Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus
Abstract
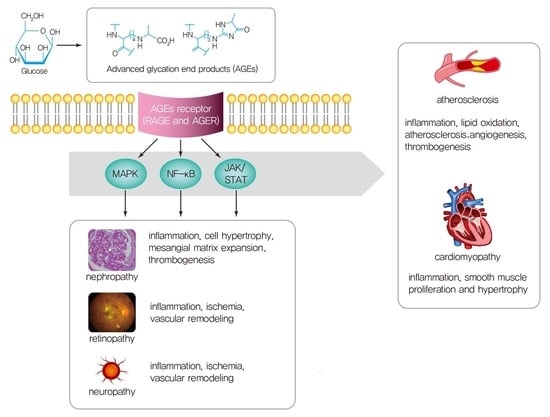
1. Introduction
2. Sources and Formation of AGEs
3. AGEs Interactions with Receptors
4. Pathogenesis of Diabetic Vascular Complications and AGEs in Type 2 DM
4.1. Microvascular Complications and AGEs
Clinical Studies on Microvascular Complications and AGEs
4.2. Macrovascular Complications and AGEs
4.2.1. Role of AGEs in Macrovascular Complications
4.2.2. Role of AGEs in Cardiomyopathy and Atherosclerosis
Clinical Studies on Macrovascular Complications and AGEs
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Son, J.W.; Kang, S.; Kim, W.J.; Kim, H.S.; Kim, H.S.; Seo, M.; Shin, H.J.; Lee, S.S.; Jeong, S.J.; et al. Diabetes Fact Sheets in Korea, 2020: An Appraisal of Current Status. Diabetes Metab. J. 2021, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [CrossRef]
- Yang, Y.S.; Han, K.; Sohn, T.S.; Kim, N.H. Young-onset type 2 diabetes in South Korea: A review of the current status and unmet need. Korean J. Intern. Med. 2021, 36, 1049–1058. [Google Scholar] [CrossRef]
- Park, J.H.; Ha, K.H.; Kim, B.Y.; Lee, J.H.; Kim, D.J. Trends in Cardiovascular Complications and Mortality among Patients with Diabetes in South Korea. Diabetes Metab. J. 2021, 45, 120–124. [Google Scholar] [CrossRef]
- Brar, P.C.; Tell, S.; Mehta, S.; Franklin, B. Hyperosmolar diabetic ketoacidosis-- review of literature and the shifting paradigm in evaluation and management. Diabetes Metab. Syndr. 2021, 15, 102313. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial (DCCT). Update. DCCT Research Group. Diabetes Care 1990, 13, 427–433. [Google Scholar] [CrossRef]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 837–853. [CrossRef]
- Study rationale and design of ADVANCE: Action in diabetes and vascular disease--preterax and diamicron MR controlled evaluation. Diabetologia 2001, 44, 1118–1120. [CrossRef]
- Buse, J.B.; Bigger, J.T.; Byington, R.P.; Cooper, L.S.; Cushman, W.C.; Friedewald, W.T.; Genuth, S.; Gerstein, H.C.; Ginsberg, H.N.; Goff, D.C., Jr.; et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: Design and methods. Am. J. Cardiol. 2007, 99, 21i–33i. [Google Scholar] [CrossRef]
- Gaede, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef]
- Choi, S.E.; Berkowitz, S.A.; Yudkin, J.S.; Naci, H.; Basu, S. Personalizing Second-Line Type 2 Diabetes Treatment Selection: Combining Network Meta-analysis, Individualized Risk, and Patient Preferences for Unified Decision Support. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2019, 39, 239–252. [Google Scholar] [CrossRef]
- Hur, K.Y.; Moon, M.K.; Park, J.S.; Kim, S.K.; Lee, S.H.; Yun, J.S.; Baek, J.H.; Noh, J.; Lee, B.W.; Oh, T.J.; et al. 2021 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association. Diabetes Metab. J. 2021, 45, 461–481. [Google Scholar] [CrossRef]
- Bae, J.; Yoon, J.H.; Lee, J.H.; Nam, J.H.; Lee, C.H.; Son, J.W.; Kim, U.; Park, J.S.; Shin, D.G. Long-term effects of the mean hemoglobin A1c levels after percutaneous coronary intervention in patients with diabetes. Korean J. Intern. Med. 2021, 36, 1365–1376. [Google Scholar] [CrossRef]
- Hoffmann, A.P.; Honigberg, M.C. Glycated Hemoglobin as an Integrator of Cardiovascular Risk in Individuals Without Diabetes: Lessons from Recent Epidemiologic Studies. Curr. Atheroscler. Rep. 2022, 24, 435–442. [Google Scholar] [CrossRef]
