Ketogenic Diet for Preoperative Weight Reduction in Bariatric Surgery: A Narrative Review
Abstract
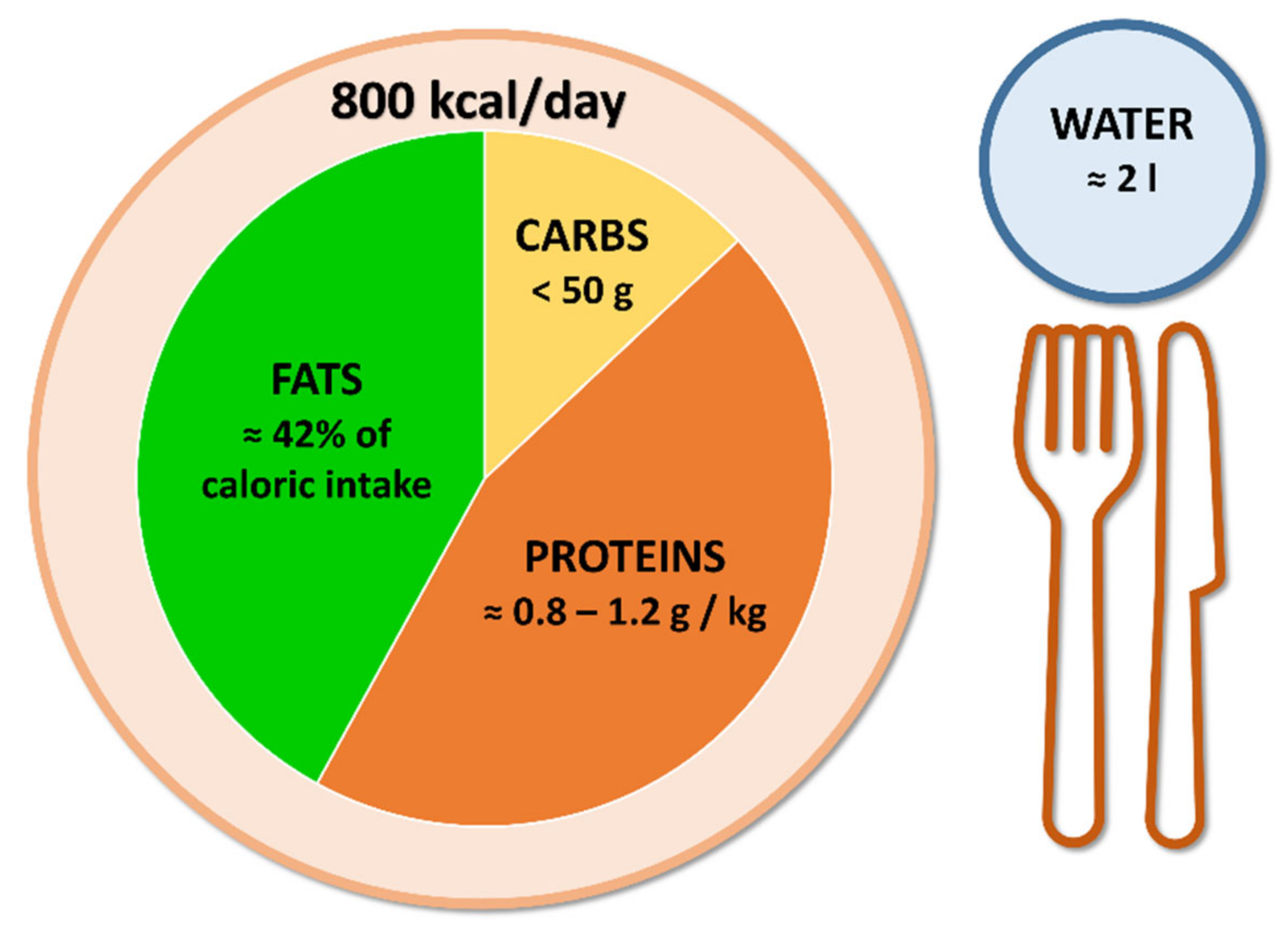
:1. Introduction
2. Concerns Regarding Pre-Operative Oxidative Stress
3. Micronutrient Deficiency
4. Pre-Operative Care of Obstructive Sleep Apnea Syndrome
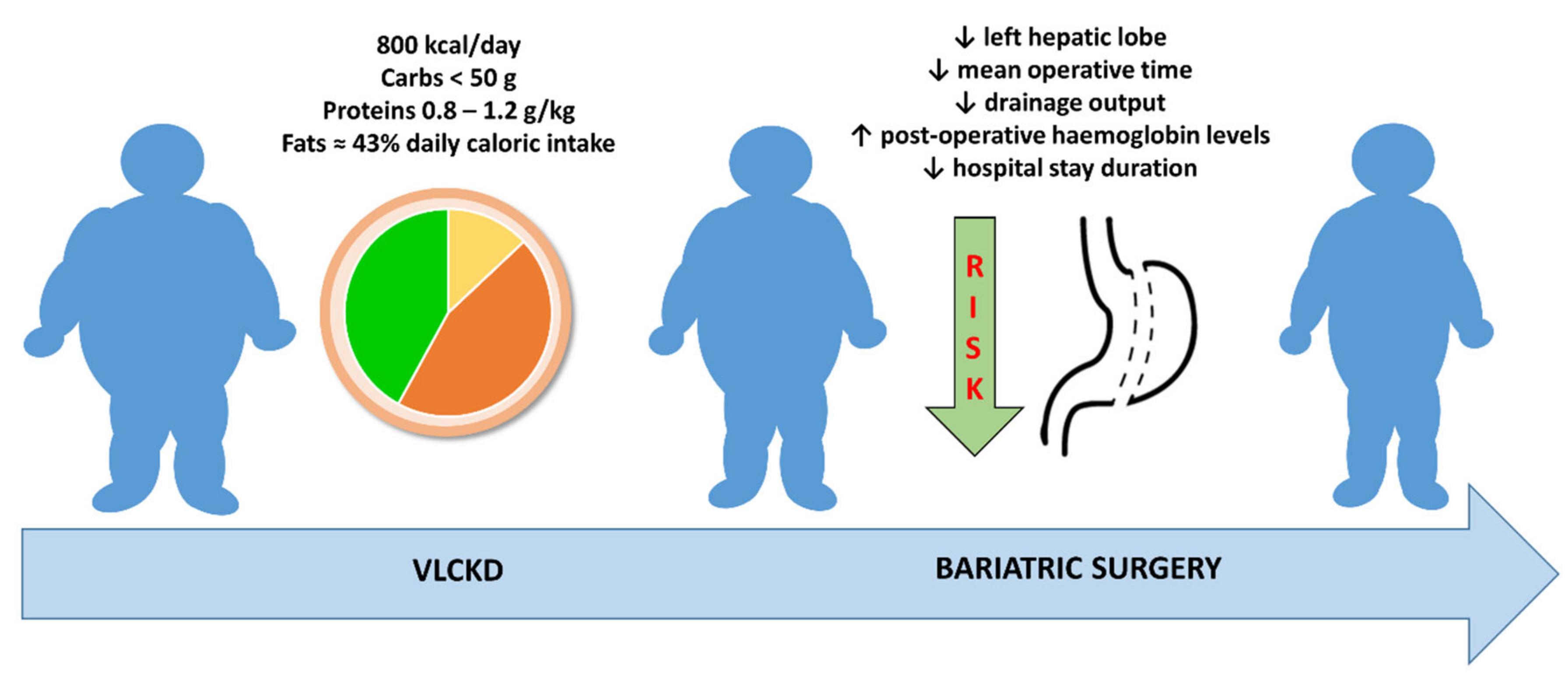
5. Evaluation of Surgical Outcomes
6. Current Evidence and Future Perspectives
| Reference | Population | Intervention Description and Duration | Control Group | Main Findings |
|---|---|---|---|---|
| Leonetti F et al., 2014 [37] | 50 patients (31 F/19 M) | OPOD regimen: VLCKD for 10 days, VLCD for 10 days, LCD for 10 days | 30 patients (18 F/12 M) standard LCD for 30 days | Reduction in BMI from 53.5 ± 8.4 kg/m2 to 49.2 ± 8.7 kg/m2 (p < 0.001); improvement in fasting plasma glucose levels; mean 30% reduction in liver volume; improvement of steatosis pattern. |
| Pilone V et al., 2018 [44] | 119 patients (75 F/44 M) | Sequential diet regimen: VLCKD for 10 days, hypocaloric scheme for 20 days. In addition: multimineral and multivitamin supplements. | Absent | Reduction in BMI from 41.5 ± 7.6 kg/m2 to 34.1 ± 5.2 kg/m2 (p < 0.001); reduction of fat mass; preservation of fat-free mass; mean 30% reduction in liver volume; improvement of steatosis pattern. |
| Schiavo L et al., 2018 [45] | 27 patients (17 F/10 M) | Ketogenic micronutrient-enriched diet for 4 weeks | Absent | Reduction in BMI from 46.9 ± 11.7 kg/m2 to 43.0 ± 13.4 kg/m2 (p < 0.001) in females; reduction in BMI from 44.5 ± 10.5 kg/m2 to 40.6 ± 6.5 kg/m2 (p < 0.001) in males; mean 19.8% reduction of left hepatic lobe; improvement in micronutrient status. |
| Albanese A et al., 2019 [50] | 72 patients (60 F/12 M) | VLCKD for 3 weeks | 106 patients (79 F/27 M) VLCD for 3 weeks | Total weight loss better in VLCKD than in VLCD group (5.8 ± 2.4 vs. 4.8 ± 2.5 kg, p = 0.008). Surgical outcomes: mean operative time slightly shorter in VLCKD group; percentage of patients requiring a longer-than-anticipated hospital stay lower in VLCKD group; lower drainage output and higher post-operative hemoglobin levels in VLCKD group. |
