Multiple Indicators of Undernutrition, Infection, and Inflammation in Lactating Women Are Associated with Maternal Iron Status and Infant Anthropometry in Panama: The MINDI Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Measures
2.3. Maternal/Infant Anthropometry
2.4. Statistical Analyses
2.4.1. Selection of Independent Variables for Regression Models Using Bootstrapping
2.4.2. Multivariate Model Construction
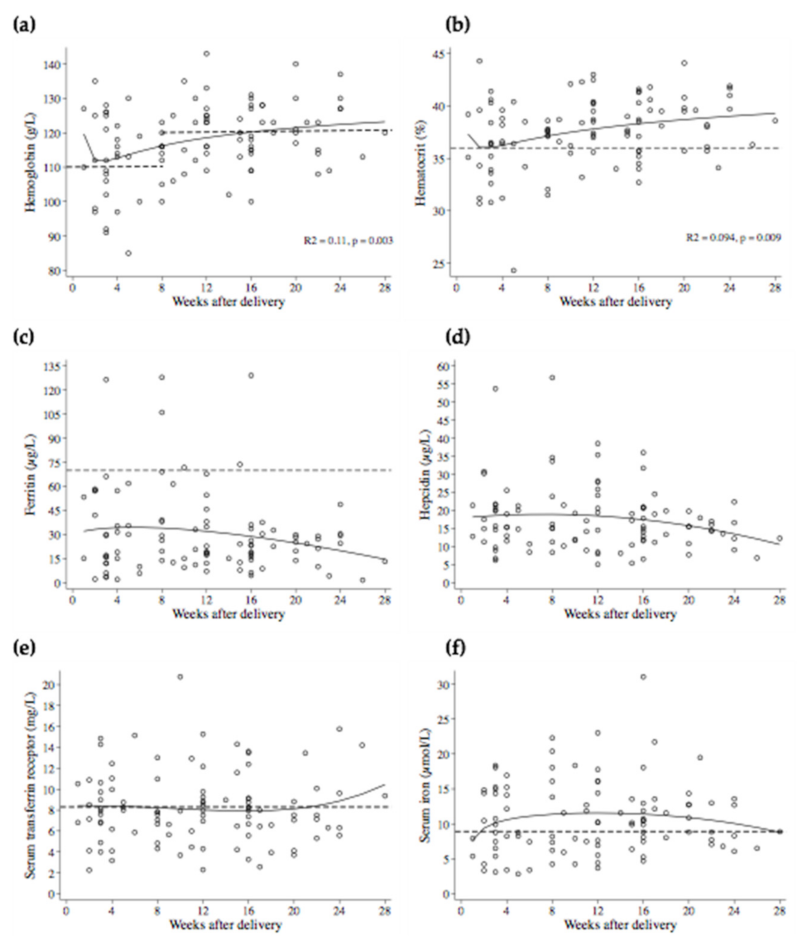
3. Results
3.1. Population Characteristics
3.2. Comparison of Women with and without Anemia
3.3. MFP Logistic Regression Models for Anemia, Elevated sTfR, and Low Serum Iron
3.4. MFP Linear Regression Model for Log Ferritin
3.5. MFP Linear Regression Model for Log Hepcidin
3.6. Maternal Nutritional and Inflammation Indicators but Not Iron Status Indicators were Associated with Infant Anthropometry
4. Discussion
4.1. Inflammation
4.1.1. CRP and Iron Indicators
4.1.2. Platelets
4.1.3. Inflammation and Ferritin
4.1.4. Inflammation and Anemia
4.2. Indicators of Malnutrition were Associated with Hepcidin, Serum Iron and sTfR
4.3. Evidence of Anemia of Malnutrition during Lactation
4.4. Nutrient Supplementation during Lactation
4.5. Maternal Determinants of Infant Anthropometry
5. Conclusions
6. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milman, N. Postpartum anemia I: Definition, prevalence, causes, and consequences. Ann. Hematol. 2011, 90, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.K.; Balogun, O.O.; Ota, E.; Takahashi, K.; Mori, R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst. Rev. 2016, 2, CD010647. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Michelazzo, F.B.; Oliveira, J.M.; Stefanello, J.; Luzia, L.A.; Rondo, P.H. The influence of vitamin A supplementation on iron status. Nutrients 2013, 5, 4399–4413. [Google Scholar] [CrossRef]
- Azizi-Soleiman, F.; Vafa, M.; Abiri, B.; Safavi, M. Effects of iron on vitamin D metabolism: A systematic review. Int. J. Prev. Med. 2016, 7, 126. [Google Scholar] [CrossRef]
- Bernát, I. Protein-Deficiency Anemia. In Iron Metabolism; Bernát, I., Ed.; Springer US: Boston, MA, USA, 1983; pp. 299–300. [Google Scholar]
- Fondu, P.; Hariga-Muller, C.; Mozes, N.; Neve, J.; Van Steirteghem, A.; Mandelbaum, I.M. Protein-energy malnutrition and anemia in Kivu. Am. J. Clin. Nutr. 1978, 31, 46–56. [Google Scholar] [CrossRef]
- Wirth, J.P.; Woodruff, B.A.; Engle-Stone, R.; Namaste, S.M.; Temple, V.J.; Petry, N.; Macdonald, B.; Suchdev, P.S.; Rohner, F.; Aaron, G.J. Predictors of anemia in women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106 (Suppl. 1), 416s–427s. [Google Scholar] [CrossRef]
- Warrier, R.P.; Dole, M.G.; Warrier, J.; Suskind, R.M. The Anemia of Malnutrition. In The Malnourished Child; Suskind, R.M., Lewinter-Suskind, L., Eds.; Nestec Ltd.: New York, NY, USA; Vevey/Raven Press, Ltd.: New York, NY, USA, 1990; pp. 61–72. [Google Scholar]
- Guralnik, J.M.; Eisenstaedt, R.S.; Ferrucci, L.; Klein, H.G.; Woodman, R.C. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood 2004, 104, 2263–2268. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Anemia of inflammation. Hematol. Oncol. Clin. N. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Atkinson, S.H.; Armitage, A.E.; Khandwala, S.; Veenemans, J.; Cox, S.E.; Eddowes, L.A.; Hayes, T.; Doherty, C.P.; Demir, A.Y.; et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci. Transl. Med. 2014, 6, 235re3. [Google Scholar] [CrossRef]
- Bah, A.; Muhammad, A.K.; Wegmuller, R.; Verhoef, H.; Goheen, M.M.; Sanyang, S.; Danso, E.; Sise, E.A.; Pasricha, S.R.; Armitage, A.E.; et al. Hepcidin-guided screen-and-treat interventions against iron-deficiency anaemia in pregnancy: A randomised controlled trial in The Gambia. Lancet Glob. Health 2019, 7, e1564–e1574. [Google Scholar] [CrossRef]
- Gebreegziabher, T.; Roice, T.; Stoecker, B.J. Chronic inflammation was a major predictor and determinant factor of anemia in lactating women in Sidama zone southern Ethiopia: A cross-sectional study. PLoS ONE 2020, 15, e0240254. [Google Scholar] [CrossRef] [PubMed]
- WHO; CDC. Assessing the Iron Status of Populations. Available online: Apps.who.int/iris/bitstream/10665/75368/1/9789241596107_eng.pdf?ua=1&ua=1 (accessed on 23 March 2016).
