Maternal Treatment with Metformin Persistently Ameliorates High-Fat Diet-Induced Metabolic Symptoms and Modulates Gut Microbiota in Rat Offspring
Abstract
:1. Introduction
2. Materials and Methods
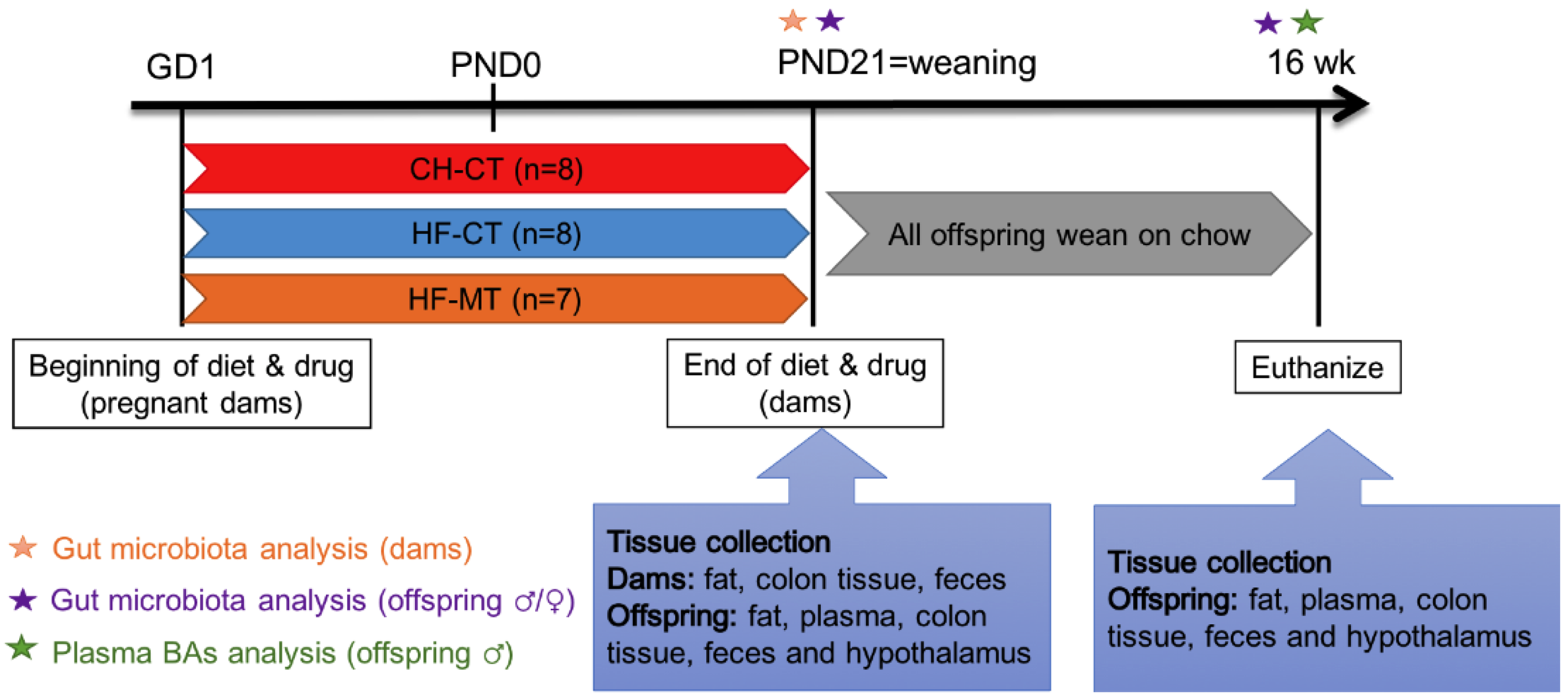
2.1. Animals
2.2. Tissue Collection
2.3. Quantitative Real-Time PCR Analysis
2.4. 16S rDNA Sequencing and Data Analysis
2.5. Targeted Metabolomics Analysis
2.6. Statistical Analysis
3. Results
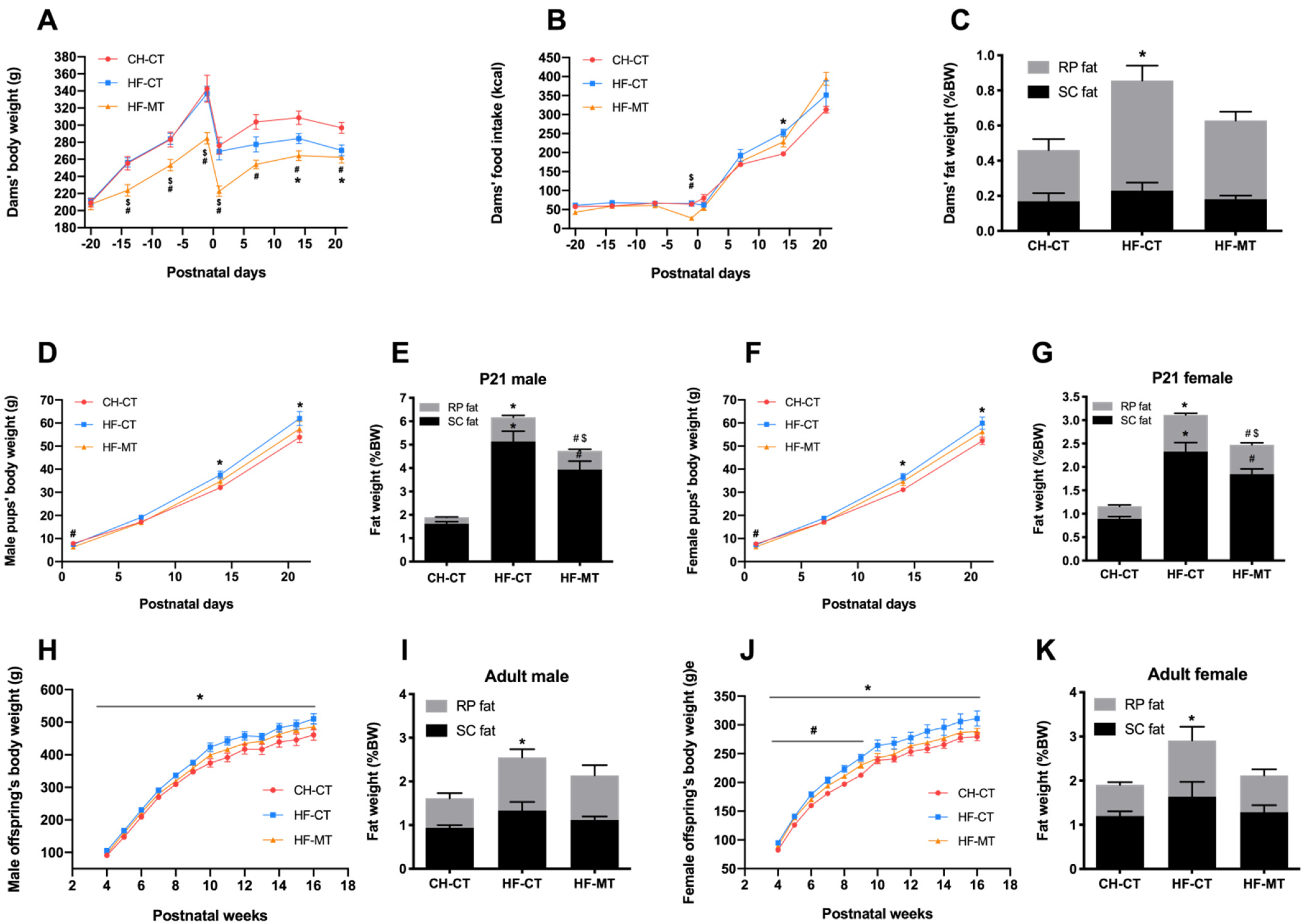
3.1. Maternal MT Treatment Improves HF-Fed Dams’ and Offspring’s Metabolic Phenotype
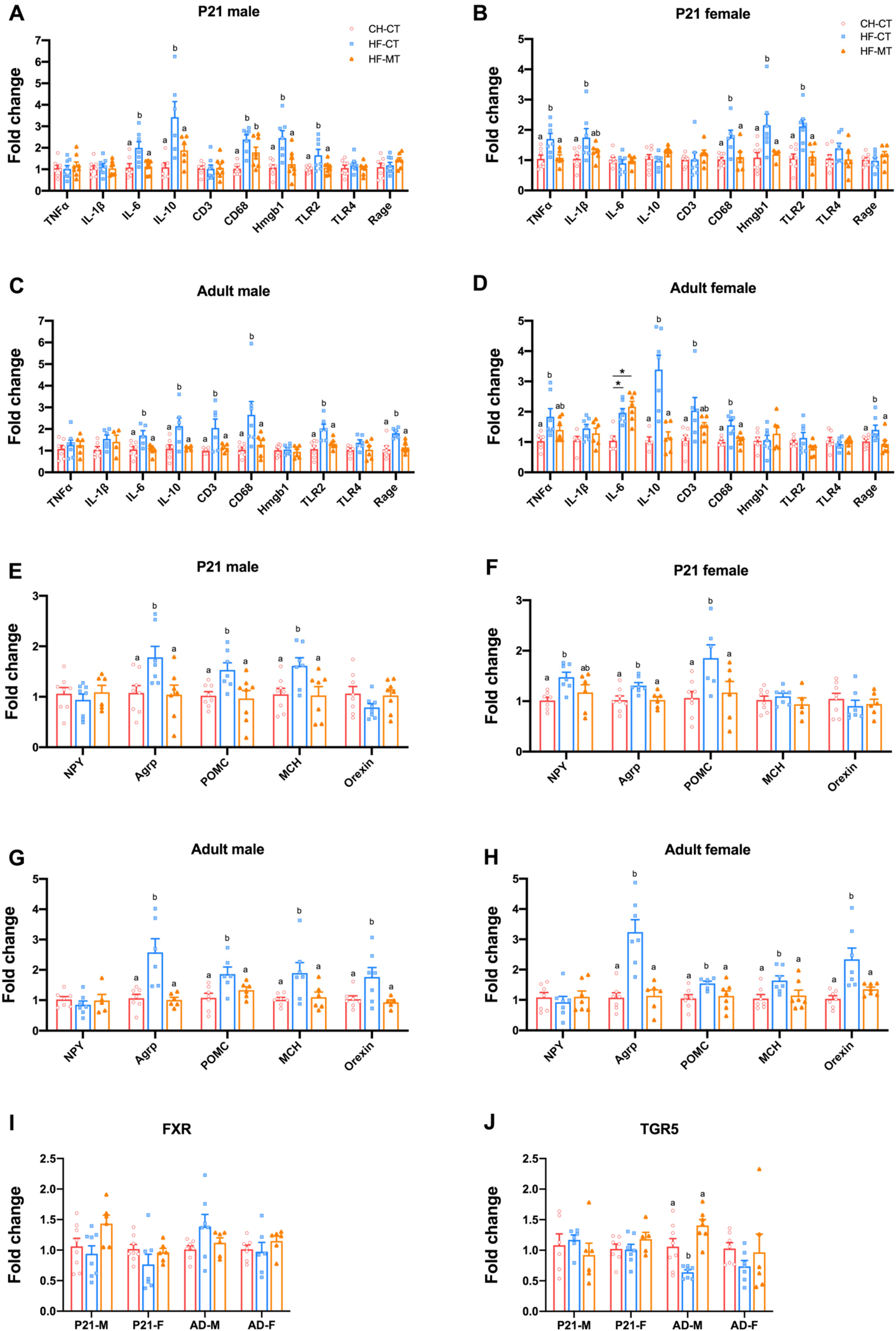
3.2. Maternal MT Treatment Restores Gut Integrity and Inflammatory Conditions in Both HF-Fed Dams and Offspring
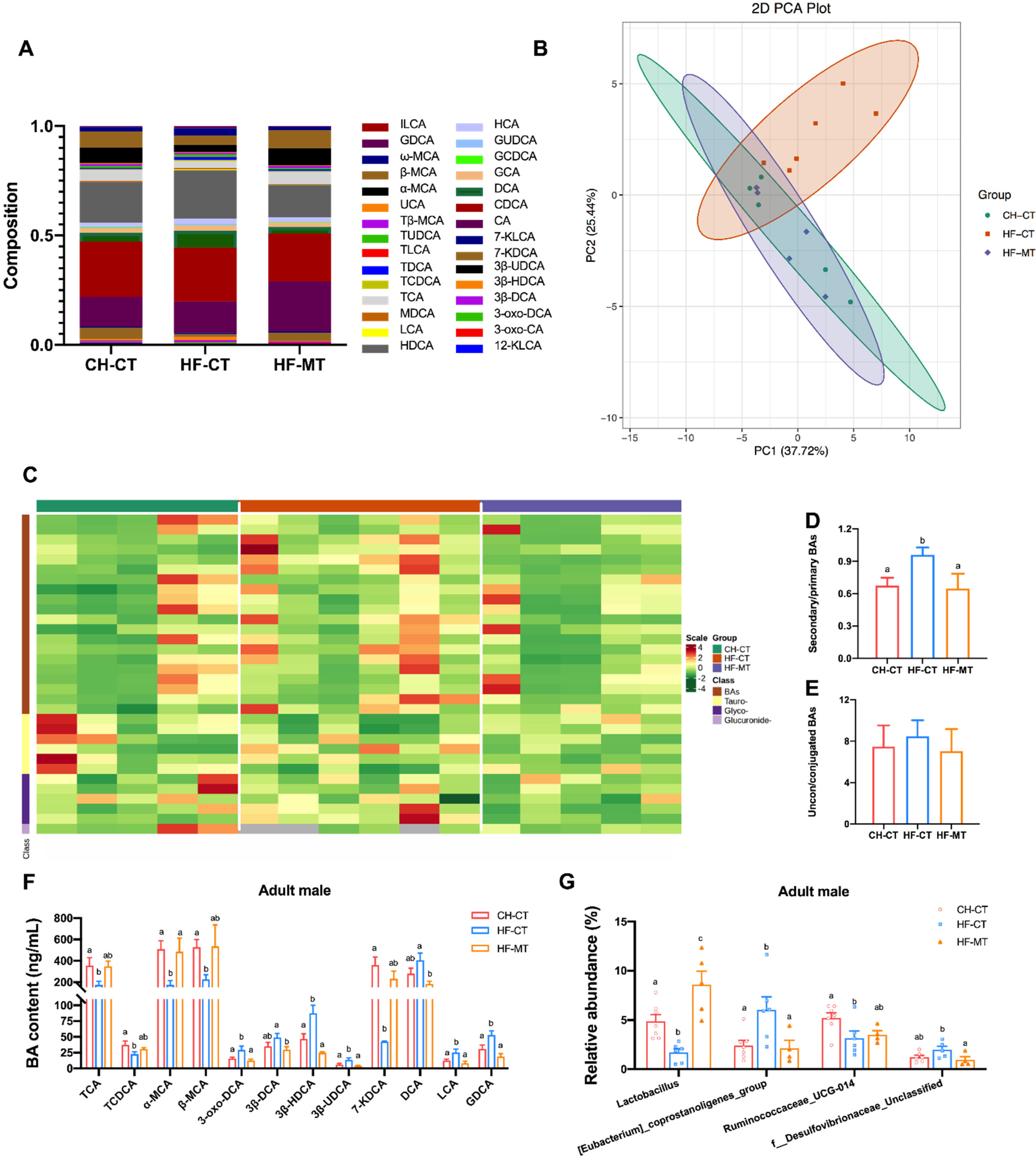
3.3. Maternal MT Treatment Reshapes Gut Microbiota in the HF-Fed Dams and Offspring
3.4. Maternal MT Treatment Improves Gene Expression of Hypothalamic Inflammatory and Appetite Markers in Offspring from HF-Fed Dams and Restores Hypothalamic Gene Expression of Bile Acid Receptor-TGR5 in Adult Male Offspring
3.5. Maternal MT Treatment Improves Maternal HF Diet-Induced Microbial BA Dysmetabolism in Adult Male Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T.; Low, F.M.; Beedle, A.S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009, 5, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.P.; Ozanne, S.E. Developmental programming of type 2 diabetes: Early nutrition and epigenetic mechanisms. Curr. Opin. Clin. Nutr. 2015, 18, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalan, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endo. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Sun, B.; Purcell, R.H.; Terrillion, C.E.; Yan, J.Q.; Moran, T.H.; Tamashiro, K.L.K. Maternal High-Fat Diet During Gestation or Suckling Differentially Affects Offspring Leptin Sensitivity and Obesity. Diabetes 2012, 61, 2833–2841. [Google Scholar] [CrossRef]
- Frias, A.E.; Morgan, T.K.; Evans, A.E.; Rasanen, J.; Oh, K.Y.; Thornburg, K.L.; Grove, K.L. Maternal High-Fat Diet Disturbs Uteroplacental Hemodynamics and Increases the Frequency of Stillbirth in a Nonhuman Primate Model of Excess Nutrition. Obstet. Gynecol. Surv. 2011, 66, 605–606. [Google Scholar] [CrossRef]
