Short-Term Fish Oil Supplementation during Adolescence Supports Sex-Specific Impact on Adulthood Visuospatial Memory and Cognitive Flexibility
Abstract
1. Introduction
Goal and Hypothesis
2. Methods
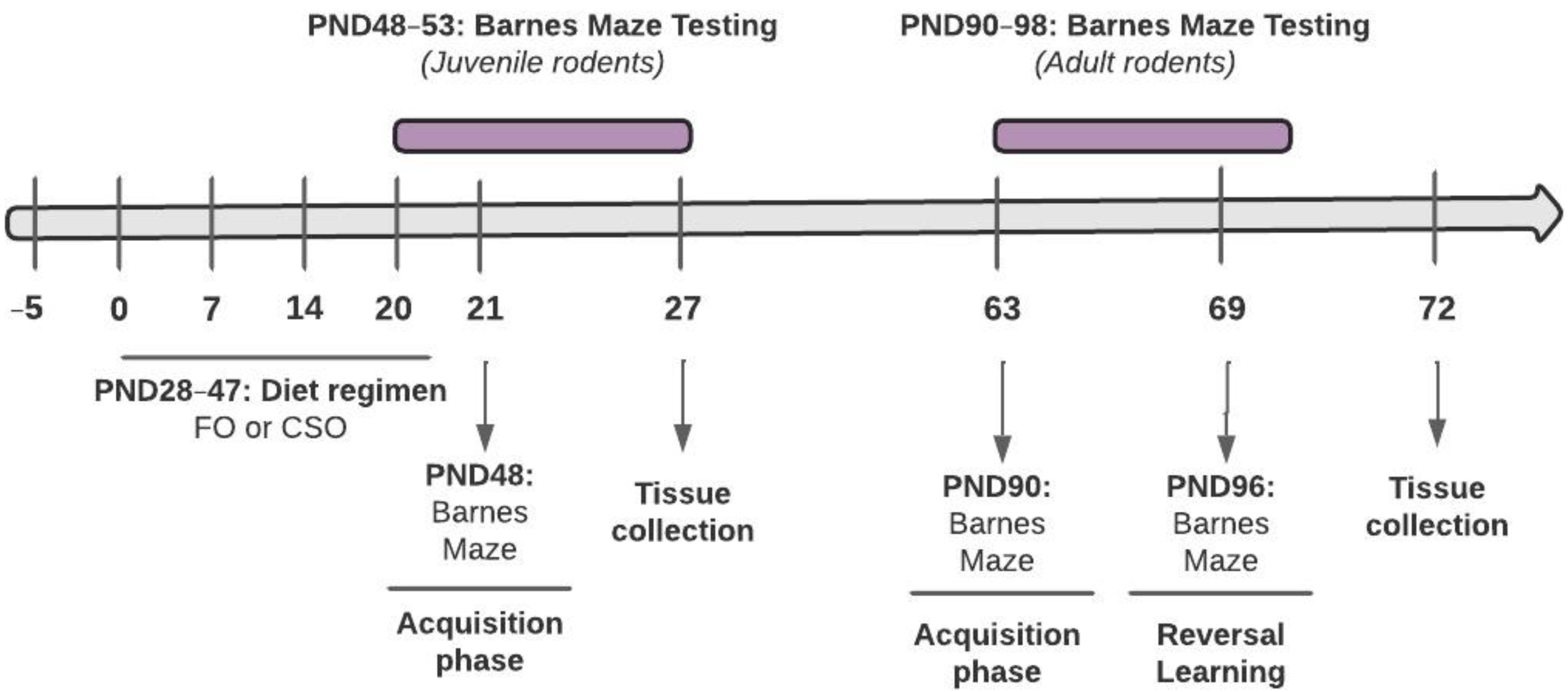
2.1. Subjects
2.2. Supplementation and Weight Monitoring
2.3. Behavioural Testing
Cognitive Testing: Barnes Maze Test (BMT) and Reversal Learning (-RL)
2.4. Statistical Analyses
3. Results
3.1. Acquisition Phase: Latency to Escape Box Entry
3.2. Acquisition Phase: Distance Travelled
3.3. Acquisition Phase: Number of Working Memory Errors (WME)
3.4. Reversal Learning: Latency to Escape Box Entry
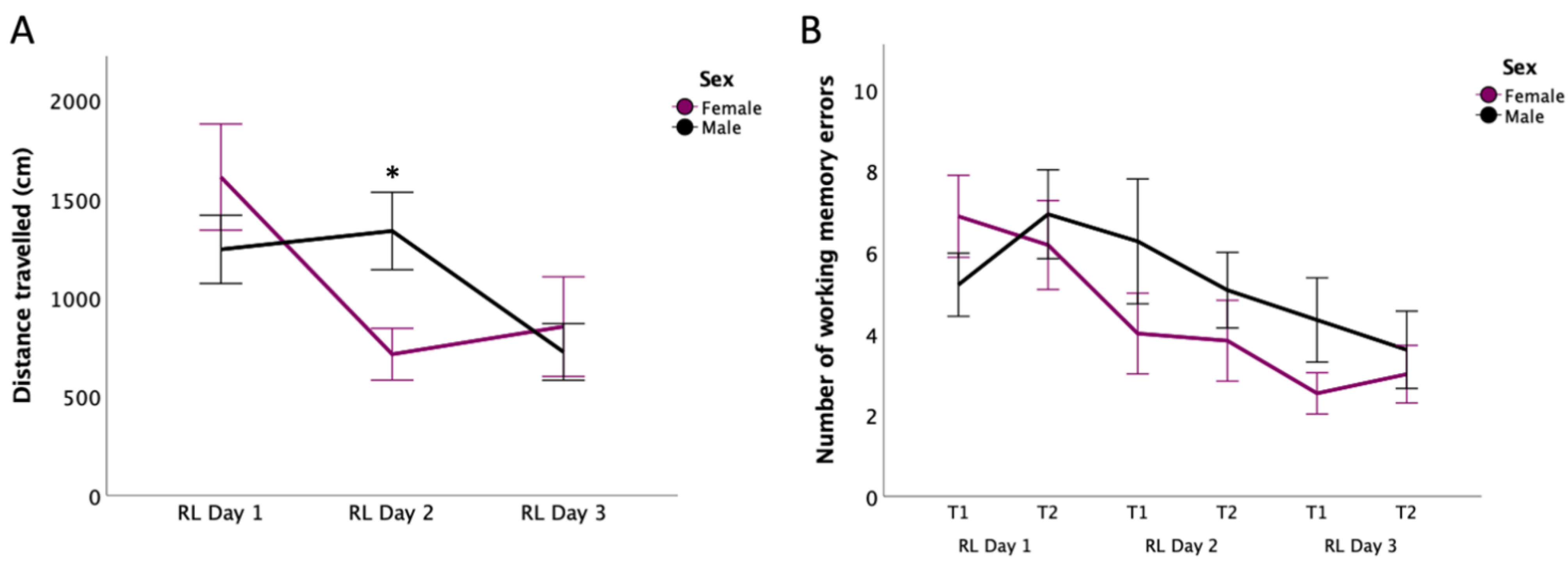
3.5. Reversal Learning: Distance Travelled
3.6. Reversal Learning: Working Memory Errors
4. Discussion
4.1. Contrasting Visuospatial Learning Abilities in Adult and Juvenile Rodents
4.2. Steady Improvements of Working Memory Performance in Adult Rats Contrasts Persistent Learning Difficulties in Juvenile Male Rats
4.3. Sex-Dependent Effect on Working Memory Errors in Adults
4.4. Changes in Search Strategy under Stressful Conditions in Male Rats
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Mani, I.; Kurpad, A.V. Fats & Fatty Acids in Indian Diets: Time for Serious Introspection. Indian J. Med. Res. 2016, 144, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain Foods: The Effects of Nutrients on Brain Function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty Acids, Eicosanoids and PPAR Gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Marangoni, F. N-3 Fatty Acids in the Mediterranean Diet. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 129–133. [Google Scholar] [CrossRef]
- Martini, D. Health Benefits of Mediterranean Diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Jump, D.B. The Biochemistry of N-3 Polyunsaturated Fatty Acids*210. J. Biol. Chem. 2002, 277, 8755–8758. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Kitajka, K.; Puskás, L.G.; Zvara, Á.; Hackler, L.; Barceló-Coblijn, G.; Yeo, Y.K.; Farkas, T. The Role of N-3 Polyunsaturated Fatty Acids in Brain: Modulation of Rat Brain Gene Expression by Dietary n-3 Fatty Acids. Proc. Natl. Acad. Sci. USA 2002, 99, 2619–2624. [Google Scholar] [CrossRef]
- Ayee, M.A.A.; Bunker, B.C.; De Groot, J.L. Chapter Two—Membrane Modulatory Effects of Omega-3 Fatty Acids: Analysis of Molecular Level Interactions. In Current Topics in Membranes; Levitan, I., Trache, A., Eds.; Membrane Biomechanics; Academic Press: Cambridge, MA, USA, 2020; Volume 86, pp. 57–81. [Google Scholar]
