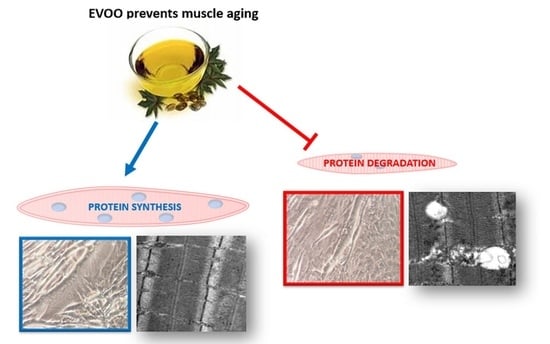
Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation
Abstract
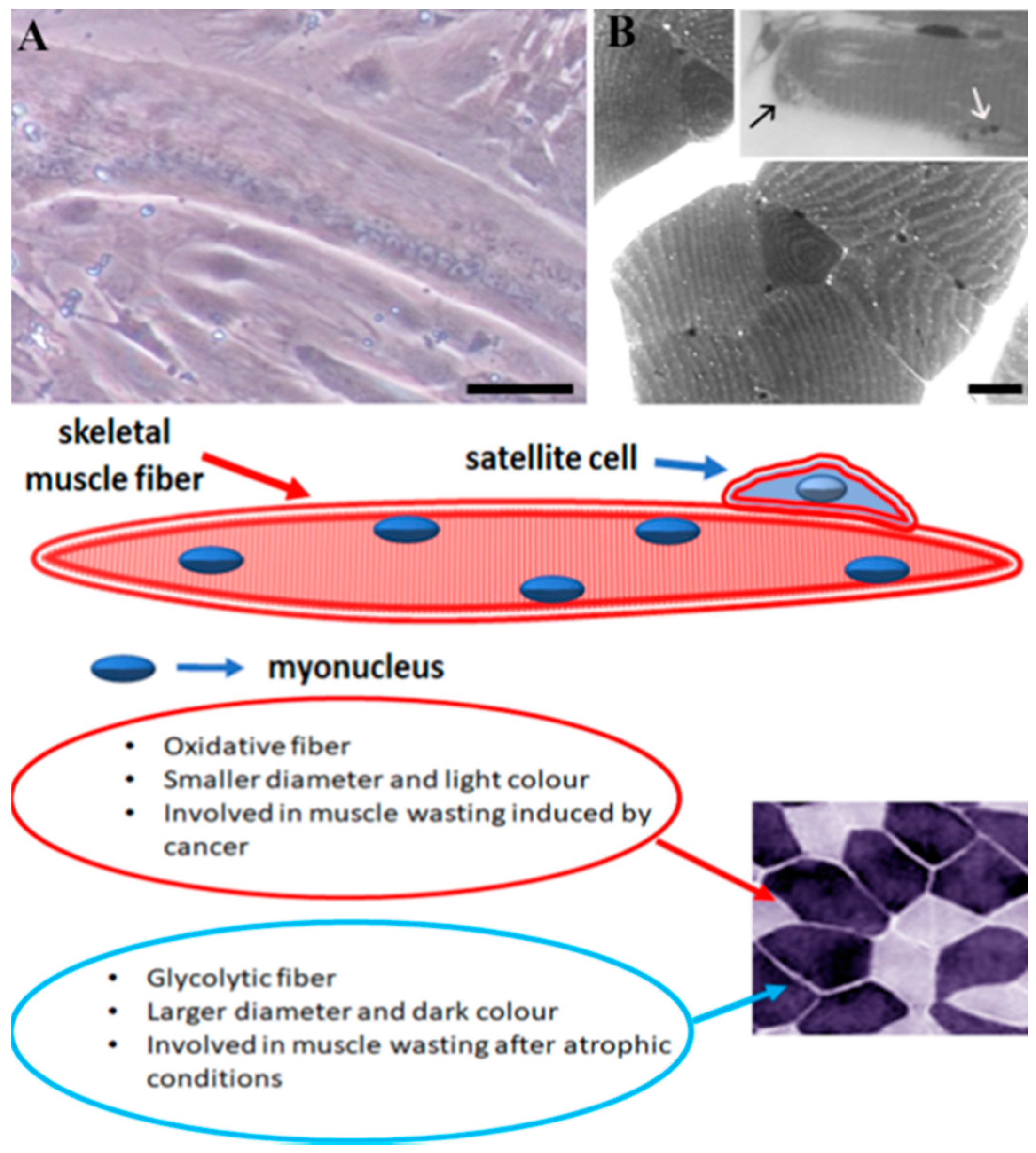
1. Introduction: Skeletal Muscle Biology
2. Materials and Methods
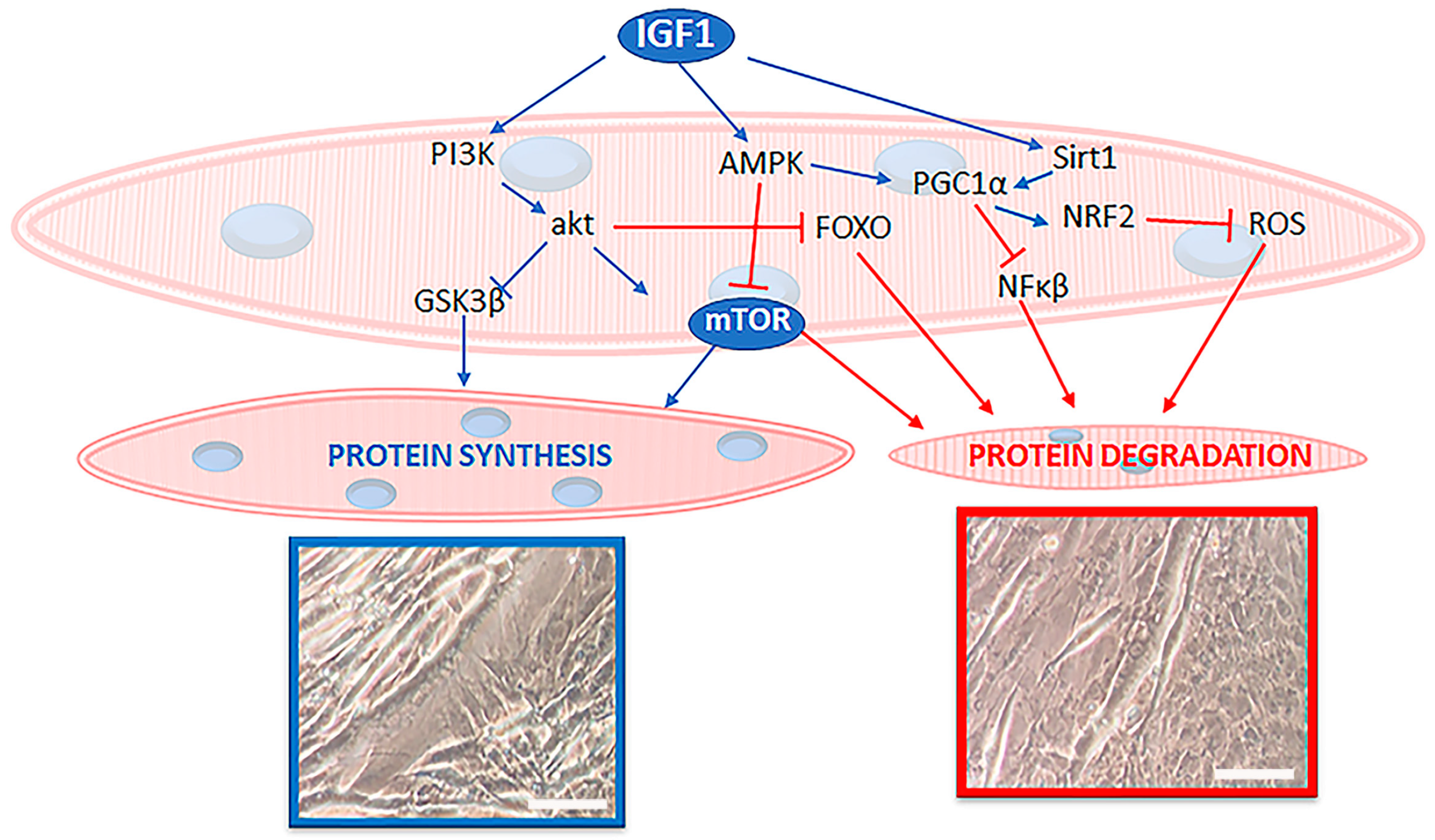
3. Protein Synthesis Regulators
4. Muscle Mass Loss during Sarcopenia
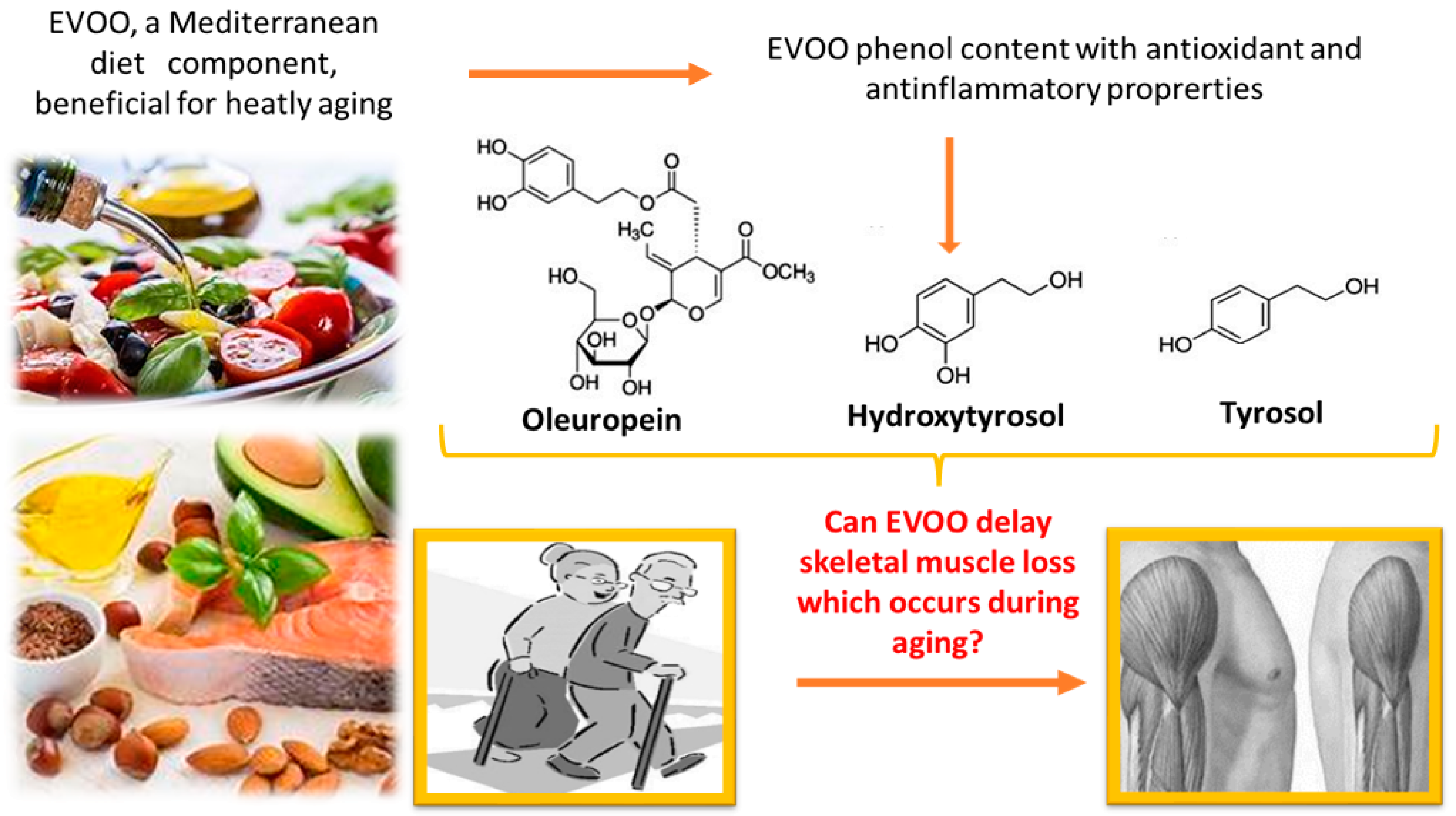
5. Extra Virgin Olive Oil (EVOO)
6. Can EVOO Prevent Sarcopenia?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J., III; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Nakamura, N. Ubiquitin System. Int. J. Mol. Sci. 2018, 19, 1080. [Google Scholar] [CrossRef]
- Burattini, S.; Battistelli, M.; Codenotti, S.; Falcieri, E.; Fanzani, A.; Salucci, S. Melatonin action in tumor skeletal muscle cells: An ultrastructural study. Acta Histochem. 2016, 118, 278–285. [Google Scholar] [CrossRef]
- Snijders, T.; Nederveen, J.P.; McKay, B.R.; Joanisse, S.; Verdijk, L.B.; van Loon, L.J.; Parise, G. Satellite cells in human skeletal muscle plasticity. Front. Physiol. 2015, 6, 283. [Google Scholar] [CrossRef]
- Salucci, S.; Taurone, S.; Burattini, S.; Gobbi, P.; Clausi, J.; Battistelli, M. Melatonin role in skeletal muscle disorders. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1024–1033. [Google Scholar]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Giordano, F.M.; Burattini, S.; Buontempo, F.; Canonico, B.; Martelli, A.M.; Papa, S.; Sampaolesi, M.; Falcieri, E.; Salucci, S. Diet Modulation Restores Autophagic Flux in Damaged Skeletal Muscle Cells. J. Nutr. Health Aging 2019, 23, 739–745. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Yue, F.; Bi, P.; Wang, C.; Shan, T.; Nie, Y.; Ratliff, T.L.; Gavin, T.P.; Kuang, S. Pten is necessary for the quiescence and maintenance of adult muscle stem cells. Nat. Commun. 2017, 8, 14328. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Millay, D.P.; Olson, E.N. Making muscle or mitochondria by selective splicing of PGC-1α. Cell Metab. 2013, 17, 3–4. [Google Scholar] [CrossRef][Green Version]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef]
- Comai, G.; Tajbakhsh, S. Molecular and cellular regulation of skeletal myogenesis. Curr. Top. Dev. Biol. 2014, 110, 1–73. [Google Scholar]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.D.P.; Howell, S.L.; Teixeira, F.J.; Pimentel, G.D. Dietary Amino Acids and Immunonutrition Supplementation in Cancer-Induced Skeletal Muscle Mass Depletion: A Mini-Review. Curr. Pharm. Des. 2020, 26, 970–978. [Google Scholar] [CrossRef]
- Pham, T.; MacRae, C.L.; Broome, S.C.; D’souza, R.F.; Narang, R.; Wang, H.W.; Mori, T.A.; Hickey, A.J.R.; Mitchell, C.J.; Merry, T.L. MitoQ and CoQ10 supplementation mildly suppresses skeletal muscle mitochondrial hydrogen peroxide levels without impacting mitochondrial function in middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T. Extra Virgin Olive Oil (EVOO): Quality, Safety, Authenticity, and Adulteration. Foods 2021, 10, 995. [Google Scholar] [CrossRef]
- Dhillon, R.J.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef] [PubMed]