- Rigon, F.A.; Ronsoni, M.F.; Vianna, A.G.D.; de Lucca Schiavon, L.; Hohl, A.; van de Sande-Lee, S. Flash glucose monitoring system in special situations. Arch. Endocrinol. Metab. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, J.H. Time in Range from Continuous Glucose Monitoring: A Novel Metric for Glycemic Control. Diabetes Metab. J. 2020, 44, 828–839. [Google Scholar] [CrossRef]
- Shamsi, A.; Shahwan, M.; Husain, F.M.; Khan, M.S. Characterization of methylglyoxal induced advanced glycation end products and aggregates of human transferrin: Biophysical and microscopic insight. Int. J. Biol. Macromol. 2019, 138, 718–724. [Google Scholar] [CrossRef]
- Koska, J.; Saremi, A.; Howell, S.; Bahn, G.; De Courten, B.; Ginsberg, H.; Beisswenger, P.J.; Reaven, P.D. Advanced Glycation End Products, Oxidation Products, and Incident Cardiovascular Events in Patients With Type 2 Diabetes. Diabetes Care 2018, 41, 570–576. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef]
- Peterson, S.B.; Hart, G.W. New insights: A role for O-GlcNAcylation in diabetic complications. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 150–161. [Google Scholar] [CrossRef]
- Indyk, D.; Bronowicka-Szydełko, A.; Gamian, A.; Kuzan, A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021, 11, 13264. [Google Scholar] [CrossRef]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. (Camb. Mass.) 2018, 24, 59. [Google Scholar] [CrossRef]
- Maillard, L.C. Action des acides amines sur les sucres; formation des melanoidines par voie methodique. Comptes R. Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Garay-Sevilla, M.E.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia 2016, 59, 2181–2192. [Google Scholar] [CrossRef]
- Sergi, D.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The Role of Dietary Advanced Glycation End Products in Metabolic Dysfunction. Mol. Nutr. Food Res. 2021, 65, e1900934. [Google Scholar] [CrossRef]
- Henning, C.; Glomb, M.A. Pathways of the Maillard reaction under physiological conditions. Glycoconj. J. 2016, 33, 499–512. [Google Scholar] [CrossRef]
- Saremi, A.; Howell, S.; Schwenke, D.C.; Bahn, G.; Beisswenger, P.J.; Reaven, P.D. Advanced Glycation End Products, Oxidation Products, and the Extent of Atherosclerosis During the VA Diabetes Trial and Follow-up Study. Diabetes Care 2017, 40, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, A. Toxicity of advanced glycation end products (Review). Biomed. Rep. 2021, 14, 46. [Google Scholar] [CrossRef]
- Miroliaei, M.; Aminjafari, A.; Ślusarczyk, S.; Nawrot-Hadzik, I.; Rahimmalek, M.; Matkowski, A. Inhibition of Glycation-induced Cytotoxicity, Protein Glycation, and Activity of Proteolytic Enzymes by Extract from Perovskia atriplicifolia Roots. Pharmacogn. Mag. 2017, 13, S676–S683. [Google Scholar] [CrossRef]
- Takeuchi, M.; Yamagishi, S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med. Hypotheses 2004, 63, 449–452. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, É. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology 2019, 20, 279–301. [Google Scholar] [CrossRef]
- Prasad, K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases? Mol. Cell. Biochem. 2019, 451, 139–144. [Google Scholar] [CrossRef]
