Investigation of the Renal Protective Effect of Combined Dietary Polyphenols in Streptozotocin-Induced Diabetic Aged Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polyphenolic Compounds
2.2. Animals and Ethics
2.3. Induction of Diabetes
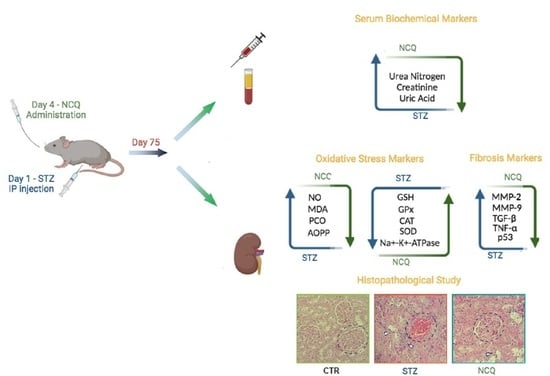
2.4. Experimental Design
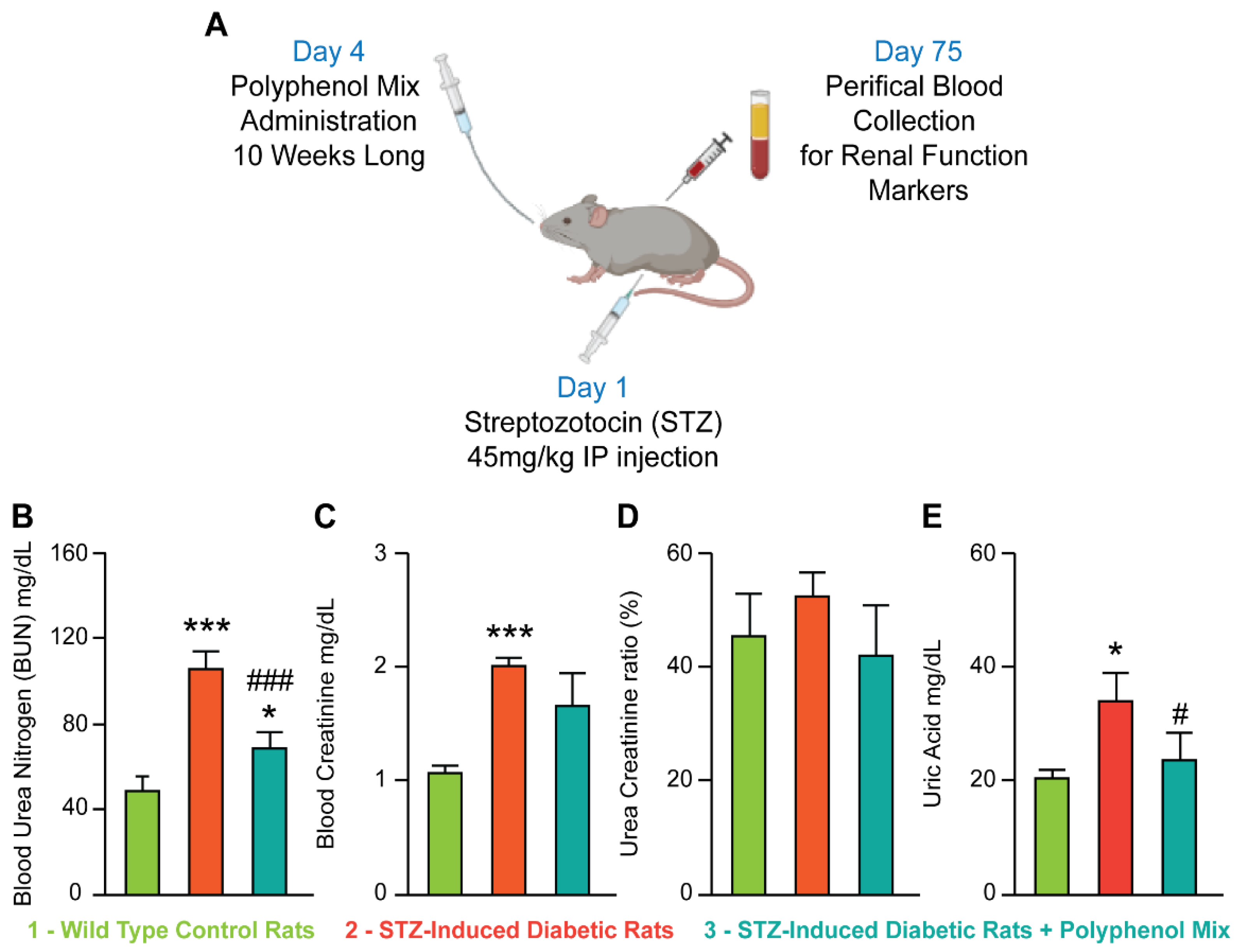
2.5. Determination of Serum Urea and Creatinine Concentration
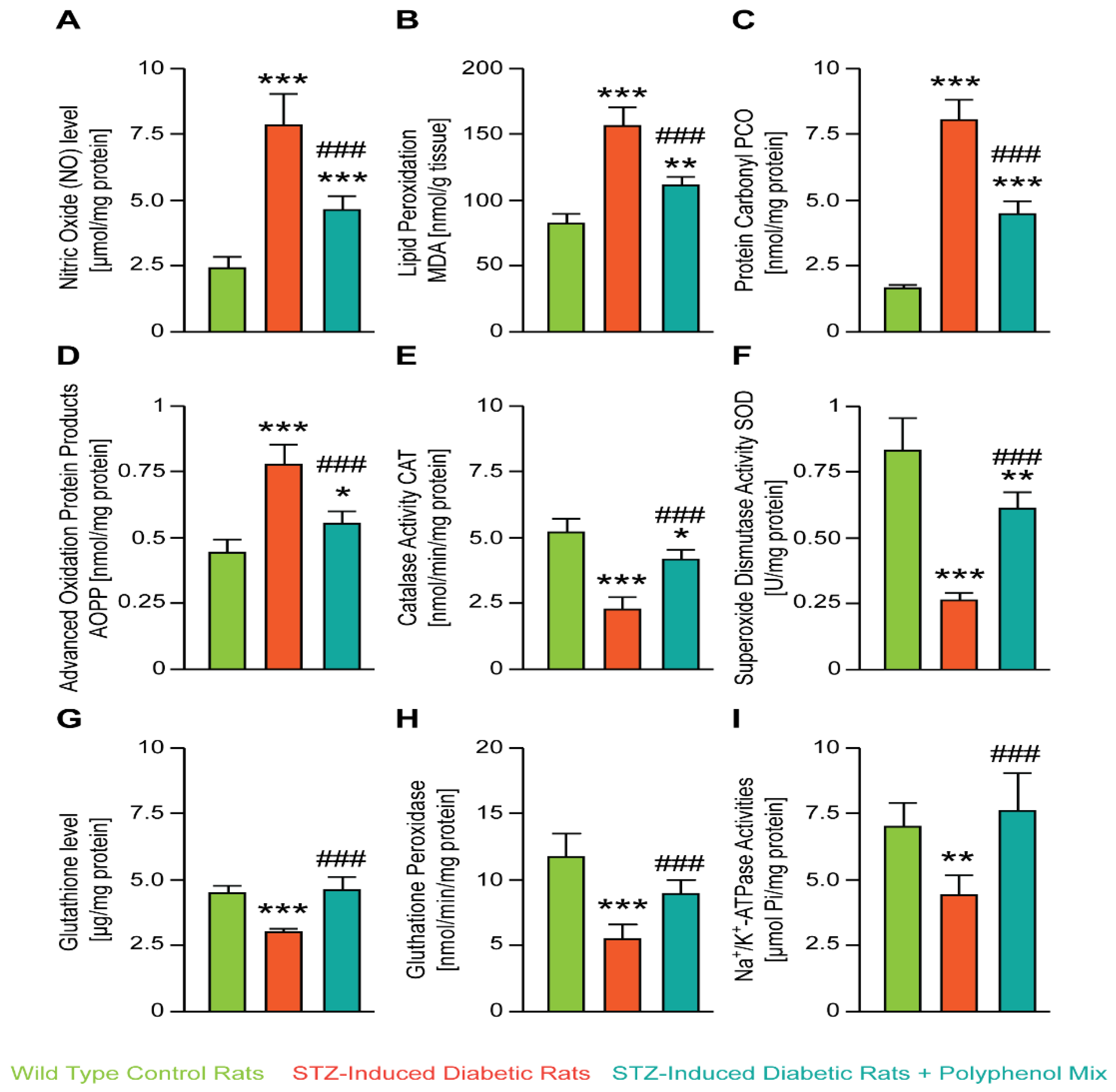
2.6. Oxidative Stress Assessment
2.6.1. Estimation of Nitric Oxide (NO) Production
2.6.2. Lipid Peroxidation
2.6.3. Carbonyl Protein Content (PCO)
2.6.4. Determination of AOPP Levels
2.6.5. Catalase Activity
2.6.6. Superoxide Dismutase SOD Activity
2.6.7. Estimation of Total GSH Levels
2.6.8. Determination of Glutathione Peroxidase Activity
2.6.9. Na+-K+-ATPase Specific Activities
2.7. Protein Quantification
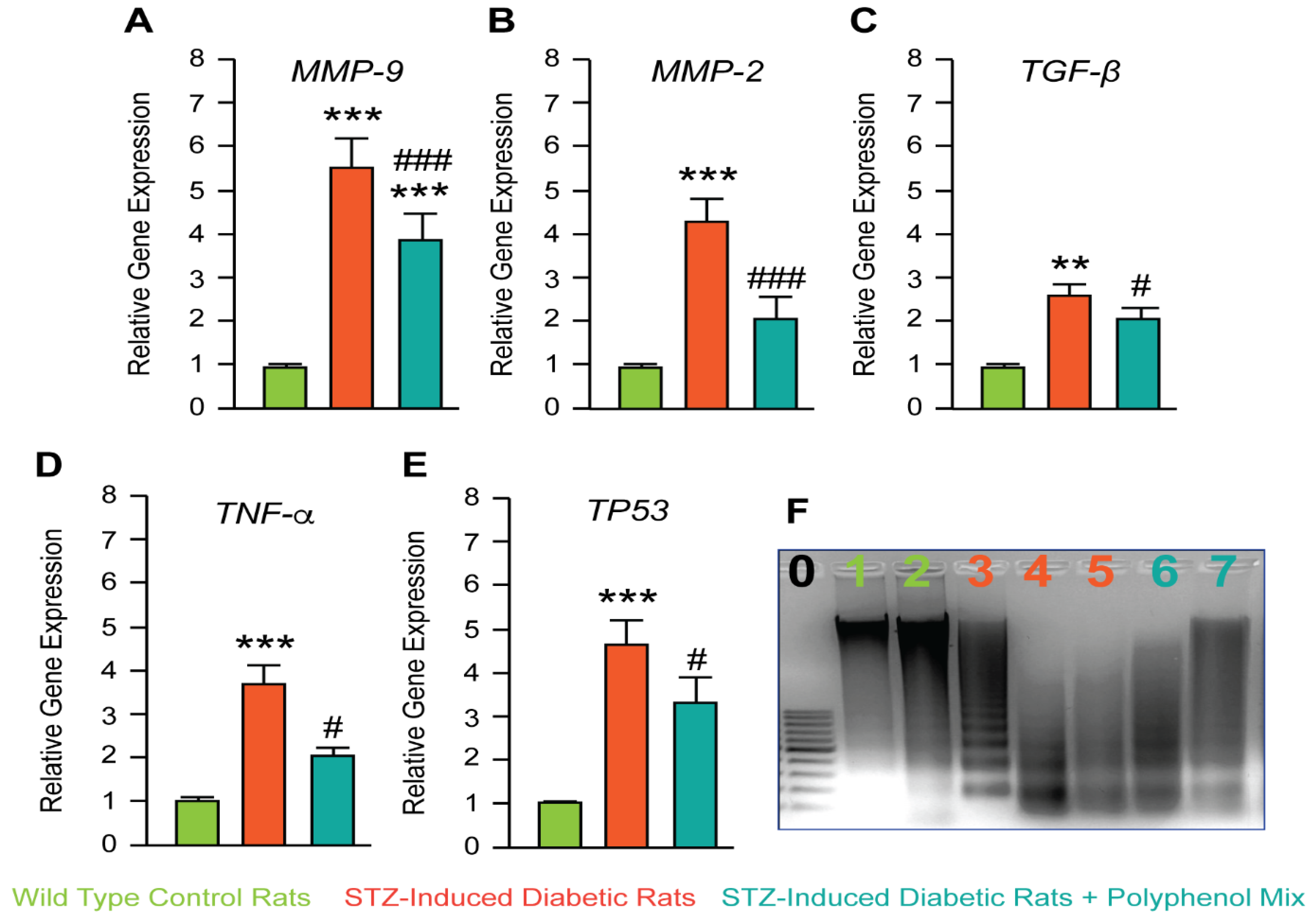
2.8. Quantitative RT-qPCR
2.9. DNA Gel Electrophoresis
2.10. Histopathology Study
2.11. Statistical Analysis
3. Results
3.1. NCQ Prevents the Alterations of Renal Biomarkers in STZ-Induced Diabetic Rats
3.2. NCQ Ameliorates Renal Lipid Peroxidation, Nitric Oxide, Protein Oxidation and Glutathione Depletion in STZ-Induced Diabetic Aged Rats
3.3. Investigation of NCQ on Genes Involved in Inflammation and Apoptosis Induced by STZ
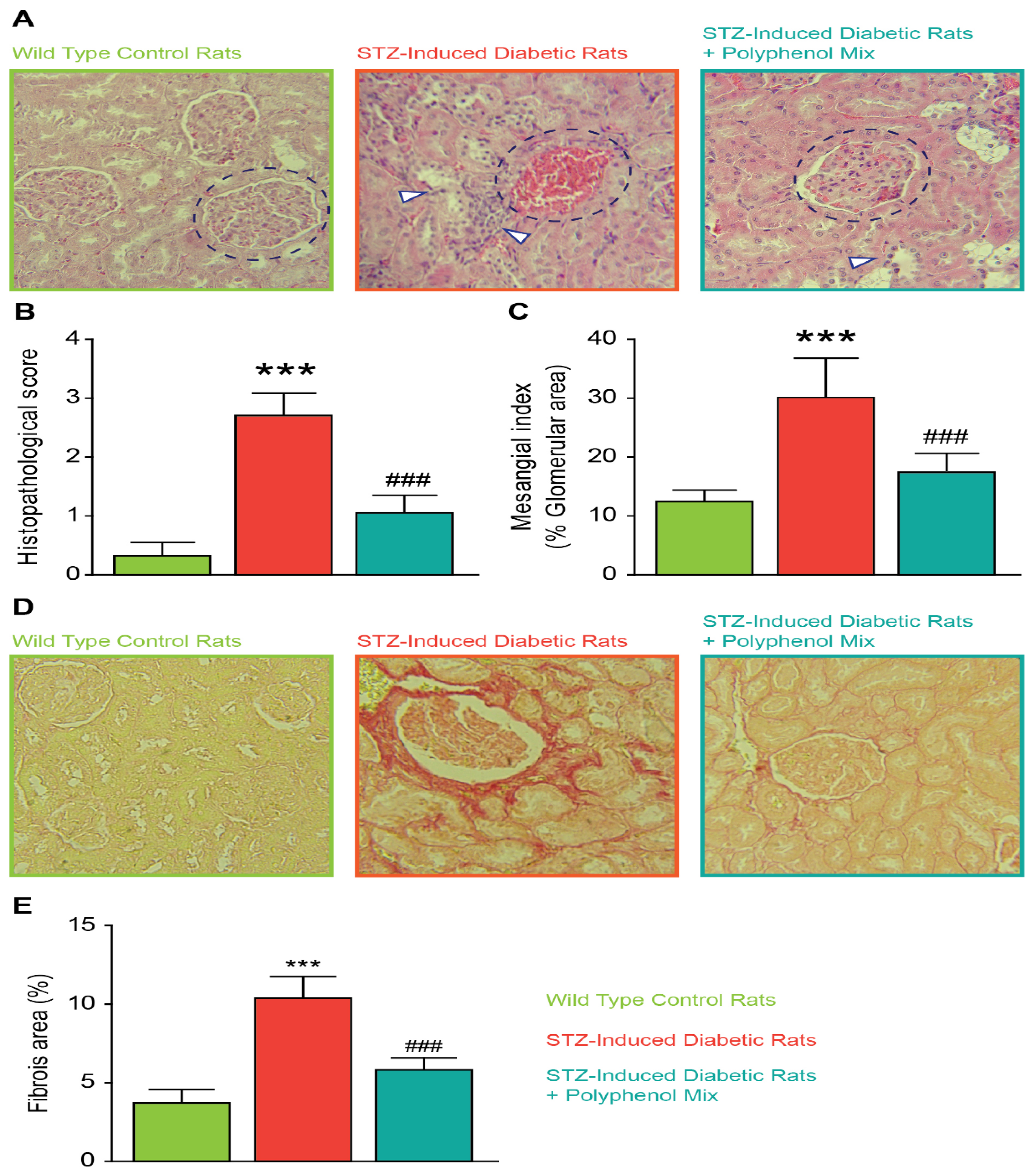
3.4. Effect of NCQ in STZ-Induced Renal Histopathological Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alhyas, L.; McKay, A.; Majeed, A. Prevalence of type 2 diabetes in the States of the co-operation council for the Arab States of the Gulf: A systematic review. PLoS ONE 2012, 7, e40948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhyas, L.; McKay, A.; Balasanthiran, A.; Majeed, A. Prevalences of overweight, obesity, hyperglycaemia, hypertension and dyslipidaemia in the Gulf: Systematic review. JRSM Short Rep. 2011, 2, 55. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar]