- Nindl, B.C.; Santtila, M.; Vaara, J.; Hakkinen, K.; Kyrolainen, H. Circulating IGF-I is associated with fitness and health outcomes in a population of 846 young healthy men. Growth Horm. IGF Res. 2011, 21, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; De Vita, F.; Lauretani, F.; Butto, V.; Bondi, G.; Cattabiani, C.; Nouvenne, A.; Meschi, T.; Dall’Aglio, E.; Ceda, G.P. IGF-1, the crossroad of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients 2013, 5, 4184–4205. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.; Schoenfeld, B.J.; Nakazato, K. The role of hormones in muscle hypertrophy. Phys. Sports Med. 2018, 46, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Musarò, A.; Scicchitano, B.M. Counteracting sarcopenia: The role of IGF-1 isoforms. Aging 2019, 11, 3410–3411. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Gruner, S.; Peter, D.; Weber, R.; Wohlbold, L.; Chung, M.Y.; Weichenrieder, O.; Valkov, E.; Igreja, C.; Izaurralde, E. The structures of eIF4E-eIF4G complexes reveal an extended interface to regulate translation initiation. Mol. Cell 2016, 64, 467–479. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef]
- Piazzi, M.; Bavelloni, A.; Cenni, V.; Faenza, I.; Blalock, W.L. Revisiting the Role of GSK3, A Modulator of Innate Immunity, in Idiopathic Inclusion Body Myositis. Cells 2021, 10, 3255. [Google Scholar] [CrossRef]
- Mirzoev, T.M.; Sharlo, K.A.; Shenkman, B.S. The Role of GSK-3β in the Regulation of Protein Turnover, Myosin Phenotype, and Oxidative Capacity in Skeletal Muscle under Disuse Conditions. Int. J. Mol. Sci. 2021, 22, 5081. [Google Scholar] [CrossRef]
- Verhees, K.J.; Schols, A.M.; Kelders, M.C.; Op den Kamp, C.M.; van der Velden, J.L.; Langen, R.C. Glycogen synthase kinase-3β is required for the induction of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2011, 301, C995–C1007. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Gesmundo, I.; Trovato, L.; Pera, G.; Gargantini, E.; Minetto, M.A.; Ghigo, E.; Granata, R. GH-Releasing Hormone Promotes Survival and Prevents TNF-α-Induced Apoptosis and Atrophy in C2C12 Myotubes. Endocrinology 2015, 156, 3239–3252. [Google Scholar] [CrossRef] [PubMed]
- Kim Do, Y.; Park, E.Y.; Chang, E.; Kang, H.G.; Koo, Y.; Lee, E.J.; Ko, J.Y.; Kong, H.K.; Chun, K.H.; Park, J.H. A novel miR-34a target, protein kinase D1, stimulates cancer stemness and drug resistance through GSK3/beta-catenin signaling in breast cancer. Oncotarget 2016, 7, 14791–14802. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Z.; Zhang, R.; Hu, Y.; Jiang, Y.; Cao, T.; Wang, J.; Gong, L.; Ji, L.; Mu, H.; et al. PKCgamma promotes axonal remodeling in the cortico-spinal tract via GSK3beta/beta-catenin signaling after traumatic brain injury. Sci. Rep. 2019, 9, 17078. [Google Scholar] [CrossRef] [PubMed]
- Barbe, C.; Kalista, S.; Loumaye, A.; Ritvos, O.; Lause, P.; Ferracin, B.; Thissen, J.P. Role of IGF-I in follistatin-induced skeletal muscle hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E557–E567. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Castets, P.; Lin, S.; Rion, N.; Fulvio, S.D.; Romanino, K.; Guridi, M.; Frank, S.; Tintignac, L.A.; Sinnreich, M.; Rüegg, M.A. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metab. 2013, 17, 731–744. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Dabrowska, A.; Venero, J.L.; Iwasawa, R.; Hankir, M.K.; Rahman, S.; Boobis, A.; Hajji, N. Erratum: PGC-1α controls mitochondrial biogenesis and dynamics in lead-induced neurotoxicity. Aging 2015, 7, 1023. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Kou, G.; Li, Z.; Wu, C.; Liu, Y.; Hu, Y.; Guo, L.; Xu, X.; Zhou, Z. Citrus Tangeretin Improves Skeletal Muscle Mitochondrial Biogenesis via Activating the AMPK-PGC1-α Pathway In Vitro and In Vivo: A Possible Mechanism for Its Beneficial Effect on Physical Performance. J. Agric. Food Chem. 2018, 66, 11917–11925. [Google Scholar] [CrossRef]