- Dozio, E.; Massaccesi, L.; Corsi Romanelli, M.M. Glycation and Glycosylation in Cardiovascular Remodeling: Focus on Advanced Glycation End Products and O-Linked Glycosylations as Glucose-Related Pathogenetic Factors and Disease Markers. J. Clin. Med. 2021, 10, 4792. [Google Scholar] [CrossRef]
- Yu, W.; Tao, M.; Zhao, Y.; Hu, X.; Wang, M. 4’-Methoxyresveratrol Alleviated AGE-Induced Inflammation via RAGE-Mediated NF-κB and NLRP3 Inflammasome Pathway. Molecules 2018, 23, 1447. [Google Scholar] [CrossRef]
- Schmidt, A.M. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vasc. Pharmacol. 2015, 72, 1–8. [Google Scholar] [CrossRef]
- Lee, A.C.; Lam, J.K.; Shiu, S.W.; Wong, Y.; Betteridge, D.J.; Tan, K.C. Serum Level of Soluble Receptor for Advanced Glycation End Products Is Associated with A Disintegrin And Metalloproteinase 10 in Type 1 Diabetes. PLoS ONE 2015, 10, e0137330. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.; Hitman, G.; Neil, A.; Livingstone, S.; Charlton-Menys, V.; Bao, W.; Demicco, D.A.; Preston, G.M.; et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: An analysis from the CARDS trial. Diabetes 2011, 60, 2379–2385. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Schmidt, A.M.; Hudson, B.I. Alternative splicing of RAGE: Roles in biology and disease. Front. Biosci. (Landmark Ed.) 2011, 16, 2756–2770. [Google Scholar] [CrossRef]
- Chawla, D.; Bansal, S.; Banerjee, B.D.; Madhu, S.V.; Kalra, O.P.; Tripathi, A.K. Role of advanced glycation end product (AGE)-induced receptor (RAGE) expression in diabetic vascular complications. Microvasc. Res. 2014, 95, 1–6. [Google Scholar] [CrossRef]
- Matsui, T.; Oda, E.; Higashimoto, Y.; Yamagishi, S. Glyceraldehyde-derived pyridinium (GLAP) evokes oxidative stress and inflammatory and thrombogenic reactions in endothelial cells via the interaction with RAGE. Cardiovasc. Diabetol. 2015, 14, 1. [Google Scholar] [CrossRef]
- Kobori, T.; Ganesh, D.; Kumano-Kuramochi, M.; Torigoe, K.; Machida, S. Assay for advanced glycation end products generating intracellular oxidative stress through binding to its receptor. Anal. Biochem. 2020, 611, 114018. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Kumar Pasupulati, A.; Chitra, P.S.; Reddy, G.B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol. Concepts 2016, 7, 293–309. [Google Scholar] [CrossRef]
- Bucciarelli, L.G.; Wendt, T.; Rong, L.; Lalla, E.; Hofmann, M.A.; Goova, M.T.; Taguchi, A.; Yan, S.F.; Yan, S.D.; Stern, D.M.; et al. RAGE is a multiligand receptor of the immunoglobulin superfamily: Implications for homeostasis and chronic disease. Cell. Mol. Life Sci. CMLS 2002, 59, 1117–1128. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, J.H. Receptor for Advanced Glycation Endproducts (RAGE), Its Ligands, and Soluble RAGE: Potential Biomarkers for Diagnosis and Therapeutic Targets for Human Renal Diseases. Genom. Inform. 2013, 11, 224–229. [Google Scholar] [CrossRef]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Goh, S.-Y.; Cooper, M.E. The Role of Advanced Glycation End Products in Progression and Complications of Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Zhu, Y.J.; Li, C.G.; Tang, Y.Z.; Jiang, Z.H.; Yang, M.; Ni, C.L.; Chen, L.M.; Niu, W.Y. The relationship between circulating irisin levels and tissues AGE accumulation in type 2 diabetes patients. Biosci. Rep. 2017, 37, BSR20170213. [Google Scholar] [CrossRef]