- Volpe, C.; Villar-Delfino, P.H.; Dos Anjos, P.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Kohnert, K.D.; Freyse, E.J.; Salzsieder, E. Glycaemic variability and pancreatic β-cell dysfunction. Curr. Diabetes Rev. 2012, 8, 345–354. [Google Scholar] [CrossRef]
- Liang, W.; Chandel, N.S. A novel damage mechanism: Contribution of the interaction between necroptosis and ROS to high glucose-induced injury and inflam-mation in H9c2 cardiac cells. Int. J. Mol. Med. 2017, 40, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Priante, G.; Gianesello, L.; Ceol, M.; Del Prete, D.; Anglani, F. Cell Death in the Kidney. Int. J. Mol. Sci. 2019, 20, 3598. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.-D.; Distefano, G.; Gagliano, C.; Rusciano, D.; Malaguarnera, L. Autophagy in diabetic retinopathy. Curr. Neuropharmacol. 2016, 14, 810–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Kashihara, N.; Haruna, Y.; Kondeti, V.K.; Kanwar, Y.S. Oxidative stress in diabetic nephropathy. Curr. Med. Chem. 2010, 17, 4256–4269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novelli, M.; D’Aleo, V.; Lupi, R.; Paolini, M.; Soleti, A.; Marchetti, P.; Masiello, P. Reduction of oxidative stress by a new low-molecular-weight antioxidant improves metabolic alterations in a nonobese mouse diabetes model. Pancreas 2007, 35, e10–e17. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants 2017, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.H. Renal fibrosis. Korean J. Pediatrics 2010, 53, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef] [Green Version]