| Schiavo L et al., 2022 [48] | 34 patients (12 F/22 M) | CPAP + LCKD for 4 weeks | 36 patients (14 F/22 M) CPAP for 4 weeks | Apnea-hypopnea score improved in both groups; reduction in BMI (from 50.1 ± 5.9 kg/m2 to 45.3 ± 6.5 kg/m2, p < 0.001) was observed only in CPAP + LCKD group; reduction in CRP levels, blood pressure, HOMA index and cholesterol levels were observed only in CPAP + LCKD group. |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, Å.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Perrone, F.; Bianciardi, E.; Ippoliti, S.; Nardella, J.; Fabi, F.; Gentileschi, P. Long-term effects of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass for the treatment of morbid obesity: A monocentric prospective study with minimum follow-up of 5 years. Updates Surg. 2017, 69, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sundbom, M. Laparoscopic revolution in bariatric surgery. World J. Gastroenterol. 2014, 20, 15135–15143. [Google Scholar] [CrossRef] [PubMed]
- Riess, K.P.; Baker, M.T.; Lambert, P.J.; Mathiason, M.A.; Kothari, S.N. Effect of preoperative weight loss on laparoscopic gastric bypass outcomes. Surg. Obes. Relat. Dis. 2008, 4, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Picot, J.; Jones, J.; Colquitt, J.L.; Gospodarevskaya, E.; Loveman, E.; Baxter, L.; Clegg, A. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: A systematic review and economic evaluation. Health Technol. Assess. 2009, 13, 1–190, 215–357. [Google Scholar] [CrossRef]
- Tarnoff, M.; Kaplan, L.M.; Shikora, S. An evidenced-based assessment of preoperative weight loss in bariatric surgery. Obes. Surg. 2008, 18, 1059–1061. [Google Scholar] [CrossRef]
- Schwartz, M.L.; Drew, R.L.; Chazin-Caldie, M. Laparoscopic Roux-en-Y gastric bypass: Preoperative determinants of prolonged operative times, conversion to open gastric bypasses, and postoperative complications. Obes. Surg. 2003, 13, 734–738. [Google Scholar] [CrossRef]
- Gonzalez, H.; Minville, V.; Delanoue, K.; Mazerolles, M.; Concina, D.; Fourcade, O. The importance of increased neck circumference to intubation difficulties in obese patients. Anesth. Analg. 2008, 106, 1132–1136. [Google Scholar] [CrossRef]
- Gerber, P.; Anderin, C.; Thorell, A. Weight loss prior to bariatric surgery: An updated review of the literature. Scand. J. Surg. 2015, 104, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Livhits, M.; Mercado, C.; Yermilov, I.; Parikh, J.A.; Dutson, E.; Mehran, A.; Ko, C.Y.; Gibbons, M.M. Does weight loss immediately before bariatric surgery improve outcomes: A systematic review. Surg. Obes. Relat. Dis. 2009, 5, 713–721. [Google Scholar] [CrossRef]