- Mishriky, B.M.; Cummings, D.M.; Powell, J.R. Diabetes-Related Microvascular Complications—A Practical Approach. Prim. Care 2022, 49, 239–254. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef]
- Arora, M.K.; Singh, U.K. Molecular mechanisms in the pathogenesis of diabetic nephropathy: An update. Vasc. Pharmacol. 2013, 58, 259–271. [Google Scholar] [CrossRef]
- Persson, F.; Rossing, P. Diagnosis of diabetic kidney disease: State of the art and future perspective. Kidney Int. Suppl. 2018, 8, 2–7. [Google Scholar] [CrossRef]
- Sun, H.; Yuan, Y.; Sun, Z. Update on Mechanisms of Renal Tubule Injury Caused by Advanced Glycation End Products. BioMed Res. Int. 2016, 2016, 5475120. [Google Scholar] [CrossRef]
- Matsui, T.; Yamagishi, S.; Takeuchi, M.; Ueda, S.; Fukami, K.; Okuda, S. Irbesartan inhibits advanced glycation end product (AGE)-induced proximal tubular cell injury in vitro by suppressing receptor for AGEs (RAGE) expression. Pharmacol. Res. 2010, 61, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Yamagishi, S.; Coughlan, M.T.; Harcourt, B.E.; Kantharidis, P.; Thallas-Bonke, V.; Okuda, S.; Cooper, M.E.; Forbes, J.M. Ramipril inhibits AGE-RAGE-induced matrix metalloproteinase-2 activation in experimental diabetic nephropathy. Diabetol. Metab. Syndr. 2014, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Ojima, A.; Matsui, T.; Nishino, Y.; Nakamura, N.; Yamagishi, S. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm. Metab. Res.=Horm.-Und Stoffwechs.=Horm. Et Metab. 2015, 47, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Sanajou, D.; Ghorbani Haghjo, A.; Argani, H.; Aslani, S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. Eur. J. Pharmacol. 2018, 833, 158–164. [Google Scholar] [CrossRef]
- Matoba, K.; Kawanami, D.; Tsukamoto, M.; Kinoshita, J.; Ito, T.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Murai, N.; Matsufuji, S.; et al. Rho-kinase regulation of TNF-α-induced nuclear translocation of NF-κB RelA/p65 and M-CSF expression via p38 MAPK in mesangial cells. Am. J. Physiol. Ren. Physiol. 2014, 307, F571–F580. [Google Scholar] [CrossRef]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3393. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Brosius, F.C., 3rd; Adler, S.G.; Kretzler, M.; Mehta, R.L.; Tumlin, J.A.; Tanaka, Y.; Haneda, M.; Liu, J.; Silk, M.E.; et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: Results from a Phase 2 randomized controlled clinical trial. Nephrol. Dial. Transplant. 2018, 33, 1950–1959. [Google Scholar] [CrossRef]
- Zhang, M.H.; Feng, L.; Zhu, M.M.; Gu, J.F.; Jiang, J.; Cheng, X.D.; Ding, S.M.; Wu, C.; Jia, X.B. The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats. J. Ethnopharmacol. 2014, 151, 591–600. [Google Scholar] [CrossRef]
- Anil Kumar, P.; Welsh, G.I.; Saleem, M.A.; Menon, R.K. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front. Endocrinol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Yadav, A.; Vallabu, S.; Arora, S.; Tandon, P.; Slahan, D.; Teichberg, S.; Singhal, P.C. ANG II promotes autophagy in podocytes. Am. J. Physiol. Cell Physiol. 2010, 299, C488–C496. [Google Scholar] [CrossRef]
- Seong, S.B.; Ha, D.S.; Min, S.Y.; Ha, T.S. Autophagy Precedes Apoptosis in Angiotensin II-Induced Podocyte Injury. Cell. Physiol. Biochem. 2019, 53, 747–759. [Google Scholar] [CrossRef]
