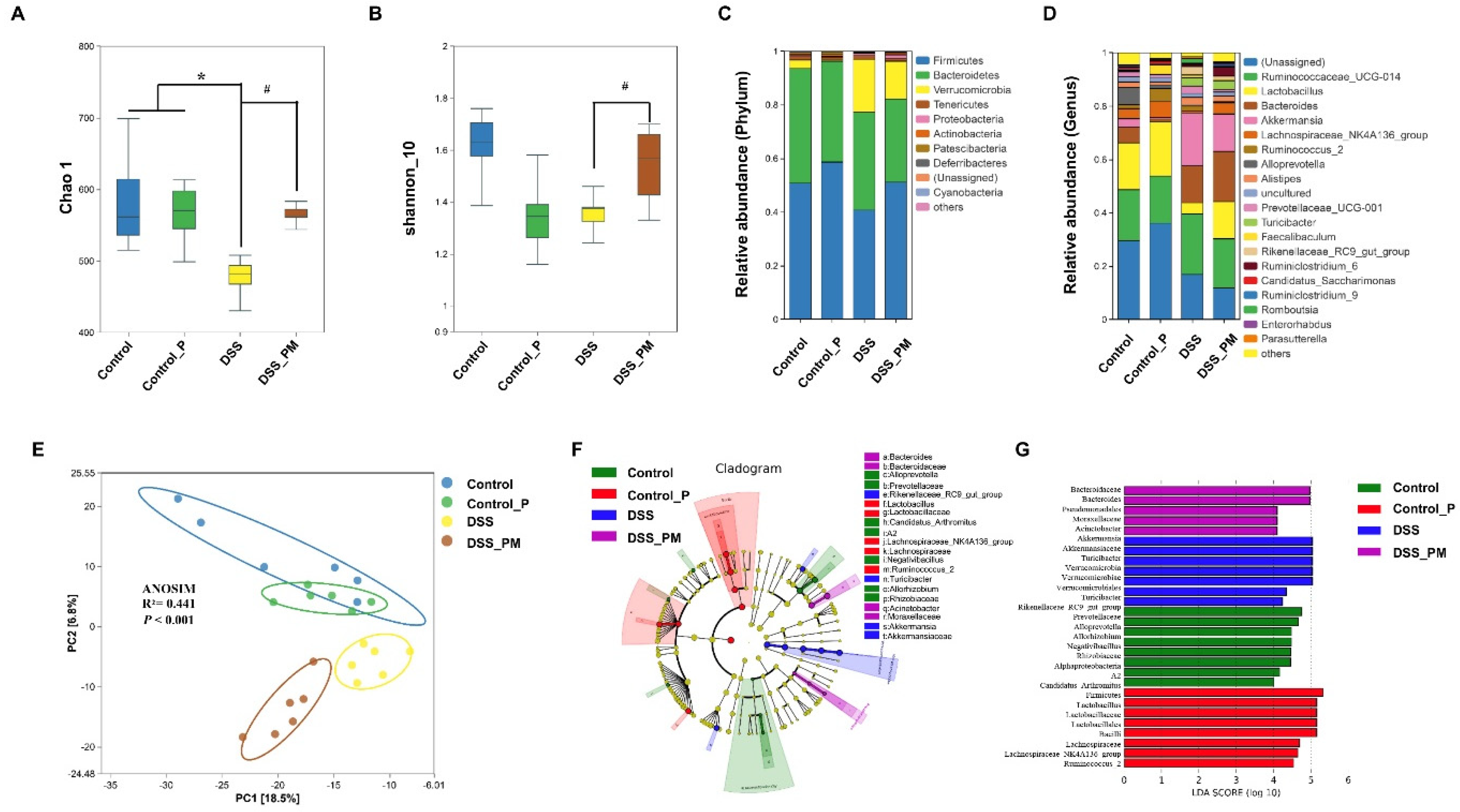
3.5. Pea Albumin Restored Gut Microbiota Community
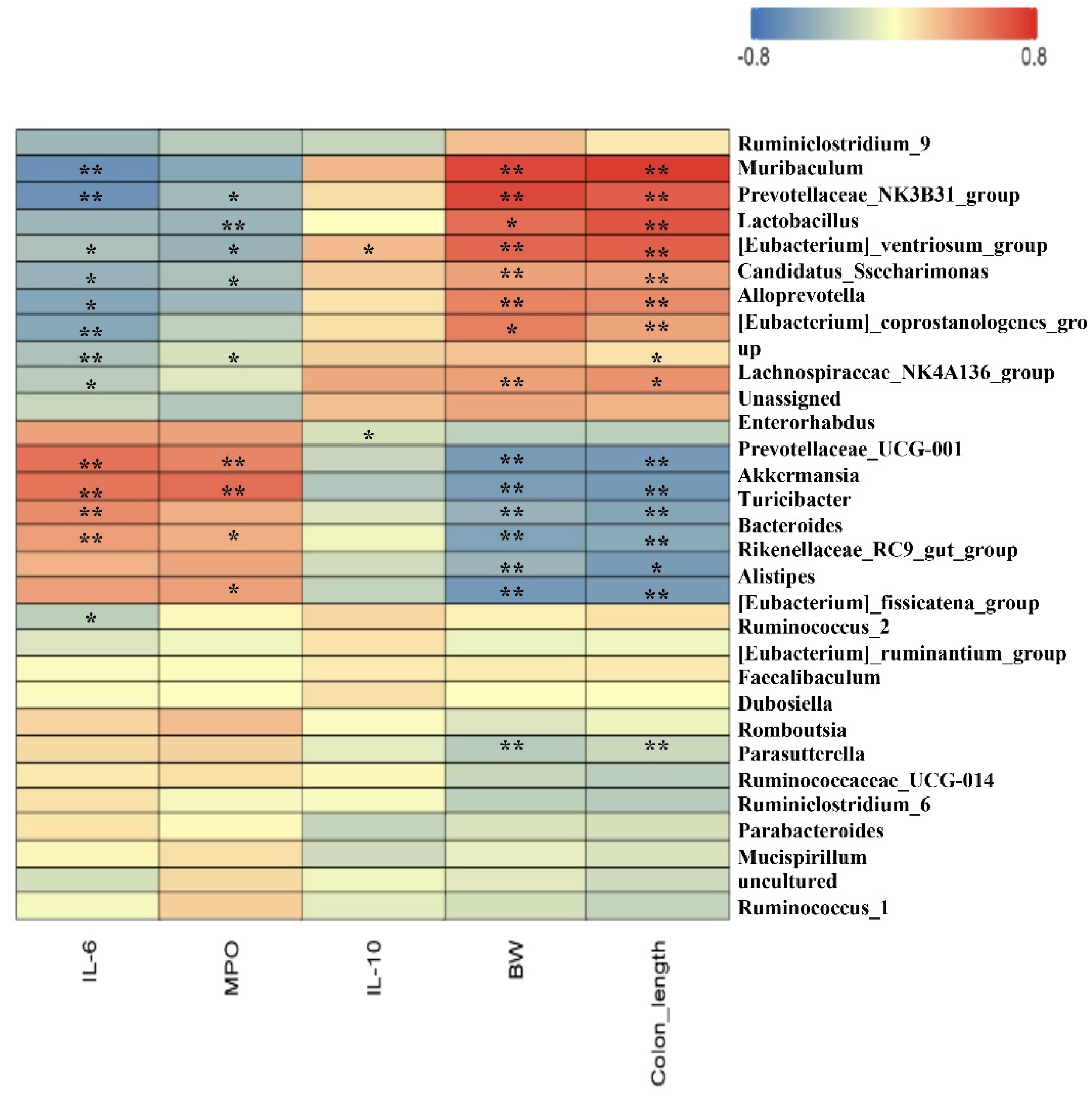
3.6. Spearman Correlation between Intestinal Microbiota and Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nature reviews. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Park, J.H.; Peyrin-Biroulet, L.; Eisenhut, M.; Shin, J.I. IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun. Rev. 2017, 16, 416–426. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kim, D.H.; Cheon, J.H. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef]
- Si, X.; Jia, H.; Liu, N.; Li, J.; Pan, L.; Wang, J.; Wu, Z. Alpha-Ketoglutarate Attenuates Colitis in Mice by Increasing Lactobacillus Abundance and Regulating Stem Cell Proliferation via Wnt-Hippo Signaling. Mol. Nutr. Food Res. 2022, 66, e2100955. [Google Scholar] [CrossRef]
- Perler, B.K.; Ungaro, R.; Baird, G.; Mallette, M.; Bright, R.; Shah, S.; Shapiro, J.; Sands, B.E. Presenting symptoms in inflammatory bowel disease: Descriptive analysis of a community-based inception cohort. BMC Gastroenterol. 2019, 19, 47. [Google Scholar] [CrossRef]
- Si, X.; Liu, N.; Jia, H.; Wang, J.; Pan, L.; Dong, L.; Rong, Z.; Yang, Y.; Wu, Z. Gut relief formula attenuates dextran sulfate sodium-induced colitis by regulating NF-kappaB signaling and the intestinal microbiota in mice. Food Funct. 2021, 12, 10983–10993. [Google Scholar] [CrossRef]
- Akanda, M.R.; Nam, H.H.; Tian, W.; Islam, A.; Choo, B.K.; Park, B.Y. Regulation of JAK2/STAT3 and NF-kappaB signal transduction pathways; Veronica polita alleviates dextran sulfate sodium-induced murine colitis. Biomed. Pharmacother. 2018, 100, 296–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, D.; Jin, Y.; Jia, H.; Yang, Y.; Kim, I.H.; Dai, Z.; Zhang, J.; Ren, F.; Wu, Z. Glycine Attenuates Citrobacter rodentium-Induced Colitis by Regulating ATF6-Mediated Endoplasmic Reticulum Stress in Mice. Mol. Nutr. Food Res. 2021, 65, e2001065. [Google Scholar] [CrossRef]
- Pigneur, B.; Ruemmele, F.M. Nutritional interventions for the treatment of IBD: Current evidence and controversies. Therap. Adv. Gastroenterol. 2019, 12, 1756284819890534. [Google Scholar] [CrossRef]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef]