- Gow, R.V.; Hibbeln, J.R. Omega-3 Fatty Acid and Nutrient Deficits in Adverse Neurodevelopment and Childhood Behaviors. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 555–590. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.D.; Kim, D.W.; Hong, Y.-S.; Kim, Y.-M.; Seo, J.-H.; Choe, B.M.; Park, J.H.; Kang, J.-W.; Yoo, J.-H.; Chueh, H.W.; et al. Dietary Patterns in Children with Attention Deficit/Hyperactivity Disorder (ADHD). Nutrients 2014, 6, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal Supplementation with Very-Long-Chain n-3 Fatty Acids during Pregnancy and Lactation Augments Children’s IQ at 4 Years of Age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.J.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Montgomery, P. Docosahexaenoic Acid for Reading, Cognition and Behavior in Children Aged 7–9 Years: A Randomized, Controlled Trial (The DOLAB Study). PLoS ONE 2012, 7, e43909. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
- Catalan, J.; Toru, M.; Slotnick, B.; Murthy, M.; Greiner, R.S.; Salem, N. Cognitive Deficits in Docosahexaenoic Acid-Deficient Rats. Behav. Neurosci. 2002, 116, 1022–1031. [Google Scholar] [CrossRef]
- Fedorova, I.; Hussein, N.; Di Martino, C.; Moriguchi, T.; Hoshiba, J.; Majchrzak, S.; Salem, N. An N-3 Fatty Acid Deficient Diet Affects Mouse Spatial Learning in the Barnes Circular Maze. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 269–277. [Google Scholar] [CrossRef]
- Gamoh, S.; Hashimoto, M.; Hossain, S.; Masumura, S. Chronic Administration of Docosahexaenoic Acid Improves the Performance of Radial Arm Maze Task in Aged Rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 266–270. [Google Scholar] [CrossRef]
- Gharami, K.; Das, M.; Das, S. Essential Role of Docosahexaenoic Acid towards Development of a Smarter Brain. Neurochem. Int. 2015, 89, 51–62. [Google Scholar] [CrossRef]
- Dalton, A.; Wolmarans, P.; Witthuhn, R.C.; van Stuijvenberg, M.E.; Swanevelder, S.A.; Smuts, C.M. A Randomised Control Trial in Schoolchildren Showed Improvement in Cognitive Function after Consuming a Bread Spread, Containing Fish Flour from a Marine Source. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.E.; Alexander, J.E.; Winningham, R.G. Omega-3 Polyunsaturated Fatty Acids and Cognition throughout the Lifespan: A Review. Nutr. Neurosci. 2011, 14, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Lassek, W.; Gaulin, S. Sex Differences in the Relationship of Dietary Fatty Acids to Cognitive Measures in American Children. Front. Evol. Neurosci. 2011, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, I.; Hussein, N.; Baumann, M.H.; Di Martino, C.; Salem, N. An N-3 Fatty Acid Deficiency Impairs Rat Spatial Learning in the Barnes Maze. Behav. Neurosci. 2009, 123, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Külzow, N.; Kerti, L.; Witte, V.A.; Kopp, U.; Breitenstein, C.; Flöel, A. An Object Location Memory Paradigm for Older Adults with and without Mild Cognitive Impairment. J. Neurosci. Methods 2014, 237, 16–25. [Google Scholar] [CrossRef]