- Stitt, A.W. Advanced glycation: An important pathological event in diabetic and age related ocular disease. Br. J. Ophthalmol. 2001, 85, 746–753. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxidative Med. Cell Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Münch, G.; Westcott, B.; Menini, T.; Gugliucci, A. Advanced glycation endproducts and their pathogenic roles in neurological disorders. Amino Acids 2012, 42, 1221–1236. [Google Scholar] [CrossRef]
- Jack, M.; Wright, D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl. Res. 2012, 159, 355–365. [Google Scholar] [CrossRef]
- Saleh, A.; Smith, D.R.; Tessler, L.; Mateo, A.R.; Martens, C.; Schartner, E.; Van der Ploeg, R.; Toth, C.; Zochodne, D.W.; Fernyhough, P. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Exp. Neurol. 2013, 249, 149–159. [Google Scholar] [CrossRef]
- Skrha, J., Jr.; Soupal, J.; Loni Ekali, G.; Prázný, M.; Kalousová, M.; Kvasnička, J.; Landová, L.; Zima, T.; Skrha, J. Skin autofluorescence relates to soluble receptor for advanced glycation end-products and albuminuria in diabetes mellitus. J. Diabetes Res. 2013, 2013, 650694. [Google Scholar] [CrossRef]
- Rigalleau, V.; Cougnard-Gregoire, A.; Nov, S.; Gonzalez, C.; Maury, E.; Lorrain, S.; Gin, H.; Barberger-Gateau, P. Association of advanced glycation end products and chronic kidney disease with macroangiopathy in type 2 diabetes. J. Diabetes Its Complicat. 2015, 29, 270–274. [Google Scholar] [CrossRef]
- Yozgatli, K.; Lefrandt, J.D.; Noordzij, M.J.; Oomen, P.H.N.; Brouwer, T.; Jager, J.; Castro Cabezas, M.; Smit, A.J. Accumulation of advanced glycation end products is associated with macrovascular events and glycaemic control with microvascular complications in Type 2 diabetes mellitus. Diabet. Med. 2018, 35, 1242–1248. [Google Scholar] [CrossRef]
- Farhan, S.S.; Hussain, S.A. Advanced glycation end products (AGEs) and their soluble receptors (sRAGE) as early predictors of reno-vascular complications in patients with uncontrolled type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 2457–2461. [Google Scholar] [CrossRef]
- Nishad, R.; Tahaseen, V.; Kavvuri, R.; Motrapu, M.; Singh, A.K.; Peddi, K.; Pasupulati, A.K. Advanced-Glycation End-Products Induce Podocyte Injury and Contribute to Proteinuria. Front. Med. 2021, 8, 685447. [Google Scholar] [CrossRef]
- Koska, J.; Gerstein, H.C.; Beisswenger, P.J.; Reaven, P.D. Advanced Glycation End Products Predict Loss of Renal Function and High-Risk Chronic Kidney Disease in Type 2 Diabetes. Diabetes Care 2022, 45, 684–691. [Google Scholar] [CrossRef]
- Jin, Q.; Lau, E.S.; Luk, A.O.; Ozaki, R.; Chow, E.Y.; So, T.; Yeung, T.; Loo, K.M.; Lim, C.K.; Kong, A.P.; et al. Skin autofluorescence is associated with progression of kidney disease in type 2 diabetes: A prospective cohort study from the Hong Kong diabetes biobank. Nutr. Metab. Cardiovasc. Dis. NMCD 2022, 32, 436–446. [Google Scholar] [CrossRef]
- Ng, Z.X.; Chua, K.H.; Iqbal, T.; Kuppusamy, U.R. Soluble receptor for advanced glycation end-product (sRAGE)/pentosidine ratio: A potential risk factor determinant for type 2 diabetic retinopathy. Int. J. Mol. Sci. 2013, 14, 7480–7491. [Google Scholar] [CrossRef]
- Vouillarmet, J.; Maucort-Boulch, D.; Michon, P.; Thivolet, C. Advanced glycation end products assessed by skin autofluorescence: A new marker of diabetic foot ulceration. Diabetes Technol. Ther. 2013, 15, 601–605. [Google Scholar] [CrossRef]