- El Nahas, A.M.; Muchaneta-Kubara, E.C.; Essawy, M.; Soylemezoglu, O. Renal fibrosis: Insights into pathogenesis and treatment. Int. J. Biochem. Cell Biol. 1997, 29, 55–62. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox. Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 2, e14264. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Maksimova, V.; Milenkovic, D. Polyphenols in human nutrition: From the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability-an overview and perspective. Br. J. Nutr. 2020, 123, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, Í.; Ferreira-Pêgo, C.; Carregosa, D.; Santos, C.N.; Menezes, R.; Fernandes, A.S.; Costa, J.G. Polyphenols and Their Metabolites in Renal Diseases: An Overview. Foods 2022, 11, 1060. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islamic Repub. Iran. 2017, 31, 134. [Google Scholar] [CrossRef] [Green Version]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A brief review of phenolic compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 8, 820–897. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Health-Promoting Effects of Thymus Phenolic-Rich Extracts: Antioxidant, Anti-Inflammatory and Antitumoral Properties. Antioxidants 2020, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Che, C.T.; Lau, C.B.; Leung, P.S.; Cheng, C.H. Inhibition of intestinal and renal Na+-glucose cotransporter by naringenin. Int. J. Biochem. Cell Biol. 2006, 38, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Du, A.; Zhou, L.; Zhang, T.; Lu, B.; Wang, Z.; Ji, L. Chlorogenic acid improves diabetic retinopathy by alleviating blood-retinal-barrier dysfunction via inducing Nrf2 activation. Phytother. Res. 2022, 36, 1386–1401. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Hu, N.; Zhang, L.H.; Jiang, W.; Yan, T.; Zhang, T.; Liu, A.; Zhang, Y.Q.; Zhao, J.; Shi, L.; et al. Lonicerae japonicae flos ameliorates radiotherapy-induced mesenteric artery endothelial dysfunction through GTPCH1/BH4/eNOS pathway. Phytomedicine 2022, 102, 154146. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hamaad, F.A.; Ali, E.Y.; Ghoneum, M.H. Antidiabetic efficacy of Trifolium alexandrinum extracts hesperetin and quercetin in ameliorating carbohydrate metabolism and activating IR and AMPK signaling in the pancreatic tissues of diabetic rats. Biomed. Pharmacother. 2022, 149, 112838. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, J.H.; Pan, J.H.; Kim, J.K.; Park, T.S.; Kim, Y.J.; Lee, J.H.; Kim, J.H. Naringin Protects Pancreatic β-Cells Against Oxidative Stress-Induced Apoptosis by Inhibiting Both Intrinsic and Extrinsic Pathways in Insulin-Deficient Diabetic Mice. Mol. Nutr. Food. Res. 2018, 62, 1700810. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar]
- Kayali, R.; Cakatay, U.; Akçay, T.; Altuğ, T. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem. Funct. 2006, 24, 79–85. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Kawamoto, E.M.; Munhoz, C.D.; Glezer, I.; Bahia, V.S.; Caramelli, P.; Nitrini, R.; Gorjão, R.; Curi, R.; Scavone, C.; Marcourakis, T. Oxidative state in platelets and erythrocytes in aging and Alzheimer’s disease. Neurobiol. Aging 2005, 26, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Sellins, K.S.; Cohen, J.J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J. Immunol. 1987, 139, 3199–3206. [Google Scholar]
- Chtourou, Y.; Slima, A.B.; Makni, M.; Gdoura, R.; Fetoui, H. Naringenin protects cardiac hypercholesterolemia-induced oxidative stress and subsequent necroptosis in rats. Pharmacol. Rep. 2015, 67, 1090–1097. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bharti, S.; Ojha, S.; Bhatia, J.; Kumar, N.; Ray, R.; Kumari, S.; Arya, D.S. Up-regulation of PPARγ, heat shock protein-27 and -72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br. J. Nutr. 2011, 106, 