- Fuhrmann, D.; Knoll, L.J.; Blakemore, S.-J. Adolescence as a Sensitive Period of Brain Development. Trends Cogn. Sci. 2015, 19, 558–566. [Google Scholar] [CrossRef]
- Gerhard, D.M.; Meyer, H.C.; Lee, F.S. An Adolescent Sensitive Period for Threat Responding: Impacts of Stress and Sex. Biol. Psychiatry 2021, 89, 651–658. [Google Scholar] [CrossRef]
- Akirav, I.; Richter-Levin, G. Biphasic Modulation of Hippocampal Plasticity by Behavioral Stress and Basolateral Amygdala Stimulation in the Rat. J. Neurosci. 1999, 19, 10530–10535. [Google Scholar] [CrossRef]
- Calabro, F.J.; Murty, V.P.; Jalbrzikowski, M.; Tervo-Clemmens, B.; Luna, B. Development of Hippocampal–Prefrontal Cortex Interactions through Adolescence. Cereb. Cortex 2020, 30, 1548–1558. [Google Scholar] [CrossRef]
- Ghashghaei, H.T.; Barbas, H. Pathways for Emotion: Interactions of Prefrontal and Anterior Temporal Pathways in the Amygdala of the Rhesus Monkey. Neuroscience 2002, 115, 1261–1279. [Google Scholar] [CrossRef]
- Naneix, F.; Marchand, A.R.; Scala, G.D.; Pape, J.-R.; Coutureau, E. Parallel Maturation of Goal-Directed Behavior and Dopaminergic Systems during Adolescence. J. Neurosci. 2012, 32, 16223–16232. [Google Scholar] [CrossRef]
- Petrovich, G.D.; Canteras, N.S.; Swanson, L.W. Combinatorial Amygdalar Inputs to Hippocampal Domains and Hypothalamic Behavior Systems. Brain Res. Rev. 2001, 38, 247–289. [Google Scholar] [CrossRef]
- Park, J.; Moghaddam, B. Impact of Anxiety on Prefrontal Cortex Encoding of Cognitive Flexibility. Neuroscience 2017, 345, 193–202. [Google Scholar] [CrossRef]
- Reinert, S.; Hübener, M.; Bonhoeffer, T.; Goltstein, P.M. Mouse Prefrontal Cortex Represents Learned Rules for Categorization. Nature 2021, 593, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.D.; Benford, V.J.; Han, B.; Cantor, A.B. The Relationship between Age and the Fatty Acid Composition of Cerebral Cortex and Erythrocytes in Human Subjects. Brain Res. Bull. 2001, 56, 79–85. [Google Scholar] [CrossRef]
- Bos, D.J.; van Montfort, S.J.T.; Oranje, B.; Durston, S.; Smeets, P.A.M. Effects of Omega-3 Polyunsaturated Fatty Acids on Human Brain Morphology and Function: What Is the Evidence? Eur. Neuropsychopharmacol. 2016, 26, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Pravosudov, V.V.; Lavenex, P.; Omanska, A. Nutritional Deficits during Early Development Affect Hippocampal Structure and Spatial Memory Later in Life. Behav. Neurosci. 2005, 119, 1368–1374. [Google Scholar] [CrossRef][Green Version]
- Ahmad, A.; Murthy, M.; Greiner, R.S.; Moriguchi, T.; Salem, N. A Decrease in Cell Size Accompanies a Loss of Docosahexaenoate in the Rat Hippocampus. Nutr. Neurosci. 2002, 5, 103–113. [Google Scholar] [CrossRef]