- Petrocelli, J.J.; Drummond, M.J. PGC-1α-Targeted Therapeutic Approaches to Enhance Muscle Recovery in Aging. Int. J. Environ. Res. Public Health 2020, 17, 8650. [Google Scholar] [CrossRef]
- von Maltzahn, J. Regulation of muscle stem cell function. Vitam. Horm. 2021, 116, 295–311. [Google Scholar]
- Zhang, L.; Wang, X.H.; Wang, H.; Du, J.; Mitch, W.E. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J. Am. Soc. Nephrol. 2010, 21, 419–427. [Google Scholar] [CrossRef]
- Brooks, N.E.; Myburgh, K.H. Skeletal muscle wasting with disuse atrophy is multi-dimensional: The response and interaction of myonuclei, satellite cells and signaling pathways. Front. Physiol. 2014, 5, 99. [Google Scholar] [CrossRef]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Song, Y.H.; Song, J.L.; Delafontaine, P.; Godard, M.P. The therapeutic potential of IGF-I in skeletal muscle repair. Trends Endocrinol. Metab. 2013, 24, 310–319. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011593. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef]
- Lim, J.; Li, L.; Shirihai, O.S.; Trudeau, K.M.; Puertollano, R.; Raben, N. Modulation of mTOR signaling as a strategy for the treatment of Pompe disease. EMBO Mol. Med. 2017, 9, 353–370. [Google Scholar] [CrossRef]
- You, J.-S.; Anderson, G.B.; Dooley, M.S.; Hornberger, T.A. The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization in mice. Dis. Models Mech. 2015, 8, 1059–1069. [Google Scholar] [CrossRef]
- Jaiswal, N.; Gavin, M.; Loro, E.; Sostre-Colón, J.; Roberson, P.A.; Uehara, K.; Rivera-Fuentes, N.; Neinast, M.; Arany, Z.; Kimball, S.R.; et al. AKT controls protein synthesis and oxidative metabolism via combined mTORC1 and FOXO1 signalling to govern muscle physiology. J. Cachexia Sarcopenia Muscle 2022, 13, 495–514. [Google Scholar] [CrossRef]
- Goodman, C.A. Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. J. Appl. Physiol. 2019, 127, 581–590. [Google Scholar] [CrossRef]
- Geremia, A.; Sartori, R.; Baraldo, M.; Nogara, L.; Balmaceda, V.; Dumitras, G.A.; Ciciliot, S.; Scalabrin, M.; Nolte, H.; Blaauw, B. Activation of Akt-mTORC1 signalling reverts cancer-dependent muscle wasting. J. Cachexia Sarcopenia Muscle 2022, 13, 648–661. [Google Scholar] [CrossRef]
- Murata, S.; Sasaki, T.; Yamauchi, Y.; Shimizu, M.; Sato, R. Maslinic acid activates mTORC1 and human TGR5 and induces skeletal muscle hypertrophy. Biosci. Biotechnol. Biochem. 2021, 85, 2311–2321. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, K.T.; Choung, S.Y. Codonopsis lanceolata and its active component Tangshenoside I ameliorate skeletal muscle atrophy via regulating the PI3K/Akt and SIRT1/PGC-1α pathways. Phytomedicine 2022, 100, 154058. [Google Scholar] [CrossRef]
- Mai, K.; Cando, P.; Trasino, S.E. mTOR1c Activation with the Leucine “Trigger” for Prevention of Sarcopenia in Older Adults During Lockdown. J. Med. Food 2022, 25, 117–120. [Google Scholar] [CrossRef]