- Aubert, C.E.; Michel, P.L.; Gillery, P.; Jaisson, S.; Fonfrede, M.; Morel, F.; Hartemann, A.; Bourron, O. Association of peripheral neuropathy with circulating advanced glycation end products, soluble receptor for advanced glycation end products and other risk factors in patients with type 2 diabetes. Diabetes/Metab. Res. Rev. 2014, 30, 679–685. [Google Scholar] [CrossRef]
- Zhao, X.W.; Yue, W.X.; Zhang, S.W.; Chen, Q. Correlation between the accumulation of skin glycosylation end products and the development of type 2 diabetic peripheral neuropathy. BMC Endocr. Disord. 2022, 22, 106. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Kim, H.C. Epidemiology of cardiovascular disease and its risk factors in Korea. Glob. Health Med. 2021, 3, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef] [PubMed]
- Avagimyan, A. The Pathophysiological Basis of Diabetic Cardiomyopathy Development. Curr. Probl. Cardiol. 2022, 19, 101156. [Google Scholar] [CrossRef] [PubMed]
- Yuen, A.; Laschinger, C.; Talior, I.; Lee, W.; Chan, M.; Birek, J.; Young, E.W.; Sivagurunathan, K.; Won, E.; Simmons, C.A.; et al. Methylglyoxal-modified collagen promotes myofibroblast differentiation. Matrix Biol. 2010, 29, 537–548. [Google Scholar] [CrossRef]
- Nabi, R.; Alvi, S.S.; Saeed, M.; Ahmad, S.; Khan, M.S. Glycation and HMG-CoA Reductase Inhibitors: Implication in Diabetes and Associated Complications. Curr. Diabetes Rev. 2019, 15, 213–223. [Google Scholar] [CrossRef]
- Di Marco, E.; Gray, S.P.; Jandeleit-Dahm, K. Diabetes alters activation and repression of pro- and anti-inflammatory signaling pathways in the vasculature. Front. Endocrinol. 2013, 4, 68. [Google Scholar] [CrossRef][Green Version]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.R.; Li, P.C.; Feng, B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: Increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis. 2016, 15, 161. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Z.; Xu, Y. HDL and Oxidation. Adv. Exp. Med. Biol. 2022, 1377, 63–77. [Google Scholar] [CrossRef]
- Bidasee, K.R.; Zhang, Y.; Shao, C.H.; Wang, M.; Patel, K.P.; Dincer, U.D.; Besch, H.R., Jr. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 2004, 53, 463–473. [Google Scholar] [CrossRef]
- Fischer, T.H.; Herting, J.; Tirilomis, T.; Renner, A.; Neef, S.; Toischer, K.; Ellenberger, D.; Förster, A.; Schmitto, J.D.; Gummert, J.; et al. Ca2+/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology. Circulation 2013, 128, 970–981. [Google Scholar] [CrossRef]
- Kosmopoulos, M.; Drekolias, D.; Zavras, P.D.; Piperi, C.; Papavassiliou, A.G. Impact of advanced glycation end products (AGEs) signaling in coronary artery disease. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2019, 1865, 611–619. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Yan, S.F.; Yan, S.D.; Belov, D.; Rong, L.L.; Sousa, M.; Andrassy, M.; Marso, S.P.; Duda, S.; Arnold, B.; et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J. Clin. Investig. 2003, 111, 959–972. [Google Scholar] [CrossRef]
- Yamagishi, S.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. Et Biophys. Acta 2012, 1820, 663–671. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Kim, Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.W.; Gong, J.; Li, E.T.S.; Wang, M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem. Pharmacol. 2019, 166, 231–241. [Google Scholar] [CrossRef]