- Dignass, A.; Farrag, K.; Stein, J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int. J. Chronic Dis. 2018, 2018, 9394060. [Google Scholar] [CrossRef]
- WHO. C-Reactive Protein Concentrations as a Marker of Inflammation or Infection for Interpreting Biomarkers of Micronutrient Status. Available online: http://apps.who.int/iris/bitstream/10665/133708/1/WHO_NMH_NHD_EPG_14.7_eng.pdf?ua=1 (accessed on 15 January 2022).
- Namaste, S.M.; Aaron, G.J.; Varadhan, R.; Peerson, J.M.; Suchdev, P.S. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106 (Suppl. 1), 333s–347s. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Cao, S.; Gao, H.; Xiao, Q.; Win, N.; Zhang, Y. Anemia among lactating mothers in Kokang, Myanmar. Southeast Asian J. Trop. Med. Public Health 2016, 47, 1298–1305. [Google Scholar] [PubMed]
- Nairz, M.; Theurl, I.; Wolf, D.; Weiss, G. Iron deficiency or anemia of inflammation? Differential diagnosis and mechanisms of anemia of inflammation. Wien Med. Wochenschr. 2016, 166, 411–423. [Google Scholar] [CrossRef]
- Beguin, Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin. Chim. Acta 2003, 329, 9–22. [Google Scholar] [CrossRef]
- Rohner, F.; Namaste, S.M.; Larson, L.M.; Addo, O.Y.; Mei, Z.; Suchdev, P.S.; Williams, A.M.; Sakr Ashour, F.A.; Rawat, R.; Raiten, D.J.; et al. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106 (Suppl. 1), 372s–382s. [Google Scholar] [CrossRef]
- Mujica-Coopman, M.F.; Brito, A.; Lopez de Romana, D.; Rios-Castillo, I.; Coris, H.; Olivares, M. Prevalence of Anemia in Latin America and the Caribbean. Food Nutr. Bull. 2015, 36 (Suppl. 2), S119–S128. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, D.; Koski, K.G.; Sinisterra, O.T.; Del Carmen Pons, E.; Murillo, E.; Scott, M.E. Interactions among urogenital, intestinal, skin, and oral infections in pregnant and lactating panamanian Ngäbe women: A neglected public health challenge. Am. J. Trop. Med. Hyg. 2015, 92, 1100–1110. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, D.; Pons, E.D.C.; Rueda, D.; Sinisterra, O.T.; Murillo, E.; Scott, M.E.; Koski, K.G. C-reactive protein is differentially modulated by co-existing infections, vitamin deficiencies and maternal factors in pregnant and lactating indigenous Panamanian women. Infect. Dis. Poverty 2017, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud de Panamá. Plan Nacional “Prevención y Control de las Deficiencias de Micronutrientes” 2008–2015. Available online: http://es.wfp.org/sites/default/files/es/file/plan__nacional_prevencion_y_control_de_las_deficiencias_de_micronutrientes_2008_2015.pdf (accessed on 13 July 2016).
- De León, J.; Barba, A.; Sinisterra, O.T.; Atencio, A. II Nutritional Monitoring in MINSA Facilities, MONINUT 2017. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjvjdzKpcLvAhXaEVkFHTjmDYQQFjABegQIAxAD&url=https%3A%2F%2Fnutricionistaspanama.com%2Fwp-content%2Fuploads%2Fpublicaciones%2FINFORME_MNINUT.pdf&usg=AOvVaw1RMKpMtoQXF1SdTy2wqs8f (accessed on 21 March 2021).
- Panamanian Ministry of Health. Sistema de Información Geográfico Interactivo de la Encuesta Nacional de Salud de Panamá (ENSPA). 2019. Available online: http://www.gorgas.gob.pa/SIGENSPA/Grafica_deplecion_ferropriva_anemia_MEF.html (accessed on 17 June 2022).
- Lewies, A.; Zandberg, L.; Baumgartner, J. Interventions to prevent iron deficiency during the first 1000 days in low-income and middle-income countries: Recent advances and challenges. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Z.B.; Fentie, M.; Abebe, Z.; Ayele, T.A.; Muchie, K.F. Maternal characteristics and nutritional status among 6-59 months of children in Ethiopia: Further analysis of demographic and health survey. BMC Pediatr. 2019, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Solomons, N.W.; Scott, M.E.; Koski, K.G. Minerals and Trace Elements in Human Breast Milk Are Associated with Guatemalan Infant Anthropometric Outcomes within the First 6 Months. J. Nutr. 2016, 146, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Saso, A.; Blyuss, O.; Munblit, D.; Faal, A.; Moore, S.E.; Le Doare, K. Breast Milk Cytokines and Early Growth in Gambian Infants. Front. Pediatr. 2018, 6, 414. [Google Scholar] [CrossRef] [PubMed]
- Tuaillon, E.; Viljoen, J.; Dujols, P.; Cambonie, G.; Rubbo, P.A.; Nagot, N.; Bland, R.M.; Badiou, S.; Newell, M.L.; Van de Perre, P. Subclinical mastitis occurs frequently in association with dramatic changes in inflammatory/anti-inflammatory breast milk components. Pediatric Res. 2017, 81, 556–564. [Google Scholar] [CrossRef]
- Wren-Atilola, H.M.; Solomons, N.W.; Scott, M.E.; Koski, K.G. Infant Anthropometry and Growth Velocity Before 6 Months are Associated with Breastfeeding Practices and the Presence of Subclinical Mastitis and Maternal Intestinal Protozoa in Indigenous Communities in Guatemala. Curr. Dev. Nutr. 2021, 5, nzab086. [Google Scholar] [CrossRef]
- Brannon, P.M.; Taylor, C.L. Iron Supplementation during Pregnancy and Infancy: Uncertainties and Implications for Research and Policy. Nutrients 2017, 9, 1327. [Google Scholar] [CrossRef]
- Halpenny, C.M.; Koski, K.G.; Valdes, V.E.; Scott, M.E. Prediction of child health by household density and asset-based indices in impoverished indigenous villages in rural Panama. Am. J. Trop. Med. Hyg. 2012, 86, 280–291. [Google Scholar] [CrossRef][Green Version]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. Available online: https://apps.who.int/iris/handle/10665/331505 (accessed on 15 August 2021).
