The Intake of Ultra-Processed Foods and Prevalence of Chronic Kidney Disease: The Health Examinees Study
Abstract
:1. Introduction
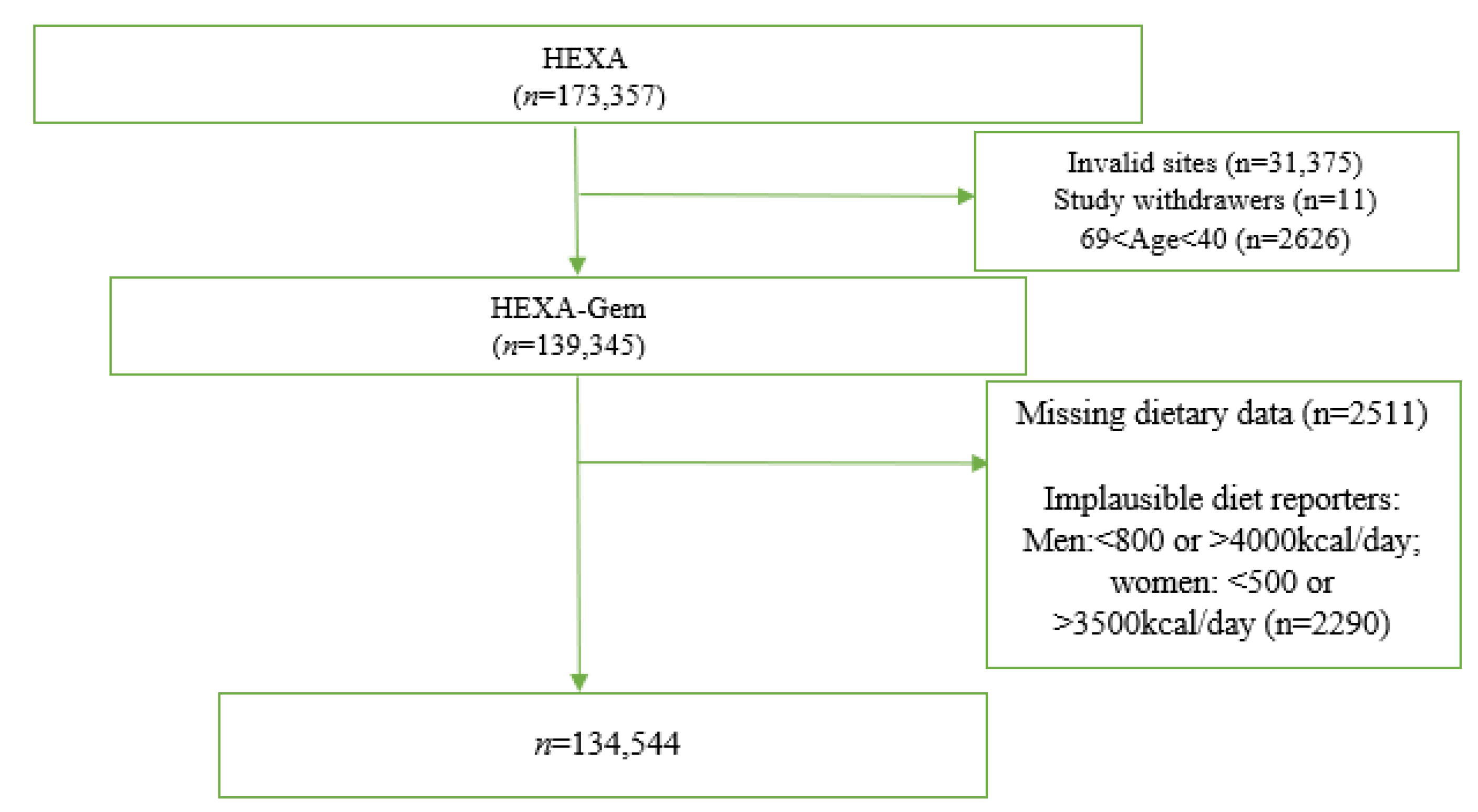
2. Materials and Methods
2.1. Study Population
2.2. Assessment of UPF Intake
2.3. Assessment of CKD [24]
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [PubMed]
- Yin, T.; Chen, Y.; Tang, L.; Yuan, H.; Zeng, X.; Fu, P. Relationship between modifiable lifestyle factors and chronic kidney disease: A bibliometric analysis of top-cited publications from 2011 to 2020. BMC Nephrol. 2022, 23, 120. [Google Scholar] [CrossRef]
- He, L.Q.; Wu, X.H.; Huang, Y.Q.; Zhang, X.Y.; Shu, L. Dietary patterns and chronic kidney disease risk: A systematic review and updated meta-analysis of observational studies. Nutr. J. 2021, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.E.; Kelly, J.T.; Campbell, K.L.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Quintela, B.C.S.F.; Carioca, A.A.F.; de Oliveira, J.G.R.; Fraser, S.D.S.; da Silva Junior, G.B. Dietary patterns and chronic kidney disease outcomes: A systematic review. Nephrology 2021, 26, 603–612. [Google Scholar] [CrossRef]
- Missikpode, C.; Ricardo, A.C.; Durazo-Arvizu, R.A.; Manoharan, A.; Mattei, J.; Isasi, C.R.; Mossavar-Rahmani, Y.; Talavera, G.A.; Sotres-Alvarez, D.; Daviglus, M.L.; et al. Association of Diet Quality Indices with Longitudinal Changes in Kidney Function in U.S. Hispanics/Latinos: Findings from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Kidney360 2021, 2, 50. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Baker, P.; Friel, S. Food systems transformations, ultra-processed food markets and the nutrition transition in Asia. Glob. Health 2016, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Shim, S.Y.; Cha, H.J.; Kim, J.; Kim, H.C. Association between Ultra-processed Food Consumption and Dietary Intake and Diet Quality in Korean Adults. J. Acad. Nutr. Diet. 2021, 122, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Shim, S.Y.; Cha, H.J.; Kim, J.; Kim, H.C. Socioeconomic Characteristics and Trends in the Consumption of Ultra-Processed Foods in Korea from 2010 to 2018. Nutrients 2021, 13, 1120. [Google Scholar] [CrossRef] [PubMed]
- Lavigne-Robichaud, M.; Moubarac, J.C.; Lantagne-Lopez, S.; Johnson-Down, L.; Batal, M.; Laouan Sidi, E.A.; Lucas, M. Diet quality indices in relation to metabolic syndrome in an Indigenous Cree (Eeyouch) population in northern Québec, Canada. Public Health Nutr. 2018, 21, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Nardocci, M.; Leclerc, B.S.; Louzada, M.L.; Monteiro, C.A.; Batal, M.; Moubarac, J.C. Consumption of ultra-processed foods and obesity in Canada. Can. J. Public Health = Rev. Can. De St. Publique 2019, 110, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Duan, M.-J.; Dekker, L.H.; Carrero, J.J.; Avesani, C.M.; Bakker, S.J.L.; de Borst, M.H.; Navis, G.J. Ultraprocessed food consumption and kidney function decline in a population-based cohort in the Netherlands. Am. J. Clin. Nutr. 2022, 116, 263–273. [Google Scholar] [CrossRef]