1713–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanello, N.; Schmatz, R.; Pereira, L.B.; Cardoso, A.M.; Passamonti, S.; Spanevello, R.M.; Thomé, G.; de Oliveira, G.M.T.; Kist, L.W.; Bogo, M.R.; et al. Effects of chlorogenic acid, caffeine and coffee on components of the purinergic system of streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2016, 38, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; He, X.; Liang, S.; Liu, S.; Liu, H.; He, Q.; Chen, L.; Jiang, H.; Zhang, Y. Quercetin ameliorates podocyte injury via inhibition of oxidative stress and the TGF-β1/Smad pathway in DN rats. RSC Adv. 2018, 8, 35413–35421. [Google Scholar] [CrossRef] [Green Version]
- Mennen, L.I.; Sapinho, D.; Ito, H.; Bertrais, S.; Galan, P.; Hercberg, S.; Scalbert, A. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br. J. Nutr. 2006, 96, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv. Nutr. 2020, 11, 602–615. [Google Scholar] [CrossRef]
- Kheriji, N.; Boukhalfa, W.; Mahjoub, F.; Hechmi, M.; Dakhlaoui, T.; Mrad, M.; Hadj Salah Bahlous, A.; Ben Amor, N.; Jamoussi, H.; Kefi, R. The Role of Dietary Intake in Type 2 Diabetes Mellitus: Importance of Macro and Micronutrients in Glucose Homeostasis. Nutrients 2022, 14, 2132. [Google Scholar] [CrossRef] [PubMed]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol.-Ren. Physiol. 2007, 292, F905–F911. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.R.; Twigg, S.M. Fibrosis in diabetes complications: Pathogenic mechanisms and circulating and urinary markers. Vasc. Health Risk Manag. 2008, 4, 575–596. [Google Scholar] [PubMed] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Giha, H.A.; Sater, M.S.; Alamin, O.A.O. Diabetes mellitus tendino-myopathy: Epidemiology, clinical features, diagnosis and management of an overlooked diabetic complication. Acta Diabetol. 2022, 59, 871–883. [Google Scholar] [CrossRef]