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers. Available online: https://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/ (accessed on 9 April 2021).
- De Benoist, B. Conclusions of a WHO Technical consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29 (Suppl. 2), S238–S244. [Google Scholar] [CrossRef] [PubMed]
- Balvers, M.G.; Brouwer-Brolsma, E.M.; Endenburg, S.; de Groot, L.C.; Kok, F.J.; Gunnewiek, J.K. Recommended intakes of vitamin D to optimize health, associated circulating 25-hydroxyvitamin D concentrations, and dosing regimens to treat deficiency: Workshop report and overview of current literature. J. Nutr. Sci. 2015, 4, e23. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, E.M.; Bar-El Dadon, S.; Reifen, R. The vicious cycle of vitamin A deficiency: A review. Crit. Rev. Food Sci. Nutr. 2016, 57, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Sinisterra, O.T.; Pons, E.d.C.; Fontes, F.; Lagrutta, F.; Carrasco, Y.; Olivares, M. Evaluación del programa de suplementación con hierro en Panamá. In Avances de Investigación en Seguridad Alimentaria y Nutricional (SAN); University of Costa Rica: San Pedro, Costa Rica, 2012; pp. 58–67. [Google Scholar]
- Jansson, L.; Nilsson, B. Serum retinol and retinol-binding protein in mothers and infants at delivery. Biol. Neonate 1983, 43, 269–271. [Google Scholar] [CrossRef]
- Fujita, M.; Brindle, E.; Rocha, A.; Shell-Duncan, B.; Ndemwa, P.; O’Connor, K.A. Assessment of the relative dose-response test based on serum retinol-binding protein instead of serum retinol in determining low hepatic vitamin A stores. Am. J. Clin. Nutr. 2009, 90, 217–224. [Google Scholar] [CrossRef][Green Version]
- Gamble, M.V.; Ramakrishnan, R.; Palafox, N.A.; Briand, K.; Berglund, L.; Blaner, W.S. Retinol binding protein as a surrogate measure for serum retinol: Studies in vitamin A-deficient children from the Republic of the Marshall Islands. Am. J. Clin. Nutr. 2001, 73, 594–601. [Google Scholar] [CrossRef]
- Feranchak, A.P.; Gralla, J.; King, R.; Ramirez, R.O.; Corkill, M.; Narkewicz, M.R.; Sokol, R.J. Comparison of indices of vitamin A status in children with chronic liver disease. Hepatology 2005, 42, 782–792. [Google Scholar] [CrossRef]
- World Health Organization. Report: Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15–17 September 2010. Available online: https://apps.who.int/iris/handle/10665/75334 (accessed on 20 June 2021).
- Panamanian Ministry of Health. Normas técnicas y administrativas del programa de salud integral del niño y la niña desde el nacimiento a los 9 años de edad. Available online: https://siteal.iiep.unesco.org/bdnp/2447/normas-tecnicas-administrativas-programa-salud-integral-nino-nina-desde-nacimiento-9-anos (accessed on 24 May 2021).
- World Health Organization. A Healthy Lifestyle-WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 16 July 2022).
- Stieglitz, E.; Huang, J. Plasmapheresis Technique. Available online: https://emedicine.medscape.com/article/1895577-technique (accessed on 6 August 2019).
- Hauser, R.G.; Kwon, R.J.; Ryder, A.; Cheng, C.; Charifa, A.; Tormey, C. Transfusion medicine equations made internet accessible. Transfus. Med. Rev. 2020, 34, 5–9. [Google Scholar] [CrossRef]
- Bruinse, H.W.; van der Berg, H.; Haspels, A.A. Maternal serum folacin levels during and after normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1985, 20, 153–158. [Google Scholar] [CrossRef]
- Vidmar, S.I.; Cole, T.J.; Pan, H. Standardizing anthropometric measures in children and adolescents with functions for egen: Update. Stata J. 2013, 13, 366–378. [Google Scholar] [CrossRef]
- Mannan, H. A practical Application of a simple bootstrapping method for assessing predictors selected for epidemiologic risk models using automated variable selection. Int. J. Stat. Appl. 2017, 7, 239–249. [Google Scholar] [CrossRef]
- Royston, P.; Sauerbrei, W. mfpa: Extension of mfp using the ACD covariate transformation for enhanced parametric multivariable modeling. Stata J. 2016, 16, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Sauerbrei, W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis based on Fractional Polynomials for Modelling Continuous Variables; John Wiley: Chichester, UK, 2008. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Model-Building Strategies and Methods for Logistic Regression. In Applied Logistic Regression; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Green, S.B. How many subjects does it take to do a regression analysis. Multivariate Behav. Res. 1991, 26, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Mayerhofer, W. Winsorizing and Trimming. Available online: https://wlm.userweb.mwn.de/Stata/wstatwin.htm (accessed on 3 June 2021).