- Rey-García, J.; Donat-Vargas, C.; Sandoval-Insausti, H.; Bayan-Bravo, A.; Moreno-Franco, B.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Ultra-Processed Food Consumption is Associated with Renal Function Decline in Older Adults: A Prospective Cohort Study. Nutrients 2021, 13, 428. [Google Scholar] [CrossRef]
- Du, S.; Kim, H.; Crews, D.C.; White, K.; Rebholz, C.M. Association Between Ultraprocessed Food Consumption and Risk of Incident CKD: A Prospective Cohort Study. Am. J. Kidney Dis. 2022. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.-G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Kang, D.; Kim, D.H.; Kim, D.H.; Lee, D.H.; Lee, D.H.; Lee, H.J.; Moon, J.D.; Leem, J.H.; Lee, J.K.; Lee, J.T.; et al. The Health Examinees (HEXA) Study: Rationale, Study Design and Baseline Characteristics. Asian Pac. J. Cancer Prev. 2015, 16, 1591–1597. [Google Scholar]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, N.; Rossato, S.; Drouin-Chartier, J.P.; Du, M.; Steele, E.M.; Sampson, L.; Monteiro, C.; Zhang, F.F.; Willett, W.; Fung, T.; et al. Categorising ultra-processed foods in large-scale cohort studies: Evidence from the Nurses’ Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. J. Nutr. Sci. 2021, 10, e77. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Yang, J.J.; Song, M.; Yoon, H.-S.; Lee, H.-W.; Lee, Y.; Lee, S.-A.; Choi, J.-Y.; Lee, J.-K.; Kang, D. What Are the Major Determinants in the Success of Smoking Cessation: Results from the Health Examinees Study. PLoS ONE 2015, 10, e0143303. [Google Scholar]
- Rauber, F.; da Louzada, M.L.C.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Shim, S.Y.; Kim, H.C.; Shim, J.-S. Consumption of Ultra-Processed Food and Blood Pressure in Korean Adults. Korean Circ. J. 2022, 52, 60–70. [Google Scholar] [CrossRef]
- Dos Santos, F.S.; Dias, M.d.S.; Mintem, G.C.; Oliveira, I.O.; Gigante, D.P. Food processing and cardiometabolic risk factors: A systematic review. Rev. De Saude Publica 2020, 54, 70. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller, E.R.; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. 2016, 68, 853–861. [Google Scholar] [CrossRef]
- Mirmiran, P.; Yuzbashian, E.; Asghari, G.; Sarverzadeh, S.; Azizi, F. Dietary fibre intake in relation to the risk of incident chronic kidney disease. Br. J. Nutr. 2018, 119, 479–485. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.A.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014, 69, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; et al. Causal effects of relative fat, protein, and carbohydrate intake on chronic kidney disease: A Mendelian randomization study. Am. J. Clin. Nutr. 2021, 113, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; et al. Observational or Genetically Predicted Higher Vegetable Intake and Kidney Function Impairment: An Integrated Population-Scale Cross-Sectional Analysis and Mendelian Randomization Study. J. Nutr. 2021, 151, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kitiyakara, C.; Chabrashvili, T.; Chen, Y.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J. Am. Soc. Nephrol. 2003, 14, 2775–2782. [Google Scholar] [CrossRef]