- Schiavo, L.; Sans, A.; Scalera, G.; Barbarisi, A.; Iannelli, A. Why Preoperative Weight Loss in Preparation for Bariatric Surgery Is Important. Obes. Surg. 2016, 26, 2790–2792. [Google Scholar] [CrossRef]
- Kim, J.J.; Rogers, A.M.; Ballem, N.; Schirmer, B. ASMBS updated position statement on insurance mandated preoperative weight loss requirements. Surg. Obes. Relat. Dis. 2016, 12, 955–959. [Google Scholar] [CrossRef]
- Bettini, S.; Belligoli, A.; Fabris, R.; Busetto, L. Diet approach before and after bariatric surgery. Rev. Endocr. Metab. Disord. 2020, 21, 297–306. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 16, 175–247. [Google Scholar]
- Fried, M.; Yumuk, V.; Oppert, J.-M.; Scopinaro, N.; Torres, A.J.; Weiner, R.; Yashkov, Y.; Frühbeck, G. Interdisciplinary European Guidelines on metabolic and bariatric surgery. Obes. Facts 2013, 6, 449–468. [Google Scholar] [CrossRef]
- Tang, T.; Abbott, S.; Le Roux, C.W.; Wilson, V.; Singhal, R.; Bellary, S.; Tahrani, A.A. Preoperative weight loss with glucagon-like peptide-1 receptor agonist treatment predicts greater weight loss achieved by the combination of medical weight management and bariatric surgery in patients with type 2 diabetes: A longitudinal analysis. Diabetes Obes. Metab. 2018, 20, 745–748. [Google Scholar] [CrossRef]
- Adrianzén Vargas, M.; Fernández, N.C.; Serrano, J.O. Preoperative weight loss in patients with indication of bariatric surgery: Which is the best method? Nutr. Hosp. 2011, 26, 1227–1230. [Google Scholar]
- Busetto, L.; Segato, G.; De Luca, M.; Bortolozzi, E.; Maccari, T.; Magon, A.; Inelmen, E.M.; Favretti, F.; Enzi, G. Preoperative weight loss by intragastric balloon in super-obese patients treated with laparoscopic gastric banding: A case-control study. Obes. Surg. 2004, 14, 671–676. [Google Scholar] [CrossRef]
- Gastaldo, I.; Casas, R.; Moizé, V. Clinical Impact of Mediterranean Diet Adherence before and after Bariatric Surgery: A Narrative Review. Nutrients 2022, 14, 393. [Google Scholar] [CrossRef]
