Progressive Metabolic Abnormalities Associated with the Development of Neonatal Bronchopulmonary Dysplasia
Abstract
1. Introduction
2. Methods
2.1. Study Cohort
2.2. Statistical Analysis
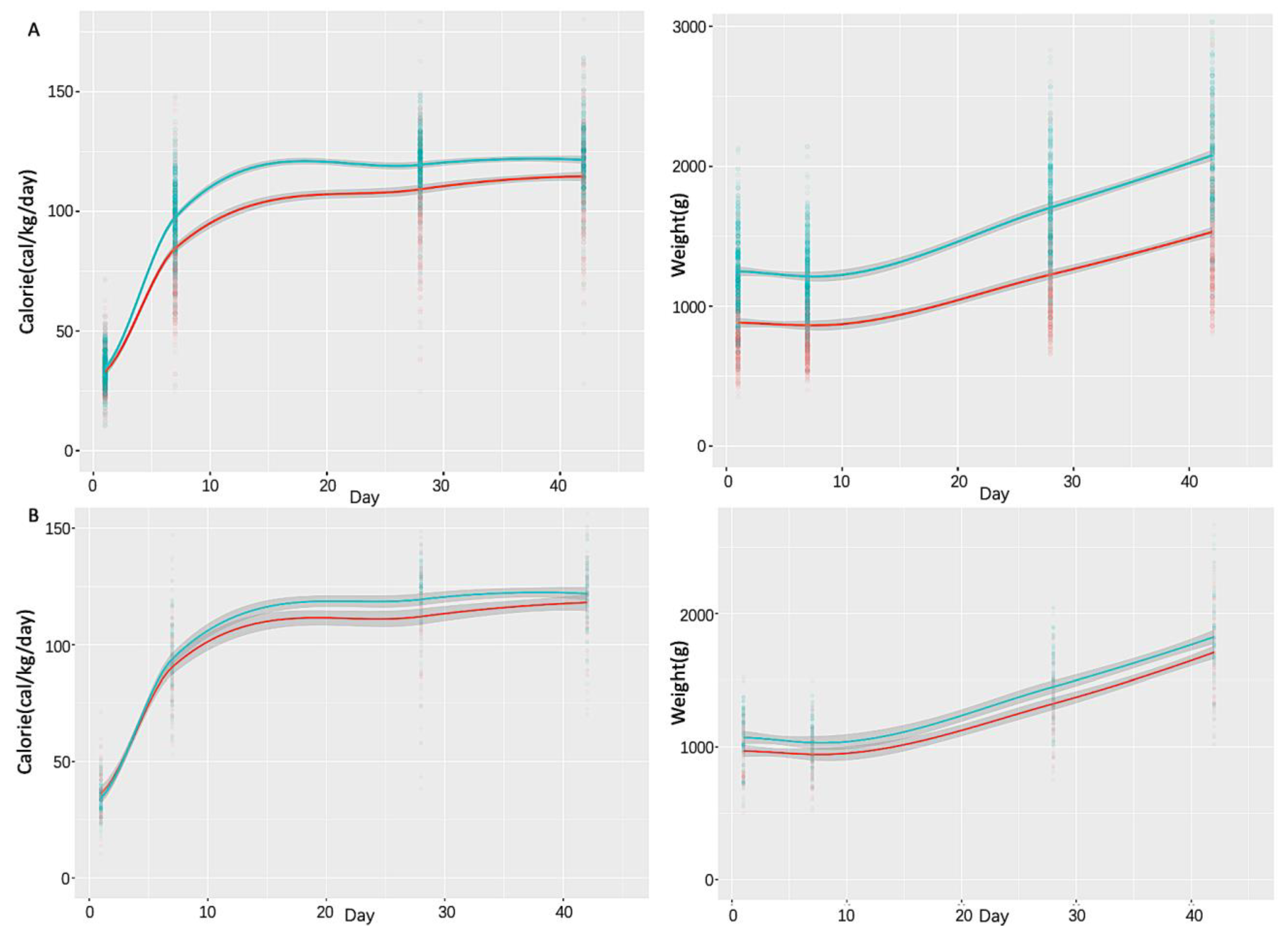
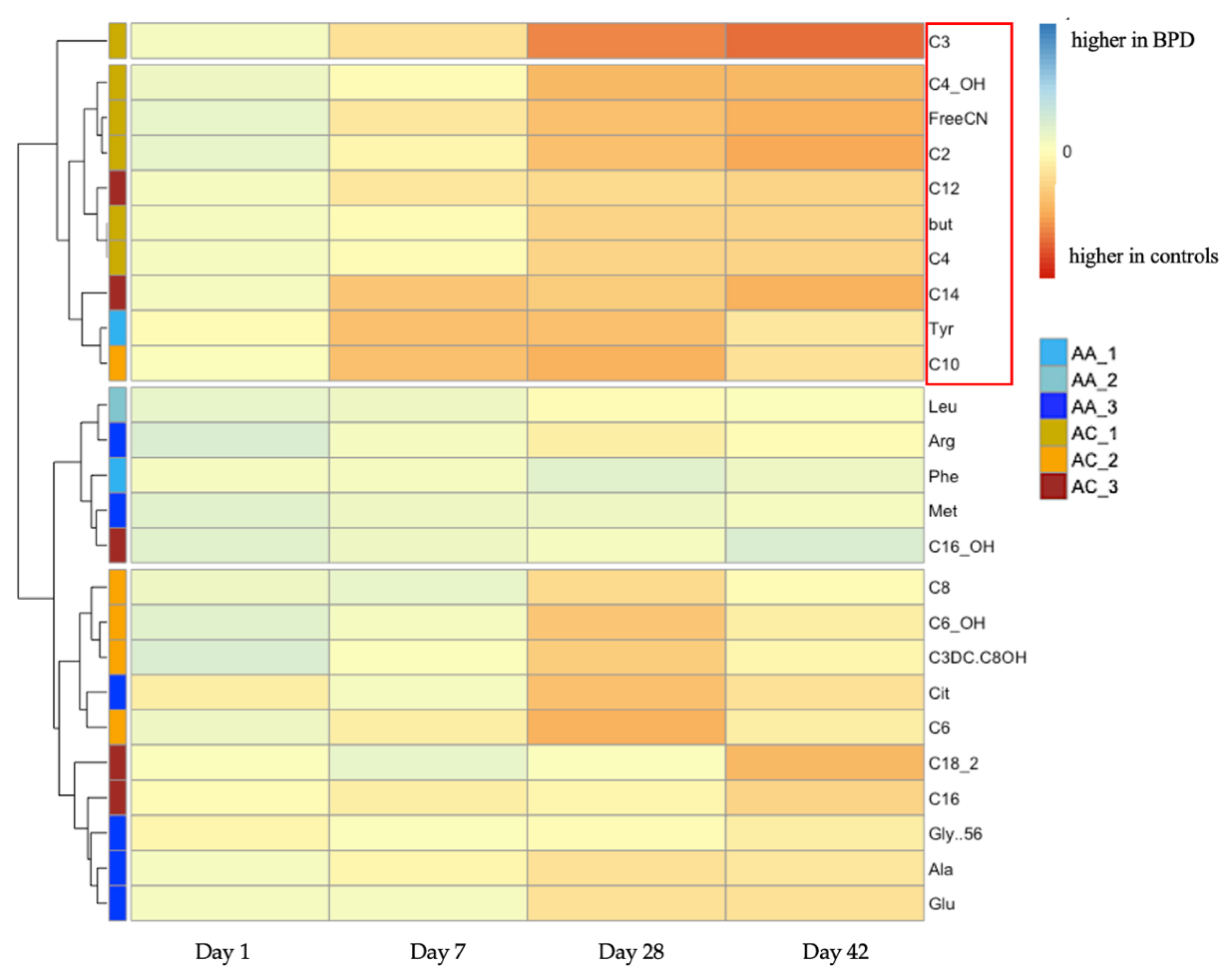
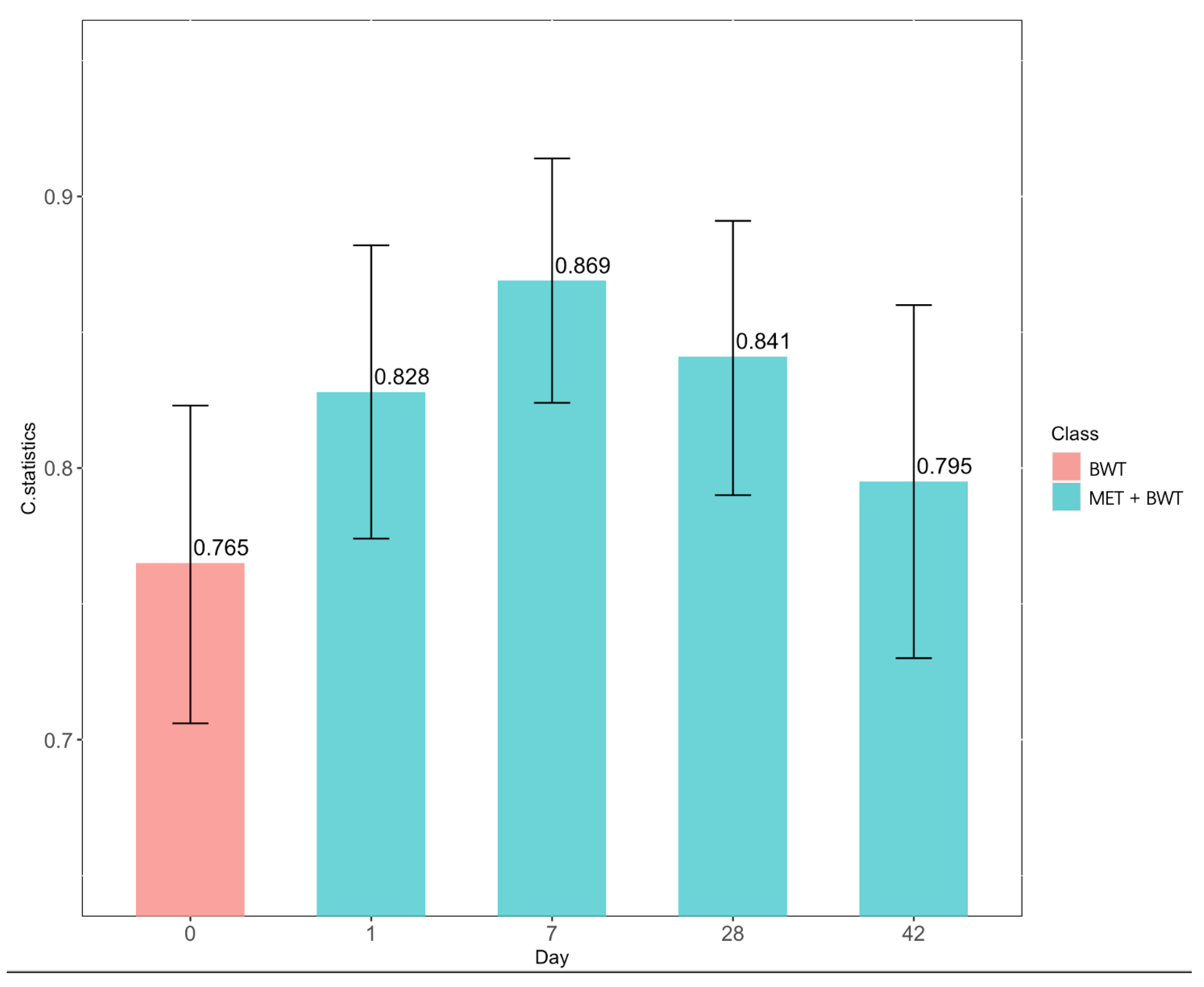
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPD | bronchopulmonary dysplasia (chronic lung disease of prematurity) |
| AA | amino acid |
| AC | acylcarnitine |
| NEC | necrotizing enterocolitis |
| IVH | intraventricular hemorrhage |
| ROP | retinopathy of prematurity |
| GA | gestational age |
References
- Martin, J.R.; Fanaroff, A.A.; Walsh, M.C. Fanaroff and Martin’s Neonatal-Perinatal Medicine, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780323567107. [Google Scholar]
- Siffel, C.; Kistler, K.D.; Lewis, J.F.M.; Sarda, S.P. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: A systematic literature review. J. Matern. Neonatal Med. 2019, 34, 1721–1731. [Google Scholar] [CrossRef]
- Hilgendorff, A.; Reiss, I.; Ehrhardt, H.; Eickelberg, O.; Alvira, C.M. Chronic Lung Disease in the Preterm Infant. Lessons Learned from Animal Models. Am. J. Respir. Cell Mol. Biol. 2014, 50, 233–245. [Google Scholar] [CrossRef]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Strnadová, K.A.; Holub, M.; Mühl, A.; Heinze, G.; Ratschmann, R.; Mascher, H.; Stöckler-Ipsiroglu, S.; Waldhauser, F.; Votava, F.; Lebl, J.; et al. Long-Term Stability of Amino Acids and Acylcarnitines in Dried Blood Spots. Clin. Chem. 2007, 53, 717–722. [Google Scholar] [CrossRef]
- Fingerhut, R.; Ensenauer, R.; Röschinger, W.; Arnecke, R.; Olgemöller, B.; Roscher, A.A. Stability of Acylcarnitines and Free Carnitine in Dried Blood Samples: Implications for Retrospective Diagnosis of Inborn Errors of Metabolism and Neonatal Screening for Carnitine Transporter Deficiency. Anal. Chem. 2009, 81, 3571–3575. [Google Scholar] [CrossRef]