- Hafner, H.; Chang, E.; Carlson, Z.; Zhu, A.; Varghese, M.; Clemente, J.; Abrishami, S.; Bagchi, D.P.; MacDougald, O.A.; Singer, K.; et al. Lactational High-Fat Diet Exposure Programs Metabolic Inflammation and Bone Marrow Adiposity in Male Offspring. Nutrients 2019, 11, 1393. [Google Scholar] [CrossRef]
- Desai, N.; Roman, A.; Rochelson, B.; Gupta, M.; Xue, X.Y.; Chatterjee, P.K.; Tam, H.T.; Metz, C.N. Maternal metformin treatment decreases fetal inflammation in a rat model of obesity and metabolic syndrome. Am. J. Obstet. Gynecol. 2013, 209, 136.e1–136.e9. [Google Scholar] [CrossRef] [PubMed]
- Ijas, H.; Vaarasmaki, M.; Morin-Papunen, L.; Keravuo, R.; Ebeling, T.; Saarela, T.; Raudaskoski, T. Metformin should be considered in the treatment of gestational diabetes: A prospective randomised study. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 880–885. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Loeken, M.R. Metformin use in pregnancy: Promises and uncertainties. Diabetologia 2017, 60, 1612–1619. [Google Scholar] [CrossRef]
- Hyer, S.; Balani, J.; Shehata, H. Metformin in Pregnancy: Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2018, 19, 1954. [Google Scholar] [CrossRef] [Green Version]
- Rowan, J.A.; Rush, E.C.; Obolonkin, V.; Battin, M.; Wouldes, T.; Hague, W.M. Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) Body composition at 2 years of age. Diabetes Care 2011, 34, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Novi, D.R.B.S.; Vidigal, C.B.; Marques, B.V.D.; Forcato, S.; Raquel, H.A.; Zaia, D.A.M.; Zaia, C.T.B.V.; Martins-Pinge, M.C.; Gerardin, D.C.C.; Ceravolo, G.S. Can maternal treatment with metformin during gestation and lactation cause metabolic and cardiovascular disorders in rat offspring? Arch. Physiol. Biochem. 2020, 126, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Forcato, S.; Regina, D.; Novi, B.D.; Costa, N.O.; Borges, L.I.; de Goes, M.L.M.; Ceravolo, G.S.; Gerardin, D.C.C. In utero and lactational exposure to metformin induces reproductive alterations in male rat offspring. Reprod. Toxicol. 2017, 74, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res. Care 2018, 6, e000456. [Google Scholar] [CrossRef] [PubMed]
- Hanem, L.G.E.; Stridsklev, S.; Juliusson, P.B.; Salvesen, O.; Roelants, M.; Carlsen, S.M.; Odegard, R.; Vanky, E. Metformin Use in PCOS Pregnancies Increases the Risk of Offspring Overweight at 4 Years of Age: Follow-Up of Two RCTs. J. Clin. Endocrinol. Metab. 2018, 103, 1612–1621. [Google Scholar] [CrossRef]