- Baraldo, M.; Nogara, L.; Dumitras, G.A.; Tchampda Dondjang, A.H.; Geremia, A.; Scalabrin, M.; Türk, C.; Telkamp, F.; Zentilin, L.; Giacca, M.; et al. Raptor is critical for increasing the mitochondrial proteome and skeletal muscle force during hypertrophy. FASEB J. 2021, 35, e22031. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Lin, S.; Romanino, K.; Castets, P.; Guridi, M.; Summermatter, S.; Handschin, C.; Tintignac, L.A.; Hall, M.N.; Rüegg, M.A. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet. Muscle 2013, 3, 6. [Google Scholar] [CrossRef]
- Rion, N.; Castets, P.; Lin, S.; Enderle, L.; Reinhard, J.R.; Rüegg, M.A. mTORC2 affects the maintenance of the muscle stem cell pool. Skelet. Muscle 2019, 9, 30. [Google Scholar] [CrossRef]
- Sanvee, G.M.; Hitzfeld, L.; Bouitbir, J.; Krähenbühl, S. mTORC2 is an important target for simvastatin-associated toxicity in C2C12 cells and mouse skeletal muscle—Roles of Rap1 geranylgeranylation and mitochondrial dysfunction. Biochem. Pharmacol. 2021, 192, 114750. [Google Scholar] [CrossRef]
- Nakashima, K.; Ishida, A. AMP-activated Protein Kinase Activation Suppresses Protein Synthesis and mTORC1 Signaling in Chick Myotube Cultures. J. Poult. Sci. 2022, 59, 81–85. [Google Scholar] [CrossRef]
- Piazzi, M.; Bavelloni, A.; Cenni, V.; Salucci, S.; Bartoletti Stella, A.; Tomassini, E.; Scotlandi, K.; Blalock, W.L.; Faenza, I. Combined Treatment with PI3K Inhibitors BYL-719 and CAL-101 Is a Promising Antiproliferative Strategy in Human Rhabdomyosarcoma Cells. Molecules 2022, 27, 2742. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Aihie Sayer, A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Dos Santos, L.; Cyrino, E.S.; Antunes, M.; Santos, D.A.; Sardinha, L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle 2017, 8, 245–250. [Google Scholar] [CrossRef]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Xu, W.; Chen, T.; Cai, Y.; Hu, Y.; Fan, L.; Wu, C. Sarcopenia in Community-Dwelling Oldest Old Is Associated with Disability and Poor Physical Function. J. Nutr. Health Aging 2020, 24, 339–345. [Google Scholar] [CrossRef]
- Tarantino, U.; Scimeca, M.; Piccirilli, E.; Tancredi, V.; Baldi, J.E.; Bonanno, E. Sarcopenia: A histological and immunohistochemical study on age-related muscle impairment. Aging Clin. Exp. Res. 2015, 27, S51–S60. [Google Scholar] [CrossRef]
- Devries, M.C.; Breen, L.; Von Allmen, M.; McDonald, M.J.; Moore, D.R.; Offord, E.A.; Horcajada, M.-N.; Breuillé, D.; Phillips, S.M. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol. Rep. 2015, 3, e12493. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwpoort, I.C.; Vlot, M.C.; Schaap, L.A.; Lips, P.; Drent, M.L. The relationship between serum IGF-1, handgrip strength, physical performance and falls in elderly men and women. Eur. J. Endocrinol. 2018, 179, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Widajanti, N.; Soelistijo, S.; Hadi, U.; Thaha, M.; Aditiawardana; Widodo; Firdausi, H.; Nurina, Y.; Asikin, M.; Srinowati, H.; et al. Association between Sarcopenia and Insulin-Like Growth Factor-1, Myostatin, and Insulin Resistance in Elderly Patients Undergoing Hemodialysis. J. Aging Res. 2022, 2022, 1327332. [Google Scholar] [CrossRef]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef]
- Livshits, G.; Kalinkovich, A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res. Rev. 2019, 56, 100980. [Google Scholar] [CrossRef]
- Beenakker, K.G.; Koopman, J.J.E.; van Bodegom, D.; Kuningas, M.; Pieternella, E.; Slagboom, P.E.; Meij, J.J.; Maier, A.B.; Westendorp, R.G.J. Variants of the IL-10 gene associate with muscle strength in elderly from rural Africa: A candidate gene study. Aging Cell 2014, 13, 862–868. [Google Scholar] [CrossRef]