- Sanders, P.W. Dietary Salt Intake, Salt Sensitivity and Cardiovascular Health. Hypertension 2009, 53, 442. [Google Scholar] [CrossRef]
- James, W.P.T.; Ralph, A.; Sanchez-Castillo, C.P. The dominance of salt in manufactured food in the sodium intake of affluent societies. Lancet 1987, 1, 426–429. [Google Scholar] [CrossRef]
- Anderson, C.A.M.; Appel, L.J.; Okuda, N.; Brown, I.J.; Chan, Q.; Zhao, L.; Ueshima, H.; Kesteloot, H.; Miura, K.; Curb, J.D.; et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: The INTERMAP study. J. Am. Diet Assoc. 2010, 110, 736–745. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, K. Methodological issues in estimating sodium intake in the Korea National Health and Nutrition Examination Survey. Epidemiol. Health 2014, 36, e2014033. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, E.S.; Lee, J.; Kim, Y. Trends in sodium intake and major contributing food groups and dishes in Korea: The Korea National Health and Nutrition Examination Survey 2013–2017. Nutr. Res. Pract. 2021, 15, 382–395. [Google Scholar] [CrossRef]
- Sarathy, S.; Sullivan, C.; Leon, J.B.; Sehgal, A.R. Fast Food, Phosphorus-Containing Additives, and the Renal Diet. J. Ren. Nutr. 2008, 18, 466–470. [Google Scholar] [CrossRef]
- Molins, R.A. Phosphates in Food [Internet], Phosphates in Food, 1st ed.; Routledge: New York, NY, USA, 1991. [Google Scholar]
- Chang, A.R.; Anderson, C. Dietary Phosphorus Intake and the Kidney. Annu. Rev. Nutr. 2017, 37, 321. [Google Scholar] [CrossRef] [PubMed]
- O’Seaghdha, C.M.; Hwang, S.J.; Muntner, P.; Melamed, M.L.; Fox, C.S. Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol. Dial. Transplant. 2011, 26, 2885–2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Srour, B.; Touvier, M. Ultra-processed foods and human health: What do we already know and what will further research tell us? eClinicalMedicine 2021, 32, 100747. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Kim, H.; Wong, E.; Rebholz, C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019, 131, 105057. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 1–150. [Google Scholar] [CrossRef]
- Vestergaard, S.V.; Christiansen, C.F.; Thomsen, R.W.; Birn, H.; Heide-Jørgensen, U. Identification of Patients with CKD in Medical Databases: A Comparison of Different Algorithms. Clin. J. Am. Soc. Nephrol. 2021, 16, 543–551. [Google Scholar] [CrossRef]
- Rule, A.D.; Bailey, K.R.; Lieske, J.C.; Peyser, P.A.; Turner, S.T. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int. 2013, 83, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