- Hanem, L.G.E.; Salvesen, O.; Juliusson, P.B.; Carlsen, S.M.; Nossum, M.C.F.; Vaage, M.O.; Odegard, R.; Vanky, E. Intrauterine metformin exposure and offspring cardiometabolic risk factors (PedMet study): A 5–10 year follow-up of the PregMet randomised controlled trial. Lancet Child Adolesc. Health 2019, 3, 166–174. [Google Scholar] [CrossRef]
- Salomaki, H.; Vahatalo, L.H.; Laurila, K.; Jappinen, N.T.; Penttinen, A.M.; Ailanen, L.; Ilyasizadeh, J.; Pesonen, U.; Koulu, M. Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood. PLoS ONE 2013, 8, e56594. [Google Scholar] [CrossRef]
- Salomaki-Myftari, H.; Vahatalo, L.H.; Ailanen, L.; Pietila, S.; Laiho, A.; Hanninen, A.; Pursiheimo, J.P.; Munukka, E.; Rintala, A.; Savontaus, E.; et al. Neuropeptide Y Overexpressing Female and Male Mice Show Divergent Metabolic but Not Gut Microbial Responses to Prenatal Metformin Exposure. PLoS ONE 2016, 11, e0163805. [Google Scholar] [CrossRef]
- Schoonejans, J.M.; Blackmore, H.L.; Ashmore, T.J.; Aiken, C.E.; Fernandez-Twinn, D.S.; Ozanne, S.E. Maternal Metformin Intervention during Obese Glucose-Intolerant Pregnancy Affects Adiposity in Young Adult Mouse Offspring in a Sex-Specific Manner. Int. J. Mol. Sci. 2021, 22, 8104. [Google Scholar] [CrossRef]
- Moeller, A.H.; Suzuki, T.A.; Phifer-Rixey, M.; Nachman, M.W. Transmission modes of the mammalian gut microbiota. Science 2018, 362, 453–456. [Google Scholar] [CrossRef] [Green Version]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Ma, W.; Chen, J.; Meng, Y.H.; Yang, J.C.; Cui, Q.H.; Zhou, Y. Metformin Alters Gut Microbiota of Healthy Mice: Implication for Its Potential Role in Gut Microbiota Homeostasis. Front. Microbiol. 2018, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Perez, O.; Cruz-Ramon, V.; Chinchilla-Lopez, P.; Mendez-Sanchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16, S21–S26. [Google Scholar] [CrossRef]
- Yanguas-Casas, N.; Barreda-Manso, M.A.; Nieto-Sampedro, M.; Romero-Ramirez, L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J. Cell. Physiol. 2017, 232, 2231–2245. [Google Scholar] [CrossRef]
- Deckmyn, B.; Domenger, D.; Blondel, C.; Ducastel, S.; Nicolas, E.; Dorchies, E.; Caron, E.; Charton, J.; Vallez, E.; Deprez, B.; et al. Farnesoid X Receptor Activation in Brain Alters Brown Adipose Tissue Function via the Sympathetic System. Front. Mol. Neurosci. 2021, 14, 808603. [Google Scholar] [CrossRef]