- Blachier, F.; Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Audebert, M.; Khodorova, N.; Andriamihaja, M.; Airinei, G.; Benamouzig, R.; et al. High-protein diets for weight management: Interactions with the intestinal microbiota and consequences for gut health. A position paper by the my new gut study group. Clin. Nutr. 2019, 38, 1012–1022. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Arroyo Hornero, R.; Hamad, I.; Corte-Real, B.; Kleinewietfeld, M. The Impact of Dietary Components on Regulatory T Cells and Disease. Front. Immunol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Gill, P.A.; Inniss, S.; Kumagai, T.; Rahman, F.Z.; Smith, A.M. The Role of Diet and Gut Microbiota in Regulating Gastrointestinal and Inflammatory Disease. Front. Immunol. 2022, 13, 866059. [Google Scholar] [CrossRef]
- Utrilla, M.P.; Peinado, M.J.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Rodriguez-Cabezas, M.E.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol. Nutr. Food Res. 2015, 59, 807–819. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Deren, J.J.; Katz, S.; Lewis, J.D.; Kennedy, A.R.; Ware, J.H. Bowman-Birk inhibitor concentrate: A novel therapeutic agent for patients with active ulcerative colitis. Dig. Dis. Sci. 2008, 53, 175–180. [Google Scholar] [CrossRef]
- Croy, R.R.; Hoque, M.S.; Gatehouse, J.A.; Boulter, D. The major albumin proteins from pea (Pisum sativum L). Purification and some properties. Biochem. J. 1984, 218, 795–803. [Google Scholar] [CrossRef]
- Rubio, L.A.; Perez, A.; Ruiz, R.; Guzman, M.A.; Aranda-Olmedo, I.; Clemente, A. Characterization of pea (Pisum sativum) seed protein fractions. J. Sci. Food Agric. 2014, 94, 280–287. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Selective Complex Coacervation of Pea Whey Proteins with Chitosan To Purify Main 2S Albumins. J. Agric. Food Chem. 2020, 68, 1698–1706. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Kataoka, T.; Yamato, K.; Taguchi, T.; Yamaoka, K. Suppression of dextran sulfate sodium-induced colitis in mice by radon inhalation. Mediat. Inflamm. 2012, 2012, 239617. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, T.W.; Baik, B.K. Relationship between proportion and composition of albumins, and in vitro protein digestibility of raw and cooked pea seeds (Pisum sativum L.). J. Sci. Food Agric. 2010, 90, 1719–1725. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Tong, L.T.; Xiao, T.; Wang, L.; Lu, C.; Liu, L.; Zhou, X.; Wang, A.; Qin, W.; Wang, F. Plant protein reduces serum cholesterol levels in hypercholesterolemia hamsters by modulating the compositions of gut microbiota and metabolites. iScience 2021, 24, 103435. [Google Scholar] [CrossRef]