- Rong, Y.D.; Bian, A.L.; Hu, H.Y.; Ma, Y.; Zhou, X.-Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef]
- Bakkar, N.; Guttridge, D.C. NF-κB signaling: A tale of two pathways in skeletal myogenesis. Physiol. Rev. 2010, 90, 495–511. [Google Scholar] [CrossRef]
- Bian, A.-L.; Hu, H.-Y.; Rong, Y.-D.; Wang, J.; Wang, J.-X.; Zhou, X.-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Welc, S.S.; Wehling-Henricks, M.; Tidball, J.G. Myeloid cell-derived tumor necrosis factor-alpha promotes sarcopenia and regulates muscle cell fusion with aging muscle fibers. Aging Cell 2018, 17, e12828. [Google Scholar] [CrossRef] [PubMed]
- Budui, S.L.; Rossi, A.P.; Zamboni, M. The pathogenetic bases of sarcopenia. Clin. Cases Miner. Bone Metab. 2015, 12, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Bourdel-Marchasson, I.; Rouland, R.; Calmettes, G.; Biran, M.; Deschodt-Arsac, V.; Miraux, S.; Thiaudiere, E.; Pasdois, P.; Detaille, D.; et al. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell 2014, 13, 39–48. [Google Scholar] [CrossRef]
- Wawrzyniak, N.R.; Joseph, A.M.; Levin, D.G.; Gundermann, D.M.; Leeuwenburgh, C.; Sandesara, B.; Manini, T.M.; Adhihetty, P.J. Idiopathic chronic fatigue in older adults is linked to impaired mitochondrial content and biogenesissignaling in skeletal muscle. Oncotarget 2016, 7, 52695–52709. [Google Scholar] [CrossRef]
- Salucci, S.; Battistelli, M.; Baldassarri, V.; Burini, D.; Falcieri, E.; Burattini, S. Melatonin prevents mitochondrial dysfunctions and death in differentiated skeletal muscle cells. Microsc. Res. Tech. 2017, 80, 1174–1181. [Google Scholar] [CrossRef]
- Derbré, F.; Gratas-Delamarche, A.; Gómez-Cabrera, M.C.; Viña, J. Inactivity-induced oxidative stress: A central role in age-related sarcopenia? Eur. J. Sport Sci. 2014, 14, S98–S108. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.-P.; Picard, M.; Pelletier, F.S.-J.; Sgarioto, N.; Auger, M.-J.; Vallée, J.; Robitaille, R.; St-Pierre, D.H.; Gouspillou, G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 2015, 6, 17923–17937. [Google Scholar] [CrossRef]
- Wenz, T.; Rossi, S.G.; Rotundo, R.L.; Spiegelman, B.M.; Moraes, C.T. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. USA. 2009, 106, 20405–20410. [Google Scholar] [CrossRef]
- Hepple, R.T. Mitochondrial involvement and impact in aging skeletal muscle. Front. Aging Neurosci. 2014, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, J.; Simon, F.; Cabrera, D.; Vilos, C.; Cabello-Verrugio, C. Mitochondrial Dysfunction in Skeletal Muscle Pathologies. Curr. Protein Pept. Sci. 2019, 20, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Marzetti, E. Musculoskeletal Aging and Sarcopenia in the Elderly. Int. J. Mol. Sci. 2022, 23, 2808. [Google Scholar] [CrossRef]
- Buckland, G.; Gonzalez, C.A. The role of olive oil in disease prevention: A focus on the recent epidemiological evidence from cohort studies and dietary intervention trials. Br. J. Nutr. 2015, 113, S94–S101. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Application of an advanced and wide scope non-target screening workflow with LC-ESI-QTOF-MS and chemometrics for the classification of the Greek olive oil varieties. Food Chem. 2018, 256, 53–61. [Google Scholar] [CrossRef]