- Perino, A.; Velazquez-Villegas, L.A.; Bresciani, N.; Sun, Y.; Huang, Q.; Fenelon, V.S.; Castellanos-Jankiewicz, A.; Zizzari, P.; Bruschetta, G.; Jin, S.; et al. Central anorexigenic actions of bile acids are mediated by TGR5. Nat. Metab. 2021, 3, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G. Development of Hypothalamic Neural Networks Controlling Appetite. Forum. Nutr. 2010, 63, 84–93. [Google Scholar] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.; Gackenheimer, S.L.; Gehlert, D.R. Functional autoradiography of neuropeptide Y Y1 and Y2 receptor subtypes in rat brain using agonist stimulated [35S]GTPgammaS binding. J. Chem. Neuroanat. 2003, 26, 179–193. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in Pregnancy: ACOG Practice Bulletin, Number 230. Obstet. Gynecol. 2021, 137, e128–e144. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.W.; Nath, M.; Khalil, A.; Webb, D.R.; Robinson, T.G.; Mousa, H.A. The effects of metformin on maternal haemodynamics in gestational diabetes mellitus: A pilot study. Diabetes Res. Clin. Pract. 2018, 139, 170–178. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, X.; Zhao, X.; Yu, X.; Wang, A.; Chen, X.; Qi, H.; Han, T.L.; Zhang, H.; Baker, P.N. Metformin administration during pregnancy attenuated the long-term maternal metabolic and cognitive impairments in a mouse model of gestational diabetes. Aging 2020, 12, 14019–14036. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Robinson, I.G.; Reynolds, R.M.; Aye, I.; Charnock-Jones, D.S.; Jenkins, B.; Koulmann, A.; Ozanne, S.E.; Aiken, C.E. Impact of Metformin Treatment on Human Placental Energy Production and Oxidative Stress. Front. Cell Dev. Biol. 2022, 10, 935403. [Google Scholar] [CrossRef]
- Huang, S.W.; Ou, Y.C.; Tang, K.S.; Yu, H.R.; Huang, L.T.; Tain, Y.L.; Lin, I.C.; Sheen, J.M.; Hou, C.Y.; Tsai, C.C.; et al. Metformin ameliorates maternal high-fat diet-induced maternal dysbiosis and fetal liver apoptosis. Lipids Health Dis. 2021, 20, 100. [Google Scholar] [CrossRef]