- De la Cruz-Ares, S.; Cardelo, M.P.; Gutiérrez-Mariscal, F.M.; Torres-Peña, J.D.; García-Rios, A.; Katsiki, N.; Malagón, M.M.; López-Miranda, J.; Pérez-Martínez, P.; Yubero-Serrano, E.M. Endothelial Dysfunction and Advanced Glycation End Products in Patients with Newly Diagnosed Versus Established Diabetes: From the CORDIOPREV Study. Nutrients 2020, 12, 238. [Google Scholar] [CrossRef]
- Stirban, A.; Kotsi, P.; Franke, K.; Strijowski, U.; Cai, W.; Götting, C.; Tschoepe, D. Acute macrovascular dysfunction in patients with type 2 diabetes induced by ingestion of advanced glycated β-lactoglobulins. Diabetes Care 2013, 36, 1278–1282. [Google Scholar] [CrossRef]
- Linkens, A.M.; Houben, A.J.; Niessen, P.M.; Wijckmans, N.E.; de Goei, E.E.; Van den Eynde, M.D.; Scheijen, J.L.; van den Waarenburg, M.P.; Mari, A.; Berendschot, T.T.; et al. A 4-week high-AGE diet does not impair glucose metabolism and vascular function in obese individuals. JCI Insight 2022, 7, e156950. [Google Scholar] [CrossRef]
- De Vos, L.C.; Mulder, D.J.; Smit, A.J.; Dullaart, R.P.; Kleefstra, N.; Lijfering, W.M.; Kamphuisen, P.W.; Zeebregts, C.J.; Lefrandt, J.D. Skin autofluorescence is associated with 5-year mortality and cardiovascular events in patients with peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 933–938. [Google Scholar] [CrossRef]
- Ninomiya, H.; Katakami, N.; Sato, I.; Osawa, S.; Yamamoto, Y.; Takahara, M.; Kawamori, D.; Matsuoka, T.A.; Shimomura, I. Association between Subclinical Atherosclerosis Markers and the Level of Accumulated Advanced Glycation End-Products in the Skin of Patients with Diabetes. J. Atheroscler. Thromb. 2018, 25, 1274–1284. [Google Scholar] [CrossRef]
| No | Authors | Year | Country | Subjects/Study Design | Finding |
|---|---|---|---|---|---|
| Diabetic nephropathy | |||||
| 1 | Skrha J. Jr., Soupal J., Loni Ekali et al. [79] | 2013 | Czechoslovakia | 41 subjects with type 2 DM (47 subjects with type 1 DM) /cross-sectional | Higher AGEs levels were correlated with the albumin–creatinine ratio |
| 2. | Galleau V., Cougnard-Gregoire A., Nov S et al. [80] | 2015 | France | 418 subjects/cohort study | AGEs accumulation was associated with renal insufficiency |
| 3 | Yozgatli K., Lefrandt, J.D., Noordzij M.J et al. [81] | 2018 | UK | 563 subjects/prospective cohort study | Development of microvascular complications was associated with HbA1c, not tissue accumulation of AGEs |
| 4 | Farhan S.S. and Hussain S.A. [82] | 2019 | Iraq | 50 subjects Cross-sectional | There was a positive correlation between AGEs ratio and urine albumin/serum ratio in type 2 DM |
| 5 | Nishad R., Tahaseen V., Kavvuri R. et al. [83] | 2021 | India | 130 subjects with albuminuria ranging from 150–450 mg/day/cross-sectional | A significant association between AGEs and impaired kidney function was observed in type 2 DM patients using AGE index. |
| 6 | Koska J, Gerstein H.C., Beisswenger P.J. et al. [84] | 2022 | USA | Action to Control Cardiovascular Risk in Diabetes (ACCORD) (n = 1150) and Veterans Affairs Diabetes Trial (VADT) (n = 447) participants/cohort study | AGEs in two different type 2 DM cohorts showed strong correlation with renal outcomes of reduced eGFR and macroalbuminuria. |
| 7 | Jin Q., Lau E.S., Luk A.O, Ozaki R, et al. [85] | 2022 | Hong Kong | 3725 subjects/cohort study | AGEs measured by higher skin autofluorescence level were associated with kidney disease progression in type 2 DM |