- Morita, T.; Oh-hashi, A.; Takei, K.; Ikai, M.; Kasaoka, S.; Kiriyama, S. Cholesterol-lowering effects of soybean, potato and rice proteins depend on their low methionine contents in rats fed a cholesterol-free purified diet. J. Nutr. 1997, 127, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Rigamonti, E.; Parolini, C.; Marchesi, M.; Diani, E.; Brambilla, S.; Sirtori, C.R.; Chiesa, G. Hypolipidemic effect of dietary pea proteins: Impact on genes regulating hepatic lipid metabolism. Mol. Nutr. Food Res. 2010, 54 (Suppl. S1), S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Han, S.I.; Won, Y.J.; Lee, E.; Park, E.; Hwang, S.Y.; Yeum, K.J. Black Rice with Giant Embryo Attenuates Obesity-Associated Metabolic Disorders in ob/ob Mice. J. Agric. Food Chem. 2016, 64, 2492–2497. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.H.; Wan, X.S.; Newberne, P.; Kennedy, A.R. Bowman-Birk inhibitor concentrate reduces colon inflammation in mice with dextran sulfate sodium-induced ulcerative colitis. Dig. Dis. Sci. 1999, 44, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Yu, H.; Wang, D.; Zhang, H.; Zhang, B.; Quezada, H.C.; Zhu, J.; Zhu, W. Bowman-Birk inhibitor concentrate suppresses experimental autoimmune neuritis via shifting macrophages from M1 to M2 subtype. Immunol. Lett. 2016, 171, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Leon-Coria, A.; Kumar, M.; Chadee, K. The delicate balance between Entamoeba histolytica, mucus and microbiota. Gut Microbes 2020, 11, 118–125. [Google Scholar] [CrossRef]
- Liu, C.; Huang, S.; Wu, Z.; Li, T.; Li, N.; Zhang, B.; Han, D.; Wang, S.; Zhao, J.; Wang, J. Cohousing-mediated microbiota transfer from milk bioactive components-dosed mice ameliorate colitis by remodeling colonic mucus barrier and lamina propria macrophages. Gut Microbes 2021, 13, 1–23. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning from Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Sommer, K.; Wiendl, M.; Muller, T.M.; Heidbreder, K.; Voskens, C.; Neurath, M.F.; Zundler, S. Intestinal Mucosal Wound Healing and Barrier Integrity in IBD-Crosstalk and Trafficking of Cellular Players. Front. Med. 2021, 8, 643973. [Google Scholar] [CrossRef]
- Wen, J.; Yang, P.R.; Chen, X.J.; Fang, Y.; Chang, Q.; Li, C.H.; Zhang, C.J. The role of Th17/Treg balance and Th22 cell in the pathogenesis of DSS-induced colitis in mice. Eur. J. Inflamm. 2015, 13, 101–108. [Google Scholar] [CrossRef]