- Schiavo, L.; Scalera, G.; Sergio, R.; De Sena, G.; Pilone, V.; Barbarisi, A. Clinical impact of Mediterranean-enriched-protein diet on liver size, visceral fat, fat mass, and fat-free mass in patients undergoing sleeve gastrectomy. Surg. Obes. Relat. Dis. 2015, 11, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, D.A.; Hale, A.L.; Ewing, J.A.; Miller, M.; Couto, F.; Bour, E.S.; Cobb, W.S.; Scott, J.D. Short-Term Preoperative Weight Loss and Postoperative Outcomes in Bariatric Surgery. J. Am. Coll. Surg. 2018, 226, 514–524. [Google Scholar] [CrossRef]
- Hollis, G.; Franz, R.; Bauer, J.; Bell, J. Implementation of a very low calorie diet program into the pre-operative model of care for obese general elective surgery patients: Outcomes of a feasibility randomised control trial. Nutr. Diet 2020, 77, 490–498. [Google Scholar] [CrossRef]
- Cicero, A.F.; Benelli, M.; Brancaleoni, M.; Dainelli, G.; Merlini, D.; Negri, R. Middle and Long-Term Impact of a Very Low-Carbohydrate Ketogenic Diet on Cardiometabolic Factors: A Multi-Center, Cross-Sectional, Clinical Study. High Blood Press. Cardiovasc. Prev. 2015, 22, 389–394. [Google Scholar] [CrossRef]
- Ricci, A.; Idzikowski, M.A.; Soares, C.N.; Brietzke, E. Exploring the mechanisms of action of the antidepressant effect of the ketogenic diet. Rev. Neurosci. 2020, 31, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.M.; Kossoff, E.H.; Hartman, A.L. The ketogenic diet: One decade later. Pediatrics 2007, 119, 535–543. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L.; Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO). European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Naso, M.; Perna, S.; Bazire, P.; Sajuox, I.; Maugeri, R.; Rigon, C. The Potential Roles of Very Low Calorie, Very Low Calorie Ketogenic Diets and Very Low Carbohydrate Diets on the Gut Microbiota Composition. Front. Endocrinol. 2021, 12, 662591. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets With Whey, Vegetable, or Animal Protein in Patients With Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef]
- Cappello, G.; Franceschelli, A.; Cappello, A.; De Luca, P. Ketogenic enteral nutrition as a treatment for obesity: Short term and long term results from 19,000 patients. Nutr. Metab. 2012, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Tabesh, M.R.; Maleklou, F.; Ejtehadi, F.; Alizadeh, Z. Nutrition, Physical Activity, and Prescription of Supplements in Pre- and Post-bariatric Surgery Patients: A Practical Guideline. Obes. Surg. 2019, 29, 3385–3400. [Google Scholar] [CrossRef]