- Yoshida, K.; Shinohara, H.; Haneji, T.; Nagata, T. Arachidonic Acid Inhibits Osteoblast Differentiation through Cytosolic Phospholipase A2-Dependent Pathway. Oral Dis. 2007, 13, 32–39. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen Regulation of Adiposity and Fuel Partitioning. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Horrobin, D.F. Sex Differences in N-3 and n-6 Fatty Acid Metabolism in EFA-Depleted Rats. Exp. Biol. Med. 1987, 185, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tammam, J.D.; Steinsaltz, D.; Bester, D.W.; Semb-Andenaes, T.; Stein, J.F. A Randomised Double-Blind Placebo-Controlled Trial Investigating the Behavioural Effects of Vitamin, Mineral and n-3 Fatty Acid Supplementation in Typically Developing Adolescent Schoolchildren. Br. J. Nutr. 2016, 115, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.A.; de Alaniz, M.J.T. Influence of Testosterone Administration on the Biosynthesis of Unsaturated Fatty Acids in Male and Female Rats. Lipids 1989, 24, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. Significance of Long-Chain Polyunsaturated Fatty Acids (PUFAs) for the Development and Behaviour of Children. Eur. J. Pediatr. 2010, 169, 149–164. [Google Scholar] [CrossRef]
- McLean, A.C.; Valenzuela, N.; Fai, S.; Bennett, S.A.L. Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. J. Vis. Exp. 2012, 67, e4389. [Google Scholar] [CrossRef]
- Goldman, J.M.; Murr, A.S.; Cooper, R.L. The Rodent Estrous Cycle: Characterization of Vaginal Cytology and Its Utility in Toxicological Studies. Birth Defects Res. B Dev. Reprod. Toxicol. 2007, 80, 84–97. [Google Scholar] [CrossRef]
- Raymond, J.; Morin, A.; Plamondon, H. Delivery Method Matters: Omega-3 Supplementation by Restricted Feeding Period and Oral Gavage Has a Distinct Impact on Corticosterone Secretion and Anxious Behavior in Adolescent Rats. Nutr. Neurosci. 2020, 25, 169–179. [Google Scholar] [CrossRef]
- Barnes, C.A. Memory Deficits Associated with Senescence: A Neurophysiological and Behavioral Study in the Rat. J. Comp. Physiol. Psychol. 1979, 93, 74–104. [Google Scholar] [CrossRef]
- Weinhard, L.; Neniskyte, U.; Vadisiute, A.; di Bartolomei, G.; Aygün, N.; Riviere, L.; Zonfrillo, F.; Dymecki, S.; Gross, C. Sexual Dimorphism of Microglia and Synapses during Mouse Postnatal Development. Dev. Neurobiol. 2018, 78, 618–626. [Google Scholar] [CrossRef]
- McHail, D.G.; Valibeigi, N.; Dumas, T.C. A Barnes Maze for Juvenile Rats Delineates the Emergence of Spatial Navigation Ability. Learn. Mem. 2018, 25, 138–146. [Google Scholar] [CrossRef]
- Blair, M.G.; Nguyen, N.N.-Q.; Albani, S.H.; L’Etoile, M.M.; Andrawis, M.M.; Owen, L.M.; Oliveira, R.F.; Johnson, M.W.; Purvis, D.L.; Sanders, E.M.; et al. Developmental Changes in Structural and Functional Properties of Hippocampal AMPARs Parallels the Emergence of Deliberative Spatial Navigation in Juvenile Rats. J. Neurosci. 2013, 33, 12218–12228. [Google Scholar] [CrossRef] [PubMed]