- Salomaki, H.; Heinaniemi, M.; Vahatalo, L.H.; Ailanen, L.; Eerola, K.; Ruohonen, S.T.; Pesonen, U.; Koulu, M. Prenatal metformin exposure in a maternal high fat diet mouse model alters the transcriptome and modifies the metabolic responses of the offspring. PLoS ONE 2014, 9, e115778. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Tomlinson, G.; Feig, D.S.; MiTy Collaborative, G. Gestational weight gain in women with type 2 diabetes and perinatal outcomes: A secondary analysis of the metformin in women with type 2 diabetes in pregnancy (MiTy) trial. Diabetes Res. Clin. Pract. 2022, 186, 109811. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, X.; Li, M.; Zhang, Q.; Yu, M.; Zheng, J.; Deng, M. Maternal Exercise Improves High-Fat Diet-Induced Metabolic Abnormalities and Gut Microbiota Profiles in Mouse Dams and Offspring. Front. Cell Infect. Microbiol. 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Sun, Y.; Wu, J.; Huang, S.; Jin, G.; Guo, Z.; Zhang, Y.; Liu, T.; Liu, X.; Cao, X.; et al. Maternal High Fat Diet Alters Gut Microbiota of Offspring and Exacerbates DSS-Induced Colitis in Adulthood. Front. Immunol. 2018, 9, 2608. [Google Scholar] [CrossRef]
- Mann, P.E.; Huynh, K.; Widmer, G. Maternal high fat diet and its consequence on the gut microbiome: A rat model. Gut Microbes 2018, 9, 143–154. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Lee, C.T.; Chan, J.Y.H.; Tain, Y.L. The Interplay between Maternal and Post-Weaning High-Fat Diet and Gut Microbiota in the Developmental Programming of Hypertension. Nutrients 2019, 11, 1982. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, W.; Tian, Z.; Wu, H.; Ning, H.; Yan, G.; Zhang, Z.; Li, Z.; Dong, F.; Sun, Y.; et al. Fecal g. Streptococcus and g. Eubacterium_coprostanoligenes_group combined with sphingosine to modulate the serum dyslipidemia in high-fat diet mice. Clin. Nutr. 2021, 40, 4234–4245. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Wu, K.L.H.; Lee, W.C.; Leu, S.; Chan, J.Y.H. Prenatal Metformin Therapy Attenuates Hypertension of Developmental Origin in Male Adult Offspring Exposed to Maternal High-Fructose and Post-Weaning High-Fat Diets. Int. J. Mol. Sci. 2018, 19, 1066. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Foley, M.H.; O’Flaherty, S.; Allen, G.; Rivera, A.J.; Stewart, A.K.; Barrangou, R.; Theriot, C.M. Lactobacillus bile salt hydrolase substrate specificity governs bacterial fitness and host colonization. Proc. Natl. Acad. Sci. USA 2021, 118, e2017709118. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Yang, X.; Mo, W.; Zheng, C.; Li, W.; Tang, J.; Wu, X. Alleviating effects of noni fruit polysaccharide on hepatic oxidative stress and inflammation in rats under a high-fat diet and its possible mechanisms. Food Funct. 