- Meyburg, J.; Schulze, A.; Kohlmueller, D.; Pöschl, J.; Linderkamp, O.; Hoffmann, G.F.; Mayatepek, E. Acylcarnitine Profiles of Preterm Infants Over the First Four Weeks of Life. Pediatr. Res. 2002, 52, 720–723. [Google Scholar] [CrossRef][Green Version]
- Gucciardi, A.; Zaramella, P.; Costa, I.; Pirillo, P.; Nardo, D.; Naturale, M.; Chiandetti, L.; Giordano, G. Analysis and interpretation of acylcarnitine profiles in dried blood spot and plasma of preterm and full-term newborns. Pediatr. Res. 2014, 77, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, O.O.; Weindel, A.L.; Saunders, A.N.; Dietzen, D.J. Impact of premature birth and critical illness on neonatal range of plasma amino acid concentrations determined by LC-MS/MS. Mol. Genet. Metab. 2011, 104, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.J.; Ye, C.; Chen, Y.; Zhang, D.; Li, T.; Ling, X.B.; Cohen, H.J.; Shaw, G.M.; Stevenson, D.K.; Chace, D.; et al. Progressive Metabolic Dysfunction and Nutritional Variability Precedes Necrotizing Enterocolitis. Nutrients 2020, 12, 1275. [Google Scholar] [CrossRef] [PubMed]
- Piersigilli, F.; Van Grambezen, B.; Hocq, C.; Danhaive, O. Nutrients and Microbiota in Lung Diseases of Prematurity: The Placenta-Gut-Lung Triangle. Nutrients 2020, 12, 469. [Google Scholar] [CrossRef] [PubMed]
- Kaluarachchi, D.C.; Smith, C.J.; Klein, J.M.; Murray, J.C.; Dagle, J.M.; Ryckman, K.K. Polymorphisms in urea cycle enzyme genes are associated with persistent pulmonary hypertension of the newborn. Pediatr. Res. 2017, 83, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.L.; Dawling, S.; Walsh, W.F.; Haines, J.L.; Christman, B.W.; Bazyk, A.; Scott, N.; Summar, M.L. Neonatal Pulmonary Hypertension. N. Engl. J. Med. 2001, 344, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Trittmann, J.K.; Peterson, E.; Rogers, L.K.; Chen, B.; Backes, C.H.; Klebanoff, M.A.; Nelin, L.D. Plasma Asymmetric Dimethylarginine Levels Are Increased in Neonates with Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension. J. Pediatr. 