- Grömping, U. Estimators of relative importance in linear regression based on variance decomposition. Am. Stat. 2007, 61, 139–147. [Google Scholar] [CrossRef]
- Nehring, S.M.; Goyal, A.; Bansal, P.; Patel, B.C. C Reactive Protein. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ravin, K.A.; Loy, M. The eosinophil in infection. Clin. Rev. Allergy Immunol. 2016, 50, 214–227. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Pounis, G.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. A score of low-grade inflammation and risk of mortality: Prospective findings from the Moli-sani study. Haematologica 2016, 101, 1434–1441. [Google Scholar] [CrossRef]
- Mishra, S.; Jaiswar, S.; Saad, S.; Tripathi, S.; Singh, N.; Deo, S.; Agarwal, M.; Mishra, N. Platelet indices as a predictive marker in neonatal sepsis and respiratory distress in preterm prelabor rupture of membranes. Int. J. Hematol. 2021, 113, 199–206. [Google Scholar] [CrossRef]
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J.; et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106 (Suppl. 1), 359S–371S. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef]
- Schumann, K.; Solomons, N.W. Perspective: What makes it so difficult to mitigate worldwide anemia prevalence? Adv. Nutr. 2017, 8, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.M.; Yang, Z.; Lönnerdal, B.; Chantry, C.J.; Dewey, K.G. Plasma ferritin and hepcidin are lower at 4 months postpartum among women with elevated C-reactive protein or α1-acid glycoprotein. J. Nutr. 2017, 147, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, C.; Montosi, G.; Garuti, C.; Corradini, E.; Sabelli, M.; Canali, S.; Pietrangelo, A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 2014, 146, 1060–1069. [Google Scholar] [CrossRef]
- Troutt, J.S.; Rudling, M.; Persson, L.; Ståhle, L.; Angelin, B.; Butterfield, A.M.; Schade, A.E.; Cao, G.; Konrad, R.J. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin. Chem. 2012, 58, 1225–1232. [Google Scholar] [CrossRef]
- Skikne, B.S. Serum transferrin receptor. Am. J. Hematol. 2008, 83, 872–875. [Google Scholar] [CrossRef]
- Canete, A.; Cano, E.; Munoz-Chapuli, R.; Carmona, R. Role of vitamin A/retinoic acid in regulation of embryonic and adult hematopoiesis. Nutrients 2017, 9, 159. [Google Scholar] [CrossRef]
- Santos, E.W.; Oliveira, D.C.; Silva, G.B.; Tsujita, M.; Beltran, J.O.; Hastreiter, A.; Fock, R.A.; Borelli, P. Hematological alterations in protein malnutrition. Nutr. Rev. 2017, 75, 909–919. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Vitamin A Review. J. Nutr. 2016, 146, 1816s–1848s. [Google Scholar] [CrossRef]
- Haidar, J.; Muroki, N.M.; Omwega, A.M.; Ayana, G. Malnutrition and iron deficiency in lactating women in urban slum communities from Addis Ababa, Ethiopia. East Afr. Med. J. 2003, 80, 191–194. [Google Scholar] [CrossRef]
- Vinoy, S.; Rosetta, L.; Mascie-Taylor, C.G. Repeated measurements of energy intake, energy expenditure and energy balance in lactating Bangladeshi mothers. Eur. J. Clin. Nutr. 2000, 54, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Roba, K.T.; O’Connor, T.P.; Belachew, T.; O’Brien, N.M. Seasonal variation in nutritional status and anemia among lactating mothers in two agro-ecological zones of rural Ethiopia: A longitudinal study. Nutrition 2015, 31, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, C. Insulin-like growth factor-I (IGF-I) and clinical nutrition. Clin. Sci. 2013, 125, 265–280. [Google Scholar] [CrossRef]
- Succurro, E.; Arturi, F.; Caruso, V.; Rudi, S.; Sciacqua, A.; Andreozzi, F.; Hribal, M.L.; Perticone, F.; Sesti, G. Low insulin-like growth factor-1 levels are associated with anaemia in adult non-diabetic subjects. Thromb. Haemost. 2011, 105, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Brent, B.; Obonyo, N.; Akech, S.; Shebbe, M.; Mpoya, A.; Mturi, N.; Berkley, J.A.; Tulloh, R.M.R.; Maitland, K. Assessment of myocardial function in Kenyan children with severe, acute malnutrition: The Cardiac Physiology in Malnutrition (CAPMAL) study. JAMA Netw. Open 2019, 2, e191054. [Google Scholar] [CrossRef] [PubMed]
- Caregaro, L.; Di Pascoli, L.; Favaro, A.; Nardi, M.; Santonastaso, P. Sodium depletion and hemoconcentration: Overlooked complications in patients with anorexia nervosa? Nutrition 2005, 21, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ali-Baya, G.; Zenile, E.; Aikins, B.O.; Amoaning, R.E.; Simpong, D.L.; Adu, P. Poor haemoglobin-haematocrit agreement in apparently healthy adult population; a cross-sectional study in Cape Coast Metropolis, Ghana. Heliyon 2021, 7, e07720. [Google Scholar] [CrossRef]
- Bardosono, S.; Morin, C.; Guelinckx, I.; Pohan, R. Pregnant and breastfeeding women: Drinking for two? Ann. Nutr. Metab. 2017, 70 (Suppl. 1), 13–17. [Google Scholar] [CrossRef]
- Heen, E.; Yassin, A.A.; Madar, A.A.; Romøren, M. Estimates of fluid intake, urine output and hydration-levels in women from Somaliland: A cross-sectional study. J. Nutr. Sci. 2021, 10, e66. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Iron Supplementation in Postpartum Women. Available online: https://www.who.int/nutrition/publications/micronutrients/guidelines/daily_iron_supp_postpartum_women/en/ (accessed on 4 June 2021).
- Gautam, C.S.; Saha, L.; Sekhri, K.; Saha, P.K. Iron deficiency in pregnancy and the rationality of iron supplements prescribed during pregnancy. Medscape J. Med. 2008, 10, 283. [Google Scholar]
- Berglund, S.K.; Domellöf, M. Iron deficiency in infancy: Current insights. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escamilla, R.; Buccini, G.S.; Segura-Pérez, S.; Piwoz, E. Perspective: Should exclusive breastfeeding still be recommended for 6 months? Adv. Nutr. 2019, 10, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.L.; Brannon, P.M. Introduction to workshop on iron screening and supplementation in iron-replete pregnant women and young children. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1547s–1554s. [Google Scholar] [CrossRef]
- Nairz, M.; Weiss, G. Infections associated with iron administration. Met. Ions Life Sci. 2019, 19, 269–274. [Google Scholar] [CrossRef]
- Wahed, M.A.; Alvarez, J.O.; Khaled, M.A.; Mahalanabis, D.; Rahman, M.M.; Habte, D. Comparison of the modified relative dose response (MRDR) and the relative dose response (RDR) in the assessment of vitamin A status in malnourished children. Am. J. Clin. Nutr. 1995, 61, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, D.; Nemeth, E.; Pons, E.d.C.; Rueda, D.; Sinisterra, O.T.; Murillo, E.; Sangkhae, V.; Starr, L.M.; Scott, M.E.; Koski, K.G. INTERGROWTH-21 identifies high prevalence of low symphysis-fundal height in indigenous pregnant women experiencing multiple infections, nutrient deficiencies and inflammation: The MINDI cohort. Curr. Dev. Nutr. 2021, 5, nzab012. [Google Scholar] [CrossRef]
- Asare, H.; Rosi, A.; Faber, M.; Smuts, C.M.; Ricci, C. Animal-source foods as a suitable complementary food for improved physical growth in 6 to 24-month-old children in low- and middle-income countries: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2022, 1–11. [Google Scholar] [CrossRef]
- De Caballero, E.A.E. Evaluation on the Acceptability and Consumption of Nutricrema in the Republic of Panama. Rev. Chil. Nutr. 2003, 30, 133–141. [Google Scholar]
- Panamanian Ministry of Health. Prácticas de cuidado y alimentación infantil en las Comarcas indígenas de Kuna-Yala, Ngöbe-Buglé, Emberá-Wounaan y los distritos de Cañazas y Las Palmas (Provincia de Veraguas). Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjY2qCokP_wAhXFc98KHYSSA1kQFjAHegQIDBAE&url=https%3A%2F%2Fwww.paho.org%2Fhq%2Findex.php%3Foption%3Dcom_docman%26view%3Ddownload%26category_slug%3Dexperiencias-ver-mas-sitio-web-ingles-4986%26alias%3D19856-2009-panama-856%26Itemid%3D270&usg=AOvVaw2_coNrI3xk0kgLJSK9ns5u (accessed on 4 June 2021).