2020, 11, 2953–2968. [Google Scholar] [CrossRef]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, F.; Zhao, A.; Lei, S.; Zhang, Y.; Xie, G.; Chen, T.; Qu, C.; Rajani, C.; Dong, B.; et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017, 15, 120. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, M.; Takamine, F.; Imamura, T.; Benno, Y. Assignment of Eubacterium sp. VPI 12708 and related strains with high bile acid 7alpha-dehydroxylating activity to Clostridium scindens and proposal of Clostridium hylemonae sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 3, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Igarashi, M.; Li, X.; Nakatani, A.; Miyamoto, J.; Inaba, Y.; Sutou, A.; Saito, T.; Sato, T.; Tachibana, N.; et al. Dietary soybean protein ameliorates high-fat diet-induced obesity by modifying the gut microbiota-dependent biotransformation of bile acids. PLoS ONE 2018, 13, e0202083. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, M.; Zhang, Y.; Chu, S.; Huo, Y.; Zhao, J.; Wan, C. Huangjinya Black Tea Alleviates Obesity and Insulin Resistance via Modulating Fecal Metabolome in High-Fat Diet-Fed Mice. Mol Nutr. Food Res. 2020, 64, e2000353. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Milewski, K.; Albrecht, J.; Zielinska, M. The Status of Bile Acids and Farnesoid X Receptor in Brain and Liver of Rats with Thioacetamide-Induced Acute Liver Failure. Int. J. Mol. Sci. 2020, 21, 7750. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Jankiewicz, A.; Guzman-Quevedo, O.; Fenelon, V.S.; Zizzari, P.; Quarta, C.; Bellocchio, L.; Tailleux, A.; Charton, J.; Fernandois, D.; Henricsson, M.; et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021, 33, 1483–1492.e10. [Google Scholar] [CrossRef]
- Chen, X.; Lou, G.; Meng, Z.; Huang, W. TGR5: A novel target for weight maintenance and glucose metabolism. Exp. Diabetes Res. 2011, 2011, 853501. [Google Scholar] [CrossRef]
- Hu, X.; Yan, J.; Huang, L.; Araujo, C.; Peng, J.; Gao, L.; Liu, S.; Tang, J.; Zuo, G.; Zhang, J.H. INT-777 attenuates NLRP3-ASC inflammasome-mediated neuroinflammation via TGR5/cAMP/PKA signaling pathway after subarachnoid hemorrhage in rats. Brain Behav. Immun. 2021, 91, 587–600. [Google Scholar] [CrossRef]