- Zindl, C.L.; Lai, J.F.; Lee, Y.K.; Maynard, C.L.; Harbour, S.N.; Ouyang, W.; Chaplin, D.D.; Weaver, C.T. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 12768–12773. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Dai, Z.; Sun, S.; Ma, X.; Yang, Y.; Tso, P.; Wu, G.; Wu, Z. Hydroxyproline Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice: Involvment of the NF-kappaB Signaling and Oxidative Stress. Mol. Nutr. Food Res. 2018, 62, e1800494. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, S.; Sonnichsen, F.D.; Polte, T. Therapeutic potential of the peptide leucine arginine as a new nonplant bowman-birk-like serine protease inhibitor. J. Med. Chem. 2013, 56, 6732–6744. [Google Scholar] [CrossRef] [PubMed]
- Bollrath, J.; Greten, F.R. IKK/NF-kappaB and STAT3 pathways: Central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009, 10, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, S.; Liu, M.; Chen, H.; Liu, N.; Wu, Z.; Wu, G.; Dai, Z. Dietary L-Tryptophan Regulates Colonic Serotonin Homeostasis in Mice with Dextran Sodium Sulfate-Induced Colitis. J. Nutr. 2020, 150, 1966–1976. [Google Scholar] [CrossRef]
- Jin, B.R.; Kim, H.J.; Sim, S.A.; Lee, M.; An, H.J. Anti-Obesity Drug Orlistat Alleviates Western-Diet-Driven Colitis-Associated Colon Cancer via Inhibition of STAT3 and NF-kappaB-Mediated Signaling. Cells 2021, 10, 2060. [Google Scholar] [CrossRef]
- Park, D.D.; Yum, H.W.; Zhong, X.; Kim, S.H.; Kim, S.H.; Kim, D.H.; Kim, S.J.; Na, H.K.; Sato, A.; Miura, T.; et al. Perilla frutescens Extracts Protects against Dextran Sulfate Sodium-Induced Murine Colitis: NF-kappaB, STAT3, and Nrf2 as Putative Targets. Front. Pharmacol. 2017, 8, 482. [Google Scholar] [CrossRef]
- Dong, C.; Chan, S.S.M.; Jantchou, P.; Racine, A.; Oldenburg, B.; Weiderpass, E.; Heath, A.K.; Tong, T.Y.N.; Tjonneland, A.; Kyro, C.; et al. Meat intake is associated with a higher risk of ulcerative colitis in a large European prospective cohort study. J. Crohn’s Colitis 2022, jjac054. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Eindor-Abarbanel, A.; Healey, G.R.; Jacobson, K. Therapeutic Advances in Gut Microbiome Modulation in Patients with Inflammatory Bowel Disease from Pediatrics to Adulthood. Int. J. Mol. Sci. 2021, 22, 12506. [Google Scholar] [CrossRef]
- Chang, C.S.; Liao, Y.C.; Huang, C.T.; Lin, C.M.; Cheung, C.H.Y.; Ruan, J.W.; Yu, W.H.; Tsai, Y.T.; Lin, I.J.; Huang, C.H.; et al. Identification of a gut microbiota member that ameliorates DSS-induced colitis in intestinal barrier enhanced Dusp6-deficient mice. Cell Rep. 2021, 37, 110016. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.-B.; Kang, Z.-P.; Wang, M.-X.; Long, J.; Wang, H.-Y.; Huang, J.-Q.; Wei, S.-Y.; Zhou, W.; Zhao, H.-M.; Liu, D.-Y. Curcumin ameliorated dextran sulfate sodium-induced colitis via regulating the homeostasis of DCs and Treg and improving the composition of the gut microbiota. J. Funct. Foods 2021, 86, 104716. [Google Scholar] [CrossRef]