2014, 166, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Piersigilli, F.; Lam, T.T.; Vernocchi, P.; Quagliariello, A.; Putignani, L.; Aghai, Z.H.; Bhandari, V. Identification of new biomarkers of bronchopulmonary dysplasia using metabolomics. Metabolomics 2019, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Steurer, M.A.; Oltman, S.; Baer, R.J.; Feuer, S.; Liang, L.; Paynter, R.A.; Rand, L.; Ryckman, K.K.; Keller, R.L.; Jelliffe-Pawlowski, L.L. Altered metabolites in newborns with persistent pulmonary hypertension. Pediatr. Res. 2018, 84, 272–278. [Google Scholar] [CrossRef]
- Black, S.M.; Field-Ridley, A.; Sharma, S.; Kumar, S.; Keller, R.L.; Kameny, R.; Maltepe, E.; Datar, S.A.; Fineman, J.R. Altered Carnitine Homeostasis in Children With Increased Pulmonary Blood Flow Due to Ventricular Septal Defects. Pediatr. Crit. Care Med. 2017, 18, 931–934. [Google Scholar] [CrossRef]
- Sylvester, K.G.; Kastenberg, Z.J.; Moss, R.L.; Enns, G.M.; Cowan, T.M.; Shaw, G.M.; Stevenson, D.K.; Sinclair, T.J.; Scharfe, C.; Ryckman, K.K.; et al. Acylcarnitine Profiles Reflect Metabolic Vulnerability for Necrotizing Enterocolitis in Newborns Born Premature. J. Pediatr. 2016, 181, 80–85. [Google Scholar] [CrossRef]
- Clark, R.H.; Kelleher, A.S.; Chace, D.H.; Spitzer, A.R. Gestational Age and Age at Sampling Influence Metabolic Profiles in Premature Infants. Pediatrics 2014, 134, e37–e46. [Google Scholar] [CrossRef]
- Jobe, A.H. Let’s feed the preterm lung. J. Pediatr. 2006, 82, 165–166. [Google Scholar] [CrossRef][Green Version]
- Coalson, J.J. Pathology of Bronchopulmonary Dysplasia. Semin. Perinatol. 2006, 30, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Wemhöner, A.; Ortner, D.; Tschirch, E.; Strasak, A.; Rüdiger, M. Nutrition of preterm infants in relation to bronchopulmonary dysplasia. BMC Pulm. Med. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, H.; Geng, H.; Cui, N.; Huang, F.; Zhu, X.; Zhu, X. Prediction of Bronchopulmonary Dysplasia in Preterm Infants Using Postnatal Risk Factors. Front. Pediatr. 