- Wills, T.J.; Cacucci, F.; Burgess, N.; O’Keefe, J. Development of the Hippocampal Cognitive Map in Preweanling Rats. Science 2010, 328, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Kraemer, P.J. Ontogenetic Differences in Retention of Spatial Learning Tested with Morris Water Maze. Dev. Psychobiol. 1997, 30, 329–341. [Google Scholar] [CrossRef]
- Jonasson, Z. Meta-Analysis of Sex Differences in Rodent Models of Learning and Memory: A Review of Behavioral and Biological Data. Neurosci. Biobehav. Rev. 2005, 28, 811–825. [Google Scholar] [CrossRef]
- Sandstrom, N.J.; Kaufman, J.; Huettel, S.A. Males and Females Use Different Distal Cues in a Virtual Environment Navigation Task1Published on the World Wide Web on 27 January 1998.1. Cogn. Brain Res. 1998, 6, 351–360. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Konkle, A.T.M. When Is a Sex Difference Not a Sex Difference? Front. Neuroendocrinol. 2005, 26, 85–102. [Google Scholar] [CrossRef]
- Roof, R.L. Neonatal Exogenous Testosterone Modifies Sex Difference in Radial Arm and Morris Water Maze Performance in Prepubescent and Adult Rats. Behav. Brain Res. 1993, 53, 1–10. [Google Scholar] [CrossRef]
- Nemeth, M.; Millesi, E.; Wagner, K.-H.; Wallner, B. Sex-Specific Effects of Diets High in Unsaturated Fatty Acids on Spatial Learning and Memory in Guinea Pigs. PLoS ONE 2015, 10, e0140485. [Google Scholar] [CrossRef]
- Teixeira, A.M.; Pase, C.S.; Boufleur, N.; Roversi, K.; Barcelos, R.C.S.; Benvegnú, D.M.; Segat, H.J.; Dias, V.T.; Reckziegel, P.; Trevizol, F.; et al. Exercise Affects Memory Acquisition, Anxiety-like Symptoms and Activity of Membrane-Bound Enzyme in Brain of Rats Fed with Different Dietary Fats: Impairments of Trans Fat. Neuroscience 2011, 195, 80–88. [Google Scholar] [CrossRef]
- Crane, S.B.; Greenwood, C.E. Dietary Fat Source Influences Neuronal Mitochondrial Monoamine Oxidase Activity and Macronutrient Selection in Rats. Pharmacol. Biochem. Behav. 1987, 27, 1–6. [Google Scholar] [CrossRef]
- Burckhardt, M.; Herke, M.; Wustmann, T.; Watzke, S.; Langer, G.; Fink, A. Omega-3 Fatty Acids for the Treatment of Dementia. Cochrane Database Syst. Rev. 2016, 4, CD009002. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; Kelly, P.; Ariffin, N.; Cryan, J.F.; Clarke, G.; Dinan, T.G. N-3 PUFAs Have Beneficial Effects on Anxiety and Cognition in Female Rats: Effects of Early Life Stress. Psychoneuroendocrinology 2015, 58, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.E. Dietary Sources of Omega-3 Fatty Acids Versus Omega-3 Fatty Acid Supplementation Effects on Cognition and Inflammation. Curr. Nutr. Rep. 2020, 9, 264–277. [Google Scholar] [CrossRef]