- Yang, B.; Li, M.; Wang, S.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus ruminis Alleviates DSS-Induced Colitis by Inflammatory Cytokines and Gut Microbiota Modulation. Foods 2021, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from Different Geographic Origins Decreases Intestinal Inflammation and Bacteroides spp. Populations in a Model of DSS-Induced Colitis. Mol. Nutr. Food Res. 2018, 62, e1800080. [Google Scholar] [CrossRef]
| Parameters | Pea Albumin | Essential Amino Acids (%) | |
|---|---|---|---|
| Dry matter (%) | 95.05 | Lysine | 8.07 |
| Protein (%) | 79.70 | Threonine | 4.73 |
| Ash (%) | 6.10 | Arginine | 4.58 |
| Moisture (%) | 4.95 | Valine | 3.43 |
| Fat (%) | 0.25 | Leucine | 2.86 |
| Saturated fat (%) | 0.13 | Phenylalanine | 2.81 |
| Trans fat (%) | ND | Isoleucine | 2.44 |
| Cholesterol (%) | ND | Tryptophan | 0.84 |
| Carbohydrate (%) | 9.00 | Methionine | 0.78 |
| Dietary fiber (%) | 5.59 | Nonessential amino acids (%) | |
| soluble fiber | 4.86 | Glutamate | 12.18 |
| insoluble fiber | 0.73 | Aspartate | 9.42 |
| Energy (kcal/g) | 3.57 | Alanine | 5.85 |
| Vitamin D (μg/kg) | ND | Glycine | 5.14 |
| Lead (mg/kg) | 0.17 | Serine | 3.75 |
| Arsenic (mg/kg) | 0.019 | Proline | 3.53 |
| Mercury (mg/kg) | ND | Tyrosine | 3.03 |
| Cadmium (mg/kg) | 0.04 | Histidine | 2.45 |
| Chromium (mg/kg) | 2.35 | Cystine | 2.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Jin, W.; Zhang, W.; Ren, F.; Wang, P.; Liu, N. Pea Albumin Attenuates Dextran Sulfate Sodium-Induced Colitis by Regulating NF-κB Signaling and the Intestinal Microbiota in Mice. Nutrients 2022, 14, 3611. https://doi.org/10.3390/nu14173611
Zhang S, Jin W, Zhang W, Ren F, Wang P, Liu N. Pea Albumin Attenuates Dextran Sulfate Sodium-Induced Colitis by Regulating NF-κB Signaling and the Intestinal Microbiota in Mice. Nutrients. 2022; 14(17):3611. https://doi.org/10.3390/nu14173611
Chicago/Turabian StyleZhang, Shucheng, Wenhua Jin, Weibo Zhang, Fazheng Ren, Pengjie Wang, and Ning Liu. 2022. "Pea Albumin Attenuates Dextran Sulfate Sodium-Induced Colitis by Regulating NF-κB Signaling and the Intestinal Microbiota in Mice" Nutrients 14, no. 17: 3611. https://doi.org/10.3390/nu14173611
APA StyleZhang, S., Jin, W., Zhang, W., Ren, F., Wang, P., & Liu, N. (2022). Pea Albumin Attenuates Dextran Sulfate Sodium-Induced Colitis by Regulating NF-κB Signaling and the Intestinal Microbiota in Mice. Nutrients, 14(17), 3611. https://doi.org/10.3390/nu14173611