- Merra, G.; Gratteri, S.; De Lorenzo, A.; Barrucco, S.; Perrone, M.A.; Avolio, E.; Bernardini, S.; Marchetti, M.; Di Renzo, L. Effects of very-low-calorie diet on body composition, metabolic state, and genes expression: A randomized double-blind placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 329–345. [Google Scholar]
- Bueno, N.B.; de Melo, I.S.V.; de Oliveira, S.L.; da Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Rippy, N.A.; Dorenbos, K.; Concepcion, R.C.; Agarwal, A.K.; Rho, J.M. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004, 55, 576–580. [Google Scholar] [CrossRef]
- Leonetti, F.; Campanile, F.C.; Coccia, F.; Capoccia, D.; Alessandroni, L.; Puzziello, A.; Coluzzi, I.; Silecchia, G. Very low-carbohydrate ketogenic diet before bariatric surgery: Prospective evaluation of a sequential diet. Obes. Surg. 2015, 25, 64–71. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Peterson, L.A.; Cheskin, L.J.; Furtado, M.M.; Papas, K.; Schweitzer, M.A.; Magnuson, T.H.; Steele, K.E. Malnutrition in Bariatric Surgery Candidates: Multiple Micronutrient Deficiencies Prior to Surgery. Obes. Surg. 2016, 26, 833–838. [Google Scholar] [CrossRef]
- Lombardo, M.; Franchi, A.; Padua, E.; Guglielmi, V.; D’Adamo, M.; Annino, G.; Gentileschi, P.; Iellamo, F.; Bellia, A.; Sbraccia, P. Potential Nutritional Deficiencies in Obese Subjects 5 Years After Bariatric Surgery. Bariatr. Surg. Pract. Patient Care 2019, 14, 125–130. [Google Scholar] [CrossRef]
- Guglielmi, V.; D’Adamo, M.; Bellia, A.; Ciotto, R.; Federici, M.; Lauro, D.; Sbraccia, P. Iron status in obesity: An independent association with metabolic parameters and effect of weight loss. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 541–547. [Google Scholar] [CrossRef]
- Thibault, R.; Huber, O.; Azagury, D.E.; Pichard, C. Twelve key nutritional issues in bariatric surgery. Clin. Nutr. 2016, 35, 12–17. [Google Scholar] [CrossRef]
- Lombardo, M.; Franchi, A.; Rinaldi, R.B.; Rizzo, G.; D’Adamo, M.; Guglielmi, V.; Bellia, A.; Padua, E.; Caprio, M.; Sbraccia, P. Long-Term Iron and Vitamin B12 Deficiency Are Present after Bariatric Surgery, Despite the Widespread Use of Supplements. Int. J. Environ. Res. Public Health 2021, 18, 4541. [Google Scholar] [CrossRef] [PubMed]