- Tuleta, I.; Frangogiannis, N.G. Diabetic fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166044. [Google Scholar] [CrossRef]
- Al Hroob, A.M.; Abukhalil, M.H.; Hussein, O.E.; Mahmoud, A.M. Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate. Biomed. Pharmacother. 2019, 109, 2155–2172. [Google Scholar] [CrossRef]
- Silva, M.; Peng, T.; Zhao, X.; Li, S.; Farhan, M.; Zheng, W. Recent trends in drug-delivery systems for the treatment of diabetic retinopathy and associated fibrosis. Adv. Drug Deliv. Rev. 2021, 173, 439–460. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Pachado, M.P.; Cuadrado, A.; Jandeleit-Dahm, K.; Schmidt, H.H.H.W. Reactive Oxygen Comes of Age: Mechanism-Based Therapy of Diabetic End-Organ Damage. Trends Endocrinol. Metab. 2019, 30, 312–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Yuan, D.; Gai, L.; Wu, X.; Shi, Y.; He, Y.; Liu, C.; Zhang, C.; Zhou, G.; Yuan, C. Saponins from Panax japonicus ameliorate age-related renal fibrosis by inhibition of inflammation mediated by NF-κB and TGF-β1/Smad signaling and suppression of oxidative stress via activation of Nrf2-ARE signaling. J. Ginseng Res. 2021, 45, 408–419. [Google Scholar] [CrossRef]
- Wada, T.; Sakai, N.; Matsushima, K.; Kaneko, S. Fibrocytes: A new insight into kidney fibrosis. Kidney Int. 2007, 72, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misseri, R.; Meldrum, D.R.; Dagher, P.; Hile, K.; Rink, R.C.; Meldrum, K.K. Unilateral ureteral obstruction induces renal tubular cell production of tumor necrosis factor-alpha independent of inflammatory cell infiltration. J. Urol. 2004, 172, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Fogo, A.B. Fibrosis and renal aging. Kidney Int. Suppl. 2014, 4, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Yuzbashian, E.; Rahbarinejad, P.; Asghari, G.; Azizi, F. Dietary intakes of total polyphenol and its subclasses in association with the incidence of chronic kidney diseases: A prospective population-based cohort study. BMC Nephrol. 2021, 22, 84. [Google Scholar] [CrossRef]