| Quartiles of UPF Intake, % of food Weight | |||||
|---|---|---|---|---|---|
| Characteristics | Q1 | Q2 | Q3 | Q4 | |
| n | 33,635 | 33,637 | 33,637 | 33,635 | |
| Age | 55.1 ± 0.04 | 52.4 ± 0.04 | 51.7 ± 0.04 | 51.7 ± 0.04 | |
| Sex | Male | 33.6 | 33.6 | 33.6 | 33.6 |
| Education | Elementary | 24.0 | 14.9 | 12.0 | 10.5 |
| Middle | 19.6 | 16.4 | 14.6 | 13.5 | |
| High | 37.2 | 41.4 | 41.3 | 41.2 | |
| ≥College | 19.3 | 27.3 | 32.1 | 34.9 | |
| Married | 88.7 | 90.4 | 90.3 | 88.3 | |
| Income, USD | Unknown | 15.2 | 13.0 | 13.2 | 13.8 |
| <1000 | 13.7 | 8.8 | 7.2 | 7.1 | |
| 1000–3000 | 40.0 | 38.2 | 36.4 | 35.3 | |
| ≥3000 | 31.2 | 40.0 | 43.2 | 43.8 | |
| Current smoker | 11.8 | 12.4 | 12.5 | 12.2 | |
| Current drinker | 41.3 | 46.5 | 45.8 | 44.7 | |
| Regular physical exercise | 49.6 | 47.4 | 45.8 | 43.8 | |
| BMI, kg/m2 | 24.0 ± 0.02 | 24.0 ± 0.02 | 23.9 ± 0.02 | 23.6 ± 0.02 | |
| BMI categories | <18.5 | 1.6 | 1.7 | 1.8 | 2.1 |
| 18.5~23.0 | 35.1 | 37.6 | 39 | 41.4 | |
| 23.0~25.0 | 28.9 | 27.9 | 27.6 | 27.0 | |
| ≥25.0 | 34.4 | 32.9 | 31.6 | 29.5 | |
| CKD | 4.7 | 3.8 | 3.9 | 4.0 | |
| CVD | 2.9 | 2.3 | 1.8 | 1.9 | |
| High blood glucose | 10.4 | 7.5 | 6.0 | 5.4 | |
| High blood pressure | 47.8 | 42.3 | 39.5 | 38.4 | |
| Total energy intake, kcal/day | 1571.5 ± 2.61 | 1684.1 ± 2.58 | 1806.3 ± 2.58 | 1884.3 ± 2.58 | |
| UPF, % of total food, median (IQR) | 1.7 (1.1–2.3) | 4.1 (3.5–4.8) | 7.4 (6.5–8.4) | 13.0 (11.0–16.0) | |
| Unprocessed foods, % of total food | 95.1 (93.2–96.6) | 91.4 (89.3–93.1) | 87.5 (85.0–89.4) | 80.6 (76.2–84.0) | |
| Carbohydrates, % of energy | 74.0 ± 0.04 | 72.3 ± 0.04 | 70.8 ± 0.04 | 69.6 ± 0.04 | |
| Protein, % energy | 13.1 ± 0.01 | 13.4 ± 0.01 | 13.7 ± 0.01 | 13.6 ± 0.01 | |
| Fat, % energy | 11.4 ± 0.03 | 13.2 ± 0.03 | 14.6 ± 0.03 | 16.1 ± 0.03 | |
| Fibre, g/day | 6.1 ± 0.01 | 5.8 ± 0.01 | 5.7 ± 0.01 | 5.4 ± 0.01 | |
| Dietary cholesterol, mg/day | 150.2 ± 0.51 | 161.6 ± 0.5 | 171.5 ± 0.5 | 177.9 ± 0.51 | |
| Dietary sodium, mg/day | 2641.7 ± 6.58 | 2511.5 ± 6.43 | 2487.8 ± 6.45 | 2338.6 ± 6.51 | |
| Dietary phosphorus, mg/day | 10.0 ± 0.02 | 9.9 ± 0.02 | 10.0 ± 0.02 | 9.8 ± 0.02 | |
| Quartiles of UPF Intake, % Food Weight | UPF, Continuous | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p for Trend | Per IQR Increment | |
| Cases, n | 1566 | 1272 | 1341 | 1359 | ||
| Model 1 | 1.00 | 1.00 (0.93–1.08) | 1.15 (1.07–1.24) | 1.16 (1.08–1.25) | <0.001 | 1.06 (1.03–1.09) |
| Model 2 | 1.00 | 1.00 (0.92–1.07) | 1.13 (1.04–1.21) | 1.13 (1.05–1.22) | 0.008 | 1.05 (1.02–1.08) |
| Model 3 | 1.00 | 1.01 (0.93–1.08) | 1.15 (1.06–1.24) | 1.16 (1.07–1.25) | 0.003 | 1.06 (1.03–1.09) |
| Quartiles of UPF Intake, % of Food Weight | UPF, Continuous | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Per IQR Increment | |
| Cases, n | 1566 | 1272 | 1341 | 1359 | |