- Pilone, V.; Tramontano, S.; Renzulli, M.; Romano, M.; Cobellis, L.; Berselli, T.; Schiavo, L. Metabolic effects, safety, and acceptability of very low-calorie ketogenic dietetic scheme on candidates for bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Frey, W.C.; Pilcher, J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes. Surg. 2003, 13, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Tham, K.W.; Lee, P.C.; Lim, C.H. Weight Management in Obstructive Sleep Apnea: Medical and Surgical Options. Sleep Med. Clin. 2019, 14, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Pierro, R.; Asteria, C.; Calabrese, P.; Di Biasio, A.; Coluzzi, I.; Severino, L.; Giovanelli, A.; Pilone, V.; Silecchia, G. Low-Calorie Ketogenic Diet with Continuous Positive Airway Pressure to Alleviate Severe Obstructive Sleep Apnea Syndrome in Patients with Obesity Scheduled for Bariatric/Metabolic Surgery: A Pilot, Prospective, Randomized Multicenter Comparative Study. Obes. Surg. 2022, 32, 634–642. [Google Scholar] [CrossRef]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Weber, R.P.; Arvanitis, M.; Stine, A.; Lux, L.; Harris, R.P. Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017, 317, 415–433. [Google Scholar] [CrossRef]
- Albanese, A.; Prevedello, L.; Markovich, M.; Busetto, L.; Vettor, R.; Foletto, M. Pre-operative Very Low Calorie Ketogenic Diet (VLCKD) vs. Very Low Calorie Diet (VLCD): Surgical Impact. Obes. Surg. 2019, 29, 292–296. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colangeli, L.; Gentileschi, P.; Sbraccia, P.; Guglielmi, V. Ketogenic Diet for Preoperative Weight Reduction in Bariatric Surgery: A Narrative Review. Nutrients 2022, 14, 3610. https://doi.org/10.3390/nu14173610
Colangeli L, Gentileschi P, Sbraccia P, Guglielmi V. Ketogenic Diet for Preoperative Weight Reduction in Bariatric Surgery: A Narrative Review. Nutrients. 2022; 14(17):3610. https://doi.org/10.3390/nu14173610
Chicago/Turabian StyleColangeli, Luca, Paolo Gentileschi, Paolo Sbraccia, and Valeria Guglielmi. 2022. "Ketogenic Diet for Preoperative Weight Reduction in Bariatric Surgery: A Narrative Review" Nutrients 14, no. 17: 3610. https://doi.org/10.3390/nu14173610
APA StyleColangeli, L., Gentileschi, P., Sbraccia, P., & Guglielmi, V. (2022). Ketogenic Diet for Preoperative Weight Reduction in Bariatric Surgery: A Narrative Review. Nutrients, 14(17), 3610. https://doi.org/10.3390/nu14173610