- De Santis, S.; Cariello, M.; Piccinin, E.; Sabbà, C.; Moschetta, A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients 2019, 11, 2085. [Google Scholar] [CrossRef]
- Díaz-Montaña, E.J.; Barbero-López, M.; Aparicio-Ruiz, R.; Morales, M.T. Does A Flavoured Extra Virgin Olive Oil Have Higher Antioxidant Properties? Antioxidants 2022, 11, 550. [Google Scholar] [CrossRef]
- Ranalli, F.; Ranalli, A.; Contento, S.; Casanovas, M.; Antonucci, M.; Simone, G.D. Bioactives and nutraceutical phytochemicals naturally occurring in virgin olive oil. The case study of the Nocellara del Belice Italian olive cultivar. Nat. Prod. Res. 2013, 27, 1686–1690. [Google Scholar] [CrossRef]
- Salucci, S.; Falcieri, E. Polyphenols and their potential role in preventing skeletal muscle atrophy. Nutr. Res. 2020, 74, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Fernández del Río, L.; Gutiérrez-Casado, E.; Varela-López, A.; Villalba, J.M. Olive oil and the hallmarks of aging. Molecules 2016, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Soto-Alarcon, S.A.; Valenzuela, R.; Valenzuela, A.; Videla, L.A. Liver protective effects of extra virgin olive oil: Interaction between its chemical composition and the cell-signaling pathways involved in protection. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yousefzadeh, M.J.; Suh, Y.; Niedernhofer, L.J.; Robbins, P.D. Signal transduction, ageing and disease. Subcell. Biochem. 2019, 91, 227–247. [Google Scholar]
- Rovillain, E.; Mansfield, L.; Caetano, C.; Alvarez-Fernandez, M.; Caballero, O.L.; Medema, R.H.; Hummerich, H.; Jat, P.S. Activation of nuclear factor-kappa B signaling promotes cellular senescence. Oncogene 2011, 30, 2356–2366. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Zazueta, C.; Königsberg, M. Nrf2: Molecular and epigenetic regulation during aging. Ageing Res. Rev. 2018, 47, 31–40. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef]
- Martin, A.I.; Priego, T.; Lopez-Calderon, A. Hormones and Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 207–233. [Google Scholar]
- Villani, A.; Wright, H.; Slater, G.; Buckley, J. A randomised controlled intervention study investigating the efficacy of carotenoid-rich fruits and vegetables and extra-virgin olive oil on attenuating sarcopenic symptomology in overweight and obese older adults during energy intake restriction: Protocol paper. BMC Geriatr. 2018, 18, 2. [Google Scholar]
- Burton, L.A.; Sumukadas, D. Optimal management of sarcopenia. Clin. Interv. Aging 2010, 5, 217–228. [Google Scholar]
- Park, J.; Bae, J.; Lee, J. Complex Exercise Improves Anti-Inflammatory and Anabolic Effects in Osteoarthritis-Induced Sarcopenia in Elderly Women. Healthcare 2021, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Fernández, J.; Fernández-Montero, A.; Córdova-Martínez, A.; Pastor, D.; Martínez-Rodríguez, A.; Roche, E. Sarcopenia: Molecular Pathways and Potential Targets for Intervention. Int. J. Mol. Sci. 2020, 21, 8844. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-López, M.J.; Berná, G.; Jurado-Ruiz, E.; Serrana, H.L.G.; Martín, F. Consumption of extra-virgin olive oil rich in phenolic compounds has beneficial antioxidant effects in healthy human adults. J. Funct. Foods 2014, 10, 475–484. [Google Scholar] [CrossRef]
- Musumeci, G.; Imbesi, R.; Szychlinska, M.A.; Castrogiovanni, P. Apoptosis and skeletal muscle in aging. Open J. Apoptosis 2015, 4, 41–46. [Google Scholar] [CrossRef]