2020, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Malikiwi, A.I.; Lee, Y.-M.; Davies-Tuck, M.; Wong, F.Y. Postnatal nutritional deficit is an independent predictor of bronchopulmonary dysplasia among extremely premature infants born at or less than 28 weeks gestation. Early Hum. Dev. 2019, 131, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Denne, S.C. Energy expenditure in infants with pulmonary insufficiency: Is there evidence for increased energy needs? J. Nutr. 2001, 131, 935S–937S. [Google Scholar] [CrossRef]
- Kurzner, S.I.; Garg, M.; Bautista, D.B.; Sargent, C.W.; Bowman, M.; Keens, T.G. Growth failure in bronchopulmonary dysplasia: Elevated metabolic rates and pulmonary mechanics. J. Pediatr. 1988, 112, 73–80. [Google Scholar] [CrossRef]
- Embleton, N.E.; Pang, N.; Cooke, R.J.; Golden, N.H.; Seigel, W.M.; Fisher, M.; Schneider, M.; Quijano, E.; Suss, A.; Bergeson, R.; et al. Postnatal Malnutrition and Growth Retardation: An Inevitable Consequence of Current Recommendations in Preterm Infants? Pediatrics 2001, 107, 270–273. [Google Scholar] [CrossRef]
- Scaglia, F.; Longo, N. Primary and secondary alterations of neonatal carnitine metabolism. Semin. Perinatol. 1999, 23, 152–161. [Google Scholar] [CrossRef]
- Sim, K.G.; Carpenter, K.; Hammond, J.; Christodoulou, J.; Wilcken, B. Acylcarnitine profiles in fibroblasts from patients with respiratory chain defects can resemble those from patients with mitochondrial fatty acid [beta ]-oxidation disorders. Metabolism 2002, 51, 366–371. [Google Scholar] [CrossRef]
- Korkmaz, A.; Tekinalp, G.; Coskun, T.; Yigit, S.; Yurdakok, M. Plasma carnitine levels in preterm infants with respiratory distress syndrome. Pediatr. Int. 2005, 47, 49–52. [Google Scholar] [CrossRef]
- Ogger, P.P.; Byrne, A.J. Macrophage metabolic reprogramming during chronic lung disease. Mucosal Immunol. 2020, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dennery, P.A.; Yao, H. Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L544–L554. [Google Scholar] [CrossRef]