- Vargas, F.; Romecín, P.; García-Guillén, A.I.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Flavonoids in Kidney Health and Disease. Front Physiol. 2018, 9, 394. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.L.; Lin, J.H.; Hammes, H.P.; Zhang, C. Flavonoids in Treatment of Chronic Kidney Disease. Molecules 2022, 27, 2365. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Zorov, D.B.; Plotnikov, E.Y. Targeting Inflammation and Oxidative Stress as a Therapy for Ischemic Kidney Injury. Biochemistry 2020, 85, 1591–1602. [Google Scholar] [CrossRef]
- Asgharpour, M.; Alirezaei, A. Herbal antioxidants in dialysis patients: A review of potential mechanisms and medical implications. Ren. Fail. 2021, 43, 351–361. [Google Scholar] [CrossRef]
- Khor, B.H.; Narayanan, S.S.; Sahathevan, S.; Gafor, A.H.A.; Daud, Z.A.M.; Khosla, P.; Sabatino, A.; Fiaccadori, E.; Chinna, K.; Karupaiah, T. Efficacy of Nutritional Interventions on Inflammatory Markers in Haemodialysis Patients: A Systematic Review and Limited Meta-Analysis. Nutrients 2018, 10, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.C.; Maggo, J.K.; Campbell, K.L.; Craig, J.C.; Johnson, D.W.; Sutanto, B.; Ruospo, M.; Tong, A.; Strippoli, G.F. Dietary interventions for adults with chronic kidney disease. Cochrane. Database Syst. Rev. 2017, 4, CD011998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.X.J.; Gammon, C.S.; von Hurst, P.; Chepulis, L.; Page, R.A. A Narrative Review of Human Clinical Trials on the Impact of Phenolic-Rich Plant Extracts on Prediabetes and Its Subgroups. Nutrients 2021, 13, 3733. [Google Scholar] [CrossRef] [PubMed]
| Aim Gene | Oligonucleotide Sequence |