- Jin, P.; Deng, S.; Tian, M.; Lenahan, C.; Wei, P.; Wang, Y.; Tan, J.; Wen, H.; Zhao, F.; Gao, Y.; et al. INT-777 prevents cognitive impairment by activating Takeda G protein-coupled receptor 5 (TGR5) and attenuating neuroinflammation via cAMP/ PKA/ CREB signaling axis in a rat model of sepsis. Exp. Neurol. 2021, 335, 113504. [Google Scholar] [CrossRef]
- Dearden, L.; Buller, S.; Furigo, I.C.; Fernandez-Twinn, D.S.; Ozanne, S.E. Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways. Mol. Metab. 2020, 42, 101079. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, G.; Cao, Z.; Wu, F.; Lei, H.; Chen, C.; Song, Y.; Liu, C.; Li, J.; Zhou, J.; et al. Gut Microbiota and Its Metabolite Deoxycholic Acid Contribute to Sucralose Consumption-Induced Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2021, 69, 3982–3991. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, A.H.; Kim, E.; Lee, S.; Yu, K.S.; Jang, I.J.; Chung, J.Y.; Cho, J.Y. Changes in the gut microbiome influence the hypoglycemic effect of metformin through the altered metabolism of branched-chain and nonessential amino acids. Diabetes Res. Clin. Pract. 2021, 178, 108985. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Fillier, T.; Pham, T.H.; Thomas, R.; Cheema, S.K. Intraperitoneal Administration of Short-Chain Fatty Acids Improves Lipid Metabolism of Long-Evans Rats in a Sex-Specific Manner. Nutrients 2021, 13, 892. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer | Reverse Primer | Gene Bank No. |
|---|---|---|---|
| Actb | CTATCGGCAATGAGCGGTTCC | TGTGTTGGCATAGAGGTCTTTACG | NM_031144.2 |
| Tjp1 | AGCGAAGCCACCTGAAGATA | GATGGCCAGCAGGAATATGT | NM_001106266.1 |
| Ocln | CTGTCTATGCTCGTCATCG | CATTCCCGATCTAATGACGC | NM_031329.3 |
| Cldn4 | CGAGCCCTGATGGTCATCAG | CGGAGTACTTGGCGGAGTAG | NM_001012022.1 |
| Muc2 | ACCATGGGGCTGCCACTA | GATCTTCTGCATGTTCCC | XM_03910270.1 |
| Tnfa | GTCGTAGCAAACCACCAAGC | TGTGGGTGAGGAGCACATAG | NM_012675.3 |
| Il1b | GCAATGGTCGGGACATAGTT | AGACCTGACTTGGCAGAGA | NM_031512.2 |
| Il6 | TCCGCAAGAGACTTCCAGCCAGT | AGCCTCCGACTTGTGAAGTGG | NM_012589.2 |
| Il10 | TGCGACGCTGTCATCGATTT | GTAGATGCCGGGTGGTTCAA | NM_012854.2 |
| Cd3 | GTCCGGTGACTTGCCTCTAC | CTAGATGCCTGATGCTGGTGT | NM_007648.5 |
| Cd68 | ACTGGGGCTCTTGGAAACTACAC | CCTTGGTTTTGTTCGGGTTCA | NM_001031638.1 |
| Hmgb1 | CTAGCCCTGTCCTGGTGGTATT | CCAATTTACAACCCCCAGACTGT | NM_012963.3 |
| Tlr2 | GCACTTGAGCGAGTCTGCTTTC | GAACAAATAGAACTGGGGGATGTG | NM_198769.2 |
| Tlr4 | GGCTGTGGAGACAAAAATGACCTC | AGGCTTGGGCTTGAATGGAGTC | NM_019178.2 |