- Brabin, L.; Brabin, B.J.; Gies, S. Influence of iron status on risk of maternal or neonatal infection and on neonatal mortality with an emphasis on developing countries. Nutr. Rev. 2013, 71, 528–540. [Google Scholar] [CrossRef]
- Sinisterra, O.F.F.; Lagrutta, F.; Olivares, M. Situación de deficiencia de hierro y anemia. Available online: https://www.unicef.org/panama/spanish/Hierro.pdf (accessed on 3 June 2022).
- Wagner, C.L.; Hollis, B.W. Early-life effects of vitamin D: A focus on pregnancy and lactation. Ann. Nutr. Metab. 2020, 76 (Suppl. 2), 16–28. [Google Scholar] [CrossRef]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Morris, S.K.; Zlotkin, S.; Gernand, A.D.; Ahmed, T.; Shanta, S.S.; Papp, E.; Korsiak, J.; Shi, J.; Islam, M.M.; et al. Vitamin D supplementation in pregnancy and lactation and infant growth. NEJM 2018, 379, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Dawodu, A.; Salameh, K.M.; Al-Janahi, N.S.; Bener, A.; Elkum, N. The effect of high-dose postpartum maternal vitamin D supplementation alone compared with maternal plus infant vitamin D supplementation in breastfeeding infants in a high-risk population a randomized controlled trial. Nutrients 2019, 11, 1632. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Moukayed, M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D supplementation can have important patient and public health benefits. Eur. J. Clin. Nutr. 2020, 74, 366–376. [Google Scholar] [CrossRef] [PubMed]


| Variable | Mean ± SD or Median (Min, Max) |
|---|---|
| Maternal Characteristics | |
| 3 Age, years | 23 (14–42) |
| 3 Parity, # | 3 (1–11) |
| 3 Weeks post-partum | 12 (1–28) |
| Home delivery, % | 42.4 |
| Wood smoke, % | 93.9 |
| Fieldwork, % | 41.4 |
| Taking iron tablets, % | 92.9 |
| 4 Weeks taking iron during pregnancy | 10 (0–39) |
| 5 Weeks taking iron during lactation | 9 (0–28) |
| Taking multiple nutrient supplements, % | 39.4 |
| BMI, kg/m2 | 25.6 ± 3.02 |
| <18.5, % | 0 |
| 18.5–24.9, % | 48.5 |
| 25.0–29.9, % | 43.4 |
| 30.0–34.9, % | 8.1 |
| Reported Weekly Intakes | |
| 3 Animal-source foods, % | 2 (0–13) |
| 3 Orange-red fruits & vegetables, % | 1 (0–14) |
| 3 Green leafy vegetables, % | 1 (0–8) |
| Maternal Infections | |
| Caries, % | 18.2 |
| Scabies, % | 8.1 |
| 1 Urinary leukocyte esterase (+), % | 76.0 |
| Vaginal microorganisms | |
| 1 Lactobacillus, % | 26.6 |
| 2 Bacteroides/Gardnerella, % | 97.5 |
| 1 Mobiluncus, % | 87.3 |
| 1 Trichomonas, % | 91.1 |
| Yeast, % | 11.4 |
| 2 Diplococcus, % | 31.6 |
| Infant Characteristics | |
| 1 Gestational age at birth | 40 (32–42) |
| Preterm delivery (<37 weeks), % | 5.0 |
| 3 Age,weeks | 12 (1–28) |
| Exclusive breastfeeding, % | 84.8% |
| Predominantly breastfeeding, % | 15.2% |
| HCAZ | 0.05 ± 1.34 |
| HCAZ < −2 SD | 5.0% |
| LAZ | −0.36 ± 1.60 |
| LAZ < −2 SD | 16.3% |
| WAZ | 0.31 ± 1.48 |
| WAZ < −2 SD | 9.1% |
| Variable | Mean ± SD; %; Median (Min, Max) | IQR |
|---|---|---|
| Nutritional Biomarkers | ||
| Folic acid, nmol/L | 11.9 (5.4–35.9) | 8.9, 16.2 |
| Folic acid < 10 nmol/L | 31.3% | |
| Vitamin B12, pmol/L | 153 (82–505) | 128, 190 |
| B12 < 150 pmol/L | 46.5% | |
| Vitamin D, nmol/L | 42.2 (14.4–75.0) | 32.7, 52.5 |
| Vitamin D < 50 nmol/L | 68.7% | |
| Vitamin A, µmol/L | 1.4, (0.6–2.9) | 1.1, 1.8 |
| Vitamin A < 1.05 µmol/L | 18.6% | |
| Retinol binding protein (RPB), mg/L | 42.6 (3.4–169.3) | 25.6, 57.7 |
| RBP < 30 mg/L | 36.4% | |
| Retinol/RBP molar ratio | 0.9 (0.1–8.8) | 0.5, 1.1 |
| Vitamin A saturation < 0.8 | 44.4% | |