|---|---|
| MMP-2 | (F) 5′ CGTGGTGAGATCTTCTTCTTCAAGGA 3′ (R) 5′ CCTCATACACAGCGTCAATCTTTTC 3′ |
| MMP-9 | (F) 5′AATTCGACTTGAAGTCTCAGAAGG 3′ (R) 5′ATTAGGTGACCCTGTCGCTG 3′ |
| P53 | (F) 5′ CCCCTGAAGACTGGATAACTG 3′ (R) 5′ AAGTATTTGTCATGGCAGAAATAGG 3′ |
| TNF-α | (F) 5′AAATGGGCTCCCTCTCATCAGTTC 3′ (R) 5′ TCTGCTTGGTGGTTTGCTACGAC 3′ |
| TGF-β | (F) 5′GGGCTTTCGCTTCAGTGCT 3′ (R) 5′TCGGTTCATGTCATGGATGGT 3′ |
| β–actin | (F) 5′GGAGATTACTGCCCTGGCTCCTA 3′ (R) 5′GACTCATCGTACTCCTGCTTGCTG 3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chtourou, Y.; Morjen, M.; Ammar, R.; Mhiri, R.; Jemaà, M.; ELBini-Dhouib, I.; Fetoui, H.; Srairi-Abid, N.; Marrakchi, N.; Jebali, J. Investigation of the Renal Protective Effect of Combined Dietary Polyphenols in Streptozotocin-Induced Diabetic Aged Rats. Nutrients 2022, 14, 2867. https://doi.org/10.3390/nu14142867
Chtourou Y, Morjen M, Ammar R, Mhiri R, Jemaà M, ELBini-Dhouib I, Fetoui H, Srairi-Abid N, Marrakchi N, Jebali J. Investigation of the Renal Protective Effect of Combined Dietary Polyphenols in Streptozotocin-Induced Diabetic Aged Rats. Nutrients. 2022; 14(14):2867. https://doi.org/10.3390/nu14142867
Chicago/Turabian StyleChtourou, Yassine, Maram Morjen, Rahma Ammar, Rania Mhiri, Mohamed Jemaà, Ines ELBini-Dhouib, Hamadi Fetoui, Najet Srairi-Abid, Naziha Marrakchi, and Jed Jebali. 2022. "Investigation of the Renal Protective Effect of Combined Dietary Polyphenols in Streptozotocin-Induced Diabetic Aged Rats" Nutrients 14, no. 14: 2867. https://doi.org/10.3390/nu14142867
APA StyleChtourou, Y., Morjen, M., Ammar, R., Mhiri, R., Jemaà, M., ELBini-Dhouib, I., Fetoui, H., Srairi-Abid, N., Marrakchi, N., & Jebali, J. (2022). Investigation of the Renal Protective Effect of Combined Dietary Polyphenols in Streptozotocin-Induced Diabetic Aged Rats. Nutrients, 14(14), 2867. https://doi.org/10.3390/nu14142867