- Sahoo, D.; Zaramela, L.S.; Hernandez, G.E.; Mai, U.; Taheri, S.; Dang, D.; Stouch, A.N.; Medal, R.M.; McCoy, A.M.; Aschner, J.L.; et al. Transcriptional profiling of lung macrophages identifies a predictive signature for inflammatory lung disease in preterm infants. Commun. Biol. 2020, 3, 259. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2017, 223, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Goetzman, E.S.; Alcorn, J.F.; Bharathi, S.S.; Uppala, R.; McHugh, K.J.; Kosmider, B.; Chen, R.; Zuo, Y.Y.; Beck, M.E.; McKinney, R.W.; et al. Long-chain Acyl-CoA Dehydrogenase Deficiency as a Cause of Pulmonary Surfactant Dysfunction. J. Biol. Chem. 2014, 289, 10668–10679. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, C.; Bharathi, S.; Uppala, R.; Ilkayeva, O.R.; Wang, D.; McHugh, K.; Zou, Y.; Wang, J.; Alcorn, J.F.; Zuo, Y.Y.; et al. Long-chain Acylcarnitines Reduce Lung Function by Inhibiting Pulmonary Surfactant. J. Biol. Chem. 2015, 290, 23897–23904. [Google Scholar] [CrossRef]
- Montgomery, A.M.; Bazzy-Asaad, A.; Asnes, J.D.; Bizzarro, M.J.; Ehrenkranz, R.A.; Weismann, C.G. Biochemical Screening for Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Neonatology 2016, 109, 190–194. [Google Scholar] [CrossRef]
- Klinger, J.R.; Kadowitz, P.J. The Nitric Oxide Pathway in Pulmonary Vascular Disease. Am. J. Cardiol. 2017, 120, S71–S79. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef]
- Scott, J.A.; Maarsingh, H.; Holguin, F.; Grasemann, H. Arginine Therapy for Lung Diseases. Front. Pharmacol. 2021, 12, 627503. [Google Scholar] [CrossRef]
- Geary, C.; Caskey, M.; Fonseca, R.; Malloy, M. Decreased Incidence of Bronchopulmonary Dysplasia After Early Management Changes, Including Surfactant and Nasal Continuous Positive Airway Pressure Treatment at Delivery, Lowered Oxygen Saturation Goals, and Early Amino Acid Administration: A Historical Cohort Study. Pediatrics 2008, 121, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, A.; Aschner, J.L.; Rey-Parra, G.J.; Magarik, J.; Zeng, H.; Summar, M.; Eaton, F.; Thébaud, B. L-Citrulline Attenuates Arrested Alveolar Growth and Pulmonary Hypertension in Oxygen-Induced Lung Injury in Newborn Rats. Pediatr. Res. 2010, 68, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D. Bronchopulmonary dysplasia and oxidative stress: Are we closer to an understanding of the pathogenesis of BPD? Acta Paediatr. 1997, 86, 1277–1282. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Zhu, L.; Chen, J.; Tsai, M.; Martin, J.C.; Smith, C.D.; Beckman, J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992, 298, 431–437. [Google Scholar] [CrossRef]
- Banks, B.A.; Ischiropoulos, H.; McClelland, M.; Ballard, P.L.; Ballard, R.A. Plasma 3-Nitrotyrosine Is Elevated in Premature Infants Who Develop Bronchopulmonary Dysplasia. Pediatrics 1998, 101, 870–874. [Google Scholar] [CrossRef]
- Tejero, J.; Stuehr, D. Tetrahydrobiopterin in nitric oxide synthase. IUBMB Life 2013, 65, 358–365. [Google Scholar] [CrossRef]
- Khoo, J.P.; Zhao, L.; Alp, N.J.; Bendall, J.K.; Nicoli, T.; Rockett, K.; Wilkins, M.R.; Channon, K.M. Pivotal Role for Endothelial Tetrahydrobiopterin in Pulmonary Hypertension. Circulation 2005, 111, 2126–2133. [Google Scholar] [CrossRef]
- Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000, 347, 1–16. [Google Scholar] [CrossRef]
| Characteristics | Control (n = 108) | BPD (n = 97) | p-Value (BPD vs. Control) |
|---|---|---|---|
| Sex, n (%) | 0.599 | ||
| Male | 44 (40.74%) | 44 (45.36%) | |
| Race, n (%) | 0.100 | ||
| American Indian | 4 (3.07%) | 2 (2.06%) | |
| Asian | 4 (3.07%) | 4 (4.12%) | |
| Black | 28 (25.93%) | 19 (19.59%) | |
| Hispanic | 15 (13.89%) | 5 (5.15%) | |
| Other | 0 (0.00%) | 1 (1.03%) | |
| Pacific Islander | 1 (0.93%) | 0 (0.00%) | |
| White | 56 (51.85%) | 66 (68.04%) | |
| Mode of delivery, n (%) | 0.944 | ||
| Cesarean section | 82 (75.93%) | 75 (77.32%) | |
| Vaginal | 26 (24.07%) | 22 (22.68%) | |
| Multiple gestation, n (%) | 0.790 | ||