| Vitamin A saturation > 1 | 34.2% | |
| IGF-1, ng/mL | 31.0 (14.5–65.2) | 26.1, 34.6 |
| Calculated plasma volume, L | 2.0 (1.6–3.1) | 1.8, 2.1 |
| Serum iron, µmol/L | 10.2 (2.8–31.0) | 7.3, 14.1 |
| Serum iron < 8.9 µmol/L | 45.4% | |
| Ferritin, µg/L | 23.5 (1.7–129.1) | 13.9, 36.1 |
| Ferritin < 70 µg/L | 93.9% | |
| Ferritin < 30 µg/L | 63.6% | |
| Ferritin < 15 µg/L | 26.3% | |
| sTfR, mg/L | 7.9 (2.3–20.8) | 6.2, 9.8 |
| sTfR > 8.3 mg/L | 41.4% | |
| Hepcidin, µg/L | 15.6 (5.1–56.8) | 11.6, 20.8 |
| Blood Cell Indices | ||
| Hemoglobin, g/L | 117.5 ± 10.8 | |
| RBC × 106/mm3 | 4.1 ± 0.37 | |
| Hematocrit | 37.8 (24.3–44.3) | 35.7, 39.7 |
| Hematocrit < 36% | 27.3% | |
| MCV, fL | 92.8 ± 5.1 | |
| MCH, pg | 29.0 ± 1.8 | |
| MCHC, g/L | 31.3 ± 1.1 | |
| 1 RDW-CV | 14 (12–18) | 13, 14 |
| WBC × 103/mm3 | 8.2 ± 1.9 | |
| Neutrophils × 103/mm3 | 4.2 (1.8–8.8) | 3.5, 5.2 |
| Lymphocytes × 103/mm3 | 2.4 ± 0.6 | |
| Monocytes × 103/mm3 | 0.36 (0.19–0.86) | 0.32, 0.44 |
| Eosinophils × 103/mm3 | 0.61 (0.07–4.26) | 0.34, 0.91 |
| Eosinophils > 0.6 × 103/mm3 | 50.5% | |
| Basophils × 103/mm3 | 0.04 (0.01–0.12) | 0.03, 0.06 |
| Platelets × 103/mm3 | 306 (180–638) | 250, 363 |
| Platelets > 415 × 103/mm3 | 16.2% | |
| 1 Plateletcrit (%) | 26.9 (17.2–52.0) | 23.5, 31.5 |
| CRP and Cytokines, pg/mL | ||
| CRP, mg/L | 1.3 (0.1–16.9) | 0.5, 3.7 |
| CRP > 5 mg/L | 20.2% | |
| CRP > 10 mg/L | 6.1% | |
| IL-1β | 0.02 (0.01–38.6) | 0.02, 1.3 |
| IL-4 | 2.2 (0.0–37.0) | 0.2, 4.3 |
| IL-6 | 1.6 (1.6–88.9) | 1.6, 1.6 |
| IL-10 | 0.3 (0.0–23.9) | 0.1, 1.3 |
| IL-12 | 1.3 (0.0–42.8) | 0.1, 5.7 |
| IL-13 | 0.9 (0.1–15.7) | 0.6, 1.6 |
| IL-17 | 0.9 (0.1–87.1) | 0.5, 3.5 |
| IFN-γ | 1.6 (0.05–223.3) | 0.05, 3.5 |
| TNF-α | 4.7 (0.04–24.4) | 1.3, 8.2 |
| MCP-1 | 315.4 (46.0–586.0) | 256.5, 391.1 |
| Presence of Anemia (n = 44) | Absence of Anemia (n = 55) | |||
|---|---|---|---|---|
| Variables | ||||
| Maternal Characteristics | Mean ± SD; %; Median (Min, Max) | IQR | Mean ± SD; %; Median (Min, Max) | IQR |
| Weeks post-partum | 11.5 (2 − 26) | 7, 16 | 12 (1–28) | 4, 17 |
| BMI, kg/m2 | 25.2 ± 0.4 | 25.9 ± 0.4 | ||
| Supplementation | ||||
| Iron tablets | 90.9% | 94.5% | ||
| Number of wks taking iron in pregnancy | 6.5 (0–39) | 0.5, 21 | 11 (0–37) | 4, 23 |
| Number of wks taking iron in lactation | 8 (0–23) | 3, 13 | 10 (0–28) | 4, 17 |
| Number of tablets × 600 mg/d | 1 (0–4) | 1, 2 | 1 (0–3) | 1, 1 |
| Multiple nutrient supplements | 29.5% | 47.3% | ||
| Intakes ≥ 1 Portion/wk | ||||
| Animal-source foods | 79.5% | 81.8% | ||
| Orange-red fruits & vegetables | 68.1% | 54.3% | ||
| Green leafy vegetables | 54.5% | 56.4% | ||
| Nutritional Biomarkers | ||||
| Folic acid, nmol/L | 11.2 (6.6–31.9) | 8.4, 14.7 | 12.1 (5.4–35.9) | 10.2, 16.2 |
| <10 nmol/L | * 43.2% | 21.8% | ||
| Vitamin B12, pmol/L | 152 (87–505) | 131, 186.5 | 157 (82–399) | 123, 200 |
| <150 pmol/L | 45.4% | 47.3% | ||
| Vitamin D, nmol/L | 39.4 (14.4–69.7) | 31.0, 48.1 | 44.7 (17.8–75.0) | 34.2, 57.9 |
| <50 nmol/L | * 79.5% | 60.0% | ||
| Vitamin A, µmol/L | ** 1.3 (0.6–2.2) | 1.0, 1.5 | 1.6 (0.6–2.9) | 1.3, 2.0 |
| <1.05 µmol/L | 25.0% | 13.2% | ||
| Retinol Binding Protein, mg/L | 31.2 (3.4–169.3) | 23.8, 55.3 | 45.4 (5.4–118.7) | 28.2, 62.0 |
| <30 mg/L | 45.4% | 29.1% | ||
| Retinol/RBP molar ratio | 0.9 (0.1–8.8) | 0.4, 1.2 | 0.8 (0.1–7.7) | 0.5, 1.1 |
| <0.8 | 43.2% | 45.4% | ||
| >1 | 40.9% | 28.3% | ||
| IGF-1, ng/Ml | 30.5 (14.5–65.2) | 21.9, 33.9 | 31.8 (21.5–61.9) | 28.4, 36.0 |
| Serum iron, µmol/L | *** 7.8 (2.8–31.0) | 6.0, 11.2 | 12.1 (3.6–23.0) | 8.3, 15.2 |