| Diabetic retinopathy | |||||
| 1. | Ng Z.X., Chua K.H., Iqbal T, et al. [86] | 2013 | Malaya | 171 type 2 DM subjects versus 235 healthy control/case–control study | Proliferative DR patients had significantly higher levels of plasma pentosidine |
| Diabetic neuropathy | |||||
| 1. | Vouillarmet J., Maucort-Boulch D., Michon P. et al. [87] | 2013 | France | 66 subjects/prospective cohort study | AGEs measured by skin auto-fluorescence predict diabetic foot. |
| 2. | Abuert C.E., Michel P.L., Gillery P. et al. [88] | 2014 | Switzerland | 198 subjects/cohort study | CML and sRAGE were associated with DPN in patients with type 2 DM |
| 3. | Zhao X.W., Yue W.X., Zhang S.W. et al. [89] | 2022 | China | 560 subject/cohort study | Accumulation of AGEs measured using skin autofluorescence is correlated with DPN |
| No | Authors | Year | Country | Subjects/Study Design | Finding |
|---|---|---|---|---|---|
| 1. | Stirban A., Kotsi P., Franke K. et al. [109] | 2013 | Germany | 19 subjects/randomized-controlled study | Administration of a single AGE-modified protein impaired macrovascular function |
| 2. | Chawla D., Bansal S, Banerjee B.D. et al. [46] | 2014 | India | 75 subjects/cohort study | Serum AGEs levels were significantly higher in DM with vascular complications as compared to T2DM without complications |
| 3. | De Vos L.C., Mulder D.J., Smit A.J. et al. [111] | 2014 | Netherlands | 252 subjects/prospective cohort study | AGEs level measured by skin autofluorescence was significantly correlated with all-cause mortality and peripheral vascular disease |
| 4. | Yozgatli K., Lefrandt J.D., Noordzij M.J. et al. [80] | 2018 | Netherlands | 563 subjects in multicenter /cohort study | AGEs were associated with the development of macrovascular events |
| 5. | Koska J., Saremi A, Howell S. et al. [20] | 2018 | USA | 445 subjects from VADT and 271 subjects from the ACCORD study | Higher levels of select AGEs were associated with an increased incidence of CVD |
| 6. | Ninomiya H., Katakami N., Sato I. et al. [112] | 2018 | Japan | 115 type 2 DM and 25 type 2 DM subjects/prospective cohort study | AGEs can be utilized as a screening marker of atherosclerosis |
| 7. | De la Cruz-Ares S., Cardelo M.P., Gutiérrez-Mariscal F.M. et al. [108] | 2020 | Spain | 540 subjects/cross-sectional study | AGEs levels and intima-media thickness of the common carotid arteries were higher in patients with CVD and type 2 DM |
| 8. | Linkens A.M., Houben A.J., Niessen P.M. et al. [110] | 2022 | Netherlands | 82 subjects/randomized-controlled study | A 4-week diet low or high in AGEs had no effect on vascular function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yun, J.-S.; Ko, S.-H. Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 3086. https://doi.org/10.3390/nu14153086
Lee J, Yun J-S, Ko S-H. Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients. 2022; 14(15):3086. https://doi.org/10.3390/nu14153086
Chicago/Turabian StyleLee, Jeongmin, Jae-Seung Yun, and Seung-Hyun Ko. 2022. "Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus" Nutrients 14, no. 15: 3086. https://doi.org/10.3390/nu14153086
APA StyleLee, J., Yun, J.-S., & Ko, S.-H. (2022). Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients, 14(15), 3086. https://doi.org/10.3390/nu14153086