| One | 85 (78.70%) | 77 (79.38%) | |
| Two | 22 (20.37%) | 18 (18.56%) | |
| Three | 1 (0.93%) | 2 (2.06%) | |
| Antenatal steroids, n (%) | 0.890 | ||
| None | 13 (12.04%) | 9 (9.28%) | |
| Betamethasone | 86 (79.63%) | 80 (82.47%) | |
| Dexamethasone | 7 (6.48%) | 7 (7.22%) | |
| Both | 2 (1.85%) | 1 (1.03%) | |
| Birth weight (g), mean (SD) | 1071 (210) | 968 (198) | <0.001 |
| Patent ductus arteriosus (PDA), n (%) | 34 (31.5%) | 53 (54.6%) | 0.0011 |
| Small for gestational age (SGA), n (%) | 10 (9.26%) | 10 (10.31%) | 0.800 |
| Apgar at 1 min, median (25%-75%) | 6 (3–7) | 5 (3–6) | 0.113 |
| Apgar at 5 min, median (25%-75%) | 8 (7–8) | 7 (6–8) | 0.063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Wu, J.; Reiss, J.D.; Sinclair, T.J.; Stevenson, D.K.; Shaw, G.M.; Chace, D.H.; Clark, R.H.; Prince, L.S.; Ling, X.B.; et al. Progressive Metabolic Abnormalities Associated with the Development of Neonatal Bronchopulmonary Dysplasia. Nutrients 2022, 14, 3547. https://doi.org/10.3390/nu14173547
Ye C, Wu J, Reiss JD, Sinclair TJ, Stevenson DK, Shaw GM, Chace DH, Clark RH, Prince LS, Ling XB, et al. Progressive Metabolic Abnormalities Associated with the Development of Neonatal Bronchopulmonary Dysplasia. Nutrients. 2022; 14(17):3547. https://doi.org/10.3390/nu14173547
Chicago/Turabian StyleYe, Chengyin, Jinghua Wu, Jonathan D. Reiss, Tiffany J. Sinclair, David K. Stevenson, Gary M. Shaw, Donald H. Chace, Reese H. Clark, Lawrence S. Prince, Xuefeng Bruce Ling, and et al. 2022. "Progressive Metabolic Abnormalities Associated with the Development of Neonatal Bronchopulmonary Dysplasia" Nutrients 14, no. 17: 3547. https://doi.org/10.3390/nu14173547
APA StyleYe, C., Wu, J., Reiss, J. D., Sinclair, T. J., Stevenson, D. K., Shaw, G. M., Chace, D. H., Clark, R. H., Prince, L. S., Ling, X. B., & Sylvester, K. G. (2022). Progressive Metabolic Abnormalities Associated with the Development of Neonatal Bronchopulmonary Dysplasia. Nutrients, 14(17), 3547. https://doi.org/10.3390/nu14173547