| <8.9 µmol/L | * 59.1% | 34.5% | ||
| Ferritin, µg/L | * 17.8 (1.7–127.9) | 12.1, 33.4 | 28.3 (5.9–129.1) | 17.1, 42.1 |
| <70 µg/L | 95.4% | 92.7% | ||
| <30 µg/L | 72.7% | 56.4% | ||
| <15 µg/L | 34.1% | 20.0% | ||
| sTfR, mg/L | 27.8 (2.3–20.8) | 5.9, 9.7 | 7.9 (2.3–15.8) | 6.4, 10.1 |
| >8.3 mg/L | 37.7% | 45.7% | ||
| Hepcidin, µg/L | * 14.6 (5.1–56.8) | 11.3, 20.0 | 16.6 (5.4–53.7) | 12.3, 21.5 |
| Blood Cell Indices | ||||
| Hemoglobin, g/L | *** 108.7 ± 8.2 | 124.6 ± 6.8 | ||
| RBC × 106/mm3 | *** 3.8 ± 0.4 | 4.2 ± 0.3 | ||
| Hematocrit | *** 35.7 (24.3–38.8) | 33.7, 37.1 | 39.6 (34.3–44.3) | 38.4, 41.0 |
| MCV, fL | * 91.5 ± 5.5 | 93.7 ± 4.5 | ||
| MCH, pg | ** 28.5 ± 2.0 | 29.5 ± 1.5 | ||
| MCHC, g/L | 31.1 ± 1.3 | 31.5 ± 0.9 | ||
| 1 RDW-CV, % | 14 (12–18) | 13, 15 | 14 (12–16) | 13, 14 |
| WBC × 103/mm3 | 8.23 ± 2.0 | 8.20 ± 1.77 | ||
| Neutrophils, × 103/mm3 | 4.14 (2.47–8.41) | 3.52, 5.23 | 4.21 (1.80–8.83) | 3.40, 5.24 |
| Lymphocytes, × 103/mm3 | 2.47 ± 0.66 | 2.44 ± 0.60 | ||
| Monocytes, × 103/mm3 | 0.37 (0.22–0.63) | 0.33, 0.45 | 0.36 (0.19–0.86) | 0.31, 0.44 |
| Eosinophils, × 103/mm3 | 0.50 (0.09–2.04) | 0.34, 0.90 | 0.65 (0.07–4.26) | 0.34, 1.17 |
| Basophils, × 103/mm3 | 0.04 (0.01–0.12) | 0.03, 0.06 | 0.04 (0.01–0.12) | 0.03, 0.06 |
| Platelets × 103/mm3 | 317 (214–638) | 273, 407 | 296 (180–619) | 233, 343 |
| 1 Plateletcrit (%) | * 27.1 (19.7–44.1) | 25.5, 35.0 | 26.6 (17.2–52.0) | 21.8, 30.1 |
| CRP and Cytokines, pg/mL | ||||
| CRP, mg/L | 1.2 (0.1–16.9) | 0.4, 4.1 | 1.4 (0.1–14.0) | 0.7, 3.4 |
| IL-1β | 0.5 (0.01–7.4) | 0.02, 1.3 | 0.02 (0.01–38.6) | 0.01, 1.7 |
| IL-4 | 2.6 (0.0–37.0) | 0.2, 3.9 | 1.5 (0.0–34.7) | 0.2, 4.3 |
| IL-6 | 1.6 (1.6–88.9) | 1.6, 1.6 | 1.6 (1.6–51.9) | 1.6, 1.6 |
| IL-10 | 0.3 (0.0–19.9 | 0.1, 0.6 | 0.3 (0.0–23.9) | 0.0, 1.6 |
| IL-12 | 1.8 (0.0–20.8) | 0.1, 5.5 | 0.8 (0.0–42.8) | 0.1, 5.3 |
| IL-13 | 0.9 (0.1–15.7) | 0.6, 1.3 | 0.9 (0.1–8.0) | 0.9, 1.6 |
| IL-17 | 0.7 (0.1–87.1) | 0.5, 1.6 | 1.0 (0.1–69.6) | 0.6, 2.0 |
| IFN-γ | 1.6 (0.05–62.1) | 0.05, 2.8 | 1.7 (0.05–223.3 | 0.05, 5.2 |
| TNF-α | 5.4 (0.04–23.1) | 1.2, 10.0 | 4.4 (0.04–24.4) | 1.3, 7.9 |
| MCP-1 | 314.8 (46.0–586.0) | 261.0, 384.4 | 317.8 (46.0–586.0) | 238.0, 391.1 |
| Infant Anthropometry | ||||
| HCAZ | −0.01 ± 1.14 | 0.11 ± 1.49 | ||
| LAZ | −0.39 ± 1.54 | −0.34 ± 1.66 | ||
| WAZ | 0.06 ± 1.19 | 0.50 ± 1.66 | ||
| Model and Variables | MFP Logistic Regression Models | Dominance Analysis | |||
|---|---|---|---|---|---|
| (A) | |||||
| Anemia 1 | OR ± SE | p-Value | 95% CI | Rank | Standardized Dominance |
| t-Serum iron, µmol/L * | 0.86 ± 0.05 | 0.005 | 0.77, 0.95 | 1 | 0.319 |
| t-Vitamin A, µmol/L | 0.18 ± 0.11 | 0.006 | 0.05, 0.61 | 2 | 0.286 |
| t-Log IGF-1, ng/mL | 0.08 0.09 | 0.022 | 0.43, 1.15 | 3 | 0.195 |
| t-Plasma volume, dL * | 1.44 ± 0.20 | 0.010 | 1.09, 1.90 | 4 | 0.188 |
| t-Weeks post-partum | 1.04 ± 0.04 | 0.329 | 0.96, 1.11 | 5 | 0.011 |
| Constant | 0.71 0.18 | 0.166 | 0.43, 1.15 | Overall fit statistic = 0.227 | |
| (B) | |||||
| sTfR > 8.3 mg/L 2 | OR ± SE | p-Value | 95% CI | Rank | Standardized Dominance |
| t-Retinol/RBP molar ratio * | 2.69 ± 0.99 | 0.007 | 1.30, 5.57 | 1 | 0.495 |
| Home delivery (0 = no, 1 = yes) | 2.32 ± 1.15 | 0.089 | 0.88, 6.12 | 2 | 0.172 |
| t-IGF-1, ng/mL | 1.05 ± 0.03 | 0.091 | 0.99, 1.11 | 3 | 0.166 |
| t-Field work, h/d | 1.15 ± 0.10 | 0.111 | 0.97, 1.37 | 4 | 0.141 |
| t-Weeks post-partum | 1.03 ± 0.03 | 0.433 | 0.96, 1.09 | 5 | 0.026 |