- Silveira, E.A.; de Souza, J.D.; Dos Santos Rodrigues, A.P.; Lima, R.M.; de Souza Cardoso, K.; de Oliveira, C. Effects of Extra Virgin Olive Oil (EVOO) and the Traditional Brazilian Diet on Sarcopenia in Severe Obesity: A Randomized Clinical Trial. Nutrients 2020, 12, 1498. [Google Scholar] [CrossRef]
- González-Hedström, D.; Priego, T.; López-Calderón, A.; Amor, S.; de la Fuente-Fernández, M.; Inarejos-García, A.M.; García-Villalón, Á.L.; Martín, A.I.; Granado, M. Beneficial Effects of a Mixture of Algae and Extra Virgin Olive Oils on the Age-Induced Alterations of Rodent Skeletal Muscle: Role of HDAC-4. Nutrients 2020, 13, 44. [Google Scholar] [CrossRef]
- Hadrich, F.; Garcia, M.; Maalej, A.; Moldes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [CrossRef]
- Kikusato, M.; Muroi, H.; Uwabe, Y.; Furukawa, K.; Toyomizu, M. Oleuropein induces mitochondrial biogenesis and decreases reactive oxygen species generation in cultured avian muscle cells, possibly via an up-regulation of peroxisome proliferator-activated receptor γ coactivator-1α. Anim. Sci. J. 2016, 87, 1371–1378. [Google Scholar] [CrossRef]
- Muroi, H.; Hori, K.; Tokutake, Y.; Hakamata, Y.; Kawabata, F.; Toyomizu, M.; Kikusato, M. Oleuropein suppresses mitochondrial reactive oxygen species generation possibly via an activation of transient receptor potential V1 and sirtuin-1 in cultured chicken muscle cells. Anim. Sci. J. 2022, 93, e13677. [Google Scholar] [CrossRef]
- Fuentes, E.; Paucar, F.; Tapia, F.; Ortiz, J.; Jimenez, P.; Romero, N. Effect of the composition of extra virgin olive oils on the differentiation and antioxidant capacities of twelve monovarietals. Food Chem. 2018, 243, 285–294. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free. Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Bai, L.; Yan, J.; Li, Y.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, Y.; Chen, Y.; et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: Regulatory effects of hydroxytyrosol. Free Radic. Biol. Med. 2011, 50, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Zheng, A.; Yang, L.; Liu, J.; Chen, C.; Tang, Y.; Zou, X.; Li, Y.; Long, J.; et al. Mitochondrial dysfunction-associated OPA1 cleavage contributes to muscle degeneration: Preventative effect of hydroxytyrosol acetate. Cell Death Dis. 2014, 5, e1521. [Google Scholar] [CrossRef]
- Pierno, S.; Tricarico, D.; Liantonio, A.; Mele, A.; Digennaro, C.; Rolland, J.F.; Bianco, G.; Villanova, L.; Merendino, A.; Camerino, G.; et al. An olive oil-derived antioxidant mixture ameliorates the age-related decline of skeletal muscle function. Age 2014, 36, 73–88. [Google Scholar] [CrossRef][Green Version]
- Rossi, M.; Caruso, F.; Kwok, L.; Lee, G.; Caruso, A.; Gionfra, F.; Candelotti, E.; Belli, S.L.; Molasky, N.; Raley-Susman, K.M.; et al. Protection by extra virgin olive oil against oxidative stress in vitro and in vivo. Chemical and biological studies on the health benefits due to a major component of the Mediterranean diet. PLoS ONE 2017, 12, e0189341. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Plotnikova, T.M. Tyrosol as a Neuroprotector: Strong Effects of a “Weak” Antioxidant. Curr. Neuropharmacol. 2021, 19, 434–448. [Google Scholar] [CrossRef]