| Model 1 | Ref | −0.03 (−0.04, −0.02) | −0.04 (−0.05, −0.03) | −0.04 (−0.05, −0.03) | −0.02 (−0.03, −0.01) |
| Mode 2 | Ref | −0.36 (−0.56, −0.16) | −0.69 (−0.91, −0.46) | −1.04 (−1.31, −0.77) | −0.44 (−0.55, −0.33) |
| Model 3 | Ref | −0.35 (−0.56, −0.14) | −0.70 (−0.93, −0.46) | −1.07 (−1.35, −0.79) | −0.45 (−0.58, −0.32) |
| Characteristic | Stratum | β (95% CI) a | p for Interaction |
|---|---|---|---|
| Sex | Male | −0.51 (−0.83, −0.20) | <0.001 |
| Female | −0.03 (−0.04, −0.02) | ||
| Age group | 40–49 | −0.41 (−0.61, −0.21) | 0.080 |
| 50–59 | −0.43 (−0.65, −0.22) | ||
| 60–69 | −0.66 (−1.04, −0.28) | ||
| Drinking | Non-drinker | 0.10 (−1.95, 3.37) | 0.129 |
| Past drinker | −0.07 (−0.80, 0.66) | ||
| Current drinker | −0.53 (−0.71, −0.36) | ||
| Current smoker | No | −0.21 (−0.28, −0.14) | <0.001 |
| Yes | −0.81 (−1.23, −0.39) | ||
| Regular exercise | No | −0.47 (−0.63, −0.3) | 0.002 |
| Yes | −0.33 (−0.45, −0.21) | ||
| BMI, kg/m2 | <18.5 | −0.03 (−0.35, 0.28) | 0.002 |
| 18.5–22.9 | −0.12 (−0.21, −0.02) | ||
| 23.0–24.9 | −0.25 (−0.4, −0.09) | ||
| ≥25.0 | −0.39 (−0.56, −0.22) | ||
| High blood glucose | No | −0.43 (−0.54, −0.32) | 0.025 |
| Yes | −0.62 (−1.19, −0.04) | ||
| High blood pressure | No | −0.34 (−0.44, −0.25) | 0.681 |
| Yes | −0.39 (−0.6, −0.18) | ||
| Prevalent CVD | No | −0.44 (−0.56, −0.32) | 0.137 |
| Yes | 0.44 (−0.55, 1.44) |
| UPF Sub-Groups (g/day) | β (95% CI) a |
|---|---|
| Instant noodles | 0.003 (−0.002, 0.009) |
| Breads | −0.007 (−0.012, −0.002) |
| Breakfast cereals and snacks | −0.028 (−0.042, −0.014) |
| Candies and chocolate | −0.168 (−0.234, −0.102) |
| Bread spreads (jam, honey, butter, and margarine) | −0.540 (−0.836, −0.238) |
| Meat and fish | 0.004 (−0.028, 0.037) |
| Pizza and hamburgers | −0.021 (−0.035, −0.005) |
| Milk | −0.015 (−0.032, 0.002) |
| Yoghurt | −0.005 (−0.007, −0.003) |
| Ice cream | −0.031 (−0.039, −0.021) |
| Soymilk drink | −0.093 (−0.123, −0.063) |
| Soft beverages and fruit sodas | −0.003 (−0.005, −0.0001) |
| Sweet rice punch (“Sikhye”) | 0.001 (−0.001, 0.003) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kityo, A.; Lee, S.-A. The Intake of Ultra-Processed Foods and Prevalence of Chronic Kidney Disease: The Health Examinees Study. Nutrients 2022, 14, 3548. https://doi.org/10.3390/nu14173548
Kityo A, Lee S-A. The Intake of Ultra-Processed Foods and Prevalence of Chronic Kidney Disease: The Health Examinees Study. Nutrients. 2022; 14(17):3548. https://doi.org/10.3390/nu14173548
Chicago/Turabian StyleKityo, Anthony, and Sang-Ah Lee. 2022. "The Intake of Ultra-Processed Foods and Prevalence of Chronic Kidney Disease: The Health Examinees Study" Nutrients 14, no. 17: 3548. https://doi.org/10.3390/nu14173548
APA StyleKityo, A., & Lee, S.-A. (2022). The Intake of Ultra-Processed Foods and Prevalence of Chronic Kidney Disease: The Health Examinees Study. Nutrients, 14(17), 3548. https://doi.org/10.3390/nu14173548