- Sidhu, V.K.; Huang, B.X.; Kim, H.-Y. Effects of Docosahexaenoic Acid on Mouse Brain Synaptic Plasma Membrane Proteome Analyzed by Mass Spectrometry and 16O/18O Labeling. J. Proteome Res. 2011, 10, 5472–5480. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M.; Berger-Sweeney, J. Spatial Reference Memory and Neocortical Neurochemistry Vary with the Estrous Cycle in C57BL/6 Mice. Behav. Neurosci. 2001, 115, 229–237. [Google Scholar] [CrossRef]
- Packard, M.G.; Teather, L.A. Double Dissociation of Hippocampal and Dorsal-Striatal Memory Systems by Posttraining Intracerebral Injections of 2-Amino-5-Phosphonopentanoic Acid. Behav. Neurosci. 1997, 111, 543–551. [Google Scholar] [CrossRef]
- Vetter-O’Hagen, C.S.; Spear, L.P. The Effects of Gonadectomy on Sex- and Age-Typical Responses to Novelty and Ethanol-Induced Social Inhibition in Adult Male and Female Sprague-Dawley Rats. Behav. Brain Res. 2012, 227, 224–232. [Google Scholar] [CrossRef]
- Biswal, S. Phytochemical Analysis and a Study on the Antiestrogenic Antifertility Effect of Leaves of Piper Betel in Female Albino Rat. Anc. Sci. Life 2014, 34, 16. [Google Scholar] [CrossRef]
- van Goethem, N.P.; Rutten, K.; van der Staay, F.J.; Jans, L.A.W.; Akkerman, S.; Steinbusch, H.W.M.; Blokland, A.; van’t Klooster, J.; Prickaerts, J. Object Recognition Testing: Rodent Species, Strains, Housing Conditions, and Estrous Cycle. Behav. Brain Res. 2012, 232, 323–334. [Google Scholar] [CrossRef]
- Contreras, C.M.; Molina, M.; Saavedra, M.; Martínez-Mota, L. Lateral Septal Neuronal Firing Rate Increases during Proestrus-Estrus in the Rat. Physiol. Behav. 2000, 68, 279–284. [Google Scholar] [CrossRef]
- Ismail, N.; Blaustein, J.D. Pubertal Immune Challenge Blocks the Ability of Estradiol to Enhance Performance on Cognitive Tasks in Adult Female Mice. Psychoneuroendocrinology 2013, 38, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Riordan, A.J.; Schaler, A.W.; Fried, J.; Paine, T.A.; Thornton, J.E. Estradiol and Luteinizing Hormone Regulate Recognition Memory Following Subchronic Phencyclidine: Evidence for Hippocampal GABA Action. Psychoneuroendocrinology 2018, 91, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of Spatial Learning and Memory in the Barnes Maze Task in Rodents—Methodological Consideration. Naunyn. Schmiedebergs Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Dalm, S.; Schächinger, H.; Oitzl, M.S. Chronic Stress Modulates the Use of Spatial and Stimulus-Response Learning Strategies in Mice and Man. Neurobiol. Learn. Mem. 2008, 90, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Tropp, J.; Markus, E.J. Sex Differences in the Dynamics of Cue Utilization and Exploratory Behavior. Behav. Brain Res. 2001, 119, 143–154. [Google Scholar] [CrossRef]



| Variable | df | Mean Squares | F | p | Effect Size |
|---|---|---|---|---|---|
| Latency to Escape Box | |||||
| Day | 2.935 | 560,387 | 32.893 | <0.001 | 0.370 |
| Trial | 1 | 105,745 | 43.911 | <0.001 | 0.440 |