- Muriana, F.J.G.; Montserrat-de la Paz, S.; Lucas, R.; Bermudez, B.; Jaramillo, S.; Morales, J.C.; Abia, R.; Lopez, S. Tyrosol and its metabolites as antioxidative and anti-inflammatory molecules in human endothelial cells. Food Funct. 2017, 8, 2905–2914. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Yadav, T.C.; Kumar, N.; Raj, U.; Goel, N.; Vardawaj, P.K.; Prasad, R.; Pruthi, V. Exploration of interaction mechanism of tyrosol as a potent anti-inflammatory agent. J. Biomol. Struct. Dyn. 2020, 38, 382–397. [Google Scholar] [CrossRef]
- Zhang, J.; Nugrahaningrum, D.A.; Marcelina, O.; Ariyanti, A.D.; Wang, G.; Liu, C.; Wu, S.; Kasim, V. Tyrosol Facilitates Neovascularization by Enhancing Skeletal Muscle Cells Viability and Paracrine Function in Diabetic Hindlimb Ischemia Mice. Front. Pharmacol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Oliván, S.; Martínez-Beamonte, R.; Calvo, A.C.; Surra, J.C.; Manzano, R.; Arnal, C.; Osta, R.; Osada, J. Extra virgin olive oil intake delays the development of amyotrophic lateral sclerosis associated with reduced reticulum stress and autophagy in muscle of SOD1G93A mice. J. Nutr. Biochem. 2014, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.S.; Polito, A.; Peluso, I. Effects of Olive Oil on TNF-α and IL-6 in Humans: Implication in Obesity and Frailty. Endocr. Metab. Immune Disord Drug Targets 2018, 18, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Fan, Y.B.; Tao, X.H.; Pan, W.L.; Wu, Y.X.; Wang, X.H.; He, Y.Q.; Xiao, W.F.; Li, Y.S. Mitochondrial Quality Control in Sarcopenia: Updated Overview of Mechanisms and Interventions. Aging Dis. 2021, 12, 2016–2030. [Google Scholar] [CrossRef] [PubMed]
- Irazoki, A.; Martinez-Vicente, M.; Aparicio, P.; Aris, C.; Alibakhshi, E.; Rubio-Valera, M.; Castellanos, J.; Lores, L.; Palacín, M.; Gumà, A.; et al. Coordination of mitochondrial and lysosomal homeostasis mitigates inflammation and muscle atrophy during aging. Aging Cell 2022, 21, e13583. [Google Scholar] [CrossRef]

| Human Studies | Animal Studies | In Vitro Studies | |
|---|---|---|---|
| EVOO |
| Old rats (n = 8), 2.5 mL/kg EVOO for 21 days | No data |
| Oleuropein | No data | No data |
|
| Hydroxytyrosol | No data |
| L6 myotubes treated with 10 μL/mL or 50 μL/mL of Hydroxytyrosol |
| Tyrosol | No data | No data | C2C12 cells after hyperglicemia induction were treated with 50 mg/mL Tyrosol for 24 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salucci, S.; Bartoletti-Stella, A.; Bavelloni, A.; Aramini, B.; Blalock, W.L.; Fabbri, F.; Vannini, I.; Sambri, V.; Stella, F.; Faenza, I. Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation. Nutrients 2022, 14, 3567. https://doi.org/10.3390/nu14173567
Salucci S, Bartoletti-Stella A, Bavelloni A, Aramini B, Blalock WL, Fabbri F, Vannini I, Sambri V, Stella F, Faenza I. Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation. Nutrients. 2022; 14(17):3567. https://doi.org/10.3390/nu14173567
Chicago/Turabian StyleSalucci, Sara, Anna Bartoletti-Stella, Alberto Bavelloni, Beatrice Aramini, William L. Blalock, Francesco Fabbri, Ivan Vannini, Vittorio Sambri, Franco Stella, and Irene Faenza. 2022. "Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation" Nutrients 14, no. 17: 3567. https://doi.org/10.3390/nu14173567
APA StyleSalucci, S., Bartoletti-Stella, A., Bavelloni, A., Aramini, B., Blalock, W. L., Fabbri, F., Vannini, I., Sambri, V., Stella, F., & Faenza, I. (2022). Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation. Nutrients, 14(17), 3567. https://doi.org/10.3390/nu14173567