| Ager | ACAGAAACCGGTGATGAAGGA | TGTCGTTTTCGCCACAGGAT | NM_05333.6 |
| Npy | CTATCCCTGCTCGTGTGTTTGG | TGGTGATGAGATTGATGTAGTGTCG | NM_012614.1 |
| Agrp | TGAAGAAGACAGCAGCAGAC | TTGAAGAAGCGGCAGTAGC | NM_03650.1 |
| Pomc | TGCTTCAGACCTCCATAGAC | GCTGTTCATCTCCGTTGC | NM_139326.2 |
| Mch | GAATGGAGTTCAGAATACTGAGTCA | AGCATACACCTGAGCATGTCAAAT | NM_012625.2 |
| Hcrt | CCTGCCGTCTCTACGAACTG | GTTACCGTTGGCCTGAAGGA | NM_013179.3 |
| Nr1h4 | CTGATTGGGCCCTCCCATTT | CAGATTCTGCCCCAGAGGAC | NM_021745.1 |
| Gpbar1 | TACTCACAGGGTTGGCACTG | CAAAAGTTGGGGGCCAAGTG | NM_177936.1 |
| Group1 | Group2 | R-Value | p-Value | |
|---|---|---|---|---|
| Dam | CH-CT | HF-CT | 0.516 | 0.002 |
| CH-CT | HF-MT | 0.956 | 0.004 | |
| HF-CT | HF-MT | 0.286 | 0.044 | |
| PND21 male | CH-CT | HF-CT | 0.939 | 0.001 |
| CH-CT | HF-MT | 0.988 | 0.001 | |
| HF-CT | HF-MT | 0.001 | 0.412 | |
| Adult male | CH-CT | HF-CT | 0.539 | 0.004 |
| CH-CT | HF-MT | 0.518 | 0.002 | |
| HF-CT | HF-MT | 0.32 | 0.006 | |
| PND21 female | CH-CT | HF-CT | 1 | 0.001 |
| CH-CT | HF-MT | 0.993 | 0.001 | |
| HF-CT | HF-MT | 0.204 | 0.05 | |
| Adult female | CH-CT | HF-CT | 0.449 | 0.002 |
| CH-CT | HF-MT | 0.875 | 0.002 | |
| HF-CT | HF-MT | 0.465 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Cui, J.; Hu, S.; Wang, R.; Li, H.; Sun, B. Maternal Treatment with Metformin Persistently Ameliorates High-Fat Diet-Induced Metabolic Symptoms and Modulates Gut Microbiota in Rat Offspring. Nutrients 2022, 14, 3612. https://doi.org/10.3390/nu14173612
Song L, Cui J, Hu S, Wang R, Li H, Sun B. Maternal Treatment with Metformin Persistently Ameliorates High-Fat Diet-Induced Metabolic Symptoms and Modulates Gut Microbiota in Rat Offspring. Nutrients. 2022; 14(17):3612. https://doi.org/10.3390/nu14173612
Chicago/Turabian StyleSong, Lin, Jiaqi Cui, Shuyuan Hu, Rui Wang, Hongbao Li, and Bo Sun. 2022. "Maternal Treatment with Metformin Persistently Ameliorates High-Fat Diet-Induced Metabolic Symptoms and Modulates Gut Microbiota in Rat Offspring" Nutrients 14, no. 17: 3612. https://doi.org/10.3390/nu14173612
APA StyleSong, L., Cui, J., Hu, S., Wang, R., Li, H., & Sun, B. (2022). Maternal Treatment with Metformin Persistently Ameliorates High-Fat Diet-Induced Metabolic Symptoms and Modulates Gut Microbiota in Rat Offspring. Nutrients, 14(17), 3612. https://doi.org/10.3390/nu14173612