| Day × Age | 2.935 | 65,152 | 3.824 | 0.012 | 0.064 |
| Trial × Sex | 1 | 18,534 | 7.696 | 0.008 | 0.121 |
| Distance Travelled | |||||
| Day | 3.424 | 70,610,325 | 51.542 | <0.001 | 0.484 |
| Trial | 1 | 33,669,400 | 120.475 | <0.001 | 0.687 |
| Day × Sex | 3.424 | 40,94,996 | 2.989 | 0.026 | 0.052 |
| Day × Age | 3.424 | 6,324,490 | 4.617 | 0.002 | 0.077 |
| Trial × Supplementation | 1 | 2,593,904 | 9.281 | 0.004 | 0.144 |
| Day × Trial | 4.351 | 2,302,846 | 4.687 | <0.001 | 0.079 |
| Trial × Supplementation × Sex × Age | 1 | 3,816,087 | 13.655 | <0.001 | 0.199 |
| Number of Working Memory Errors | |||||
| Day | 3.37 | 192.113 | 3.795 | 0.009 | 0.062 |
| Trial | 1 | 290.854 | 31.235 | <0.001 | 0.354 |
| Day × Age | 3.37 | 728.147 | 23.095 | <0.001 | 0.288 |
| Day × Supplementation × Sex | 3.37 | 92.468 | 2.933 | 0.029 | 0.049 |
| Day × Sex × Age | 3.37 | 97.631 | 3.097 | 0.023 | 0.052 |
| Trial × Age | 1 | 65.860 | 7.073 | 0.01 | 0.110 |
| Day × Trial | 5 | 32.285 | 2.841 | 0.016 | 0.047 |
| Day × Trial × Age | 5 | 44.373 | 3.904 | 0.002 | 0.064 |
| Day × Trial × Supplementation × Sex | 5 | 34.503 | 3.036 | 0.011 | 0.051 |
| Variable | df | Mean Squares | F | p | Effect Size |
|---|---|---|---|---|---|
| Latency to Escape Box | |||||
| Day | 2 | 107,000 | 24.926 | <0.001 | 0.462 |
| Distance Travelled | |||||
| Day | 2 | 6,179,837 | 15.019 | <0.001 | 0.357 |
| Trial | 1 | 1,969,308 | 5.734 | 0.024 | 0.175 |
| Day × Sex | 2 | 4,415,398 | 10.731 | <0.001 | 0.284 |
| Day × Trial × Sex | 2 | 2,637,329 | 7.113 | 0.002 | 0.209 |
| Number of Working Memory Errors | |||||
| Day | 2 | 138.104 | 13.818 | <0.001 | 0.33 |
| Day × Trial × Sex | 2 | 17.657 | 3.195 | 0.049 | 0.102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raymond, J.; Morin, A.; Poitras, M.; Plamondon, H. Short-Term Fish Oil Supplementation during Adolescence Supports Sex-Specific Impact on Adulthood Visuospatial Memory and Cognitive Flexibility. Nutrients 2022, 14, 3513. https://doi.org/10.3390/nu14173513
Raymond J, Morin A, Poitras M, Plamondon H. Short-Term Fish Oil Supplementation during Adolescence Supports Sex-Specific Impact on Adulthood Visuospatial Memory and Cognitive Flexibility. Nutrients. 2022; 14(17):3513. https://doi.org/10.3390/nu14173513
Chicago/Turabian StyleRaymond, Julie, Alexandre Morin, Marilou Poitras, and Hélène Plamondon. 2022. "Short-Term Fish Oil Supplementation during Adolescence Supports Sex-Specific Impact on Adulthood Visuospatial Memory and Cognitive Flexibility" Nutrients 14, no. 17: 3513. https://doi.org/10.3390/nu14173513
APA StyleRaymond, J., Morin, A., Poitras, M., & Plamondon, H. (2022). Short-Term Fish Oil Supplementation during Adolescence Supports Sex-Specific Impact on Adulthood Visuospatial Memory and Cognitive Flexibility. Nutrients, 14(17), 3513. https://doi.org/10.3390/nu14173513