| Constant | 0.40 ± 0.13 | 0.007 | 0.21, 0.78 | Overall fit statistic = 0.149 | |
| (C) | |||||
| Serum Iron < 8.9 µmol/L 3 | OR ± SE | p-Value | 95% CI | Rank | Standardized Dominance |
| t-Platelets × 105/mm3 | 2.39 ± 0.83 | 0.012 | 1.21, 4.72 | 1 | 0.236 |
| t-Ferritin, µg/L | 0.95 ± 0.01 | 0.004 | 0.92, 0.98 | 2 | 0.190 |
| t-CRP, mg/L | 1.29 ± 0.14 | 0.019 | 1.04, 1.60 | 3 | 0.182 |
| t-IGF-1, ng/mL | 0.91 ± 0.04 | 0.031 | 0.83, 0.99 | 4 | 0.161 |
| t-NLR | 2.00 ± 0.78 | 0.076 | 0.93, 4.32 | 5 | 0.100 |
| t-Green/leafy vegetables, portions/wk | 0.80 ± 0.10 | 0.074 | 0.64, 1.02 | 6 | 0.089 |
| t-Weeks post-partum | 1.09 ± 0.05 | 0.057 | 0.99, 1.19 | 7 | 0.041 |
| Constant | 0.77 ± 0.22 | 0.353 | 0.44, 1.33 | Overall fit statistic = 0.357 | |
| Model and Variables | MFP Linear Regression Models | Dominance Analysis | ||||
|---|---|---|---|---|---|---|
| (A) | ||||||
| Log Ferritin (µg/L) with Hepcidin 1 | Coeff ± SE | 95% CI | β | p-Value | Ranking | Standardized Dominance |
| t-Hepcidin, µg/L | 0.04 ± 0.007 | 0.03, 0.06 | 0.47 | <0.0001 | 1 | 0.450 |
| t-Serum iron, µmol/L | 0.04 ± 0.01 | 0.01, 0.07 | 0.22 | 0.009 | 2 | 0.152 |
| t-Hemoglobin, g/L | 0.02 ± 0.01 | 0.004, 0.03 | 0.21 | 0.011 | 3 | 0.128 |
| t-sTfR, mg/L | −0.07 ± 0.02 | −0.10, −0.03 | −0.25 | 0.001 | 4 | 0.126 |
| t-CRP, mg/L | 0.06 ± 0.02 | 0.02, 0.09 | 0.24 | 0.004 | 5 | 0.083 |
| t-Weeks post-partum | 0.0004 ± 0.01 | −0.02, 0.02 | 0.003 | 0.964 | 6 | 0.011 |
| Constant | 3.10 ± 0.06 | 2.98, 3.22 | <0.0001 | Overall fit statistic = 0.589 | ||
| (B) | ||||||
| Log Ferritin (µg/L) without Hepcidin 2 | Coeff ± SE | 95% CI | β | p-Value | Ranking | Standardized Dominance |
| t-Serum iron, µmol/L | 0.06 ± 0.02 | 0.03, 0.09 | 0.35 | <0.0001 | 1 | 0.307 |
| t-Hematocrit, %* | 0.08 ± 0.02 | 0.03, 0.13 | 0.29 | 0.002 | 2 | 0.224 |
| t-sTfr, mg/L | −0.08 ± 0.02 | −0.12, −0.03 | −0.29 | 0.001 | 3 | 0.217 |
| t-CRP, mg/L | 0.08 ± 0.02 | 0.04, 0.12 | 0.34 | <0.0001 | 4 | 0.165 |
| Caries, presence | −0.29 ± 0.19 | −0.67, 0.08 | −0.13 | 0.123 | 5 | 0.066 |
| t-Weeks post-partum | −0.005 ± 0.01 | −0.03, 0.02 | −0.04 | 0.639 | 6 | 0.021 |
| Constant | 3.10 ± 0.07 | 2.96, 3.24 | <0.0001 | Overall fit statistic = 0.415 | ||
| (C) | ||||||
| Log Hepcidin (µg/L) with Ferritin 3 | Coeff ± SE | 95% CI | β | p-Value | Ranking | Standardized Dominance |
| t-Ferritin, µg/L | 1.44 ± 0.16 | 1.12, 1.76 | 0.62 | <0.0001 | 1 | 0.710 |
| t-RBC × 106/mm3 | 0.28 ± 0.09 | 0.09, 0.47 | 0.21 | 0.004 | 2 | 0.088 |
| t-Animal-source foods, portions/wk | −0.03 ± 0.01 | −0.05, −0.008 | −0.19 | 0.008 | 3 | 0.085 |
| t-IGF-1 pg/mL | −0.01 ± 0.004 | −0.02, −0.003 | −0.19 | 0.006 | 4 | 0.071 |
| t-Months on iron supplements | 0.02 ± 0.01 | −0.003, 0.04 | 0.11 | 0.094 | 5 | 0.030 |
| t-Weeks post-partum | −0.003 ± 0.005 | −0.01, 0.006 | −0.05 | 0.512 | 6 | 0.017 |
| Constant | 2.81 ± 0.03 | 2.75, 2.87 | <0.0001 | Overall fit statistic = 0.600 | ||
| (D) | ||||||
| Log Hepcidin (µg/L) without Ferritin 4 | Coeff ± SE | 95% CI | β | p-Value | Ranking | Standardized Dominance |
| t-Hemoglobin, g/L | 0.02 ± 0.004 | 0.009, 0.02 | 0.38 | <0.0001 | 1 | 0.220 |
| t-Serum iron, µmol/L | 0.03 ± 0.008 | 0.01, 0.04 | 0.32 | 0.001 | 2 | 0.211 |
| t-Animal-source portions/wk | −0.05 ± 0.01 | −0.07, −0.02 | −0.32 | <0.0001 | 3 | 0.171 |
| t-CRP, mg/L | 0.05 ± 0.01 | 0.02, 0.07 | 0.37 | <0.0001 | 4 | 0.159 |
| t-IGF-1, pg/mL | −0.01 ± 0.004 | −0.02, −0.007 | −0.29 | 0.001 | 5 | 0.140 |
| t-Months on iron supplements | 0.03 ± 0.01 | 0.006, 0.06 | 0.20 | 0.016 | 6 | 0.065 |
| t-Weeks post-partum | −0.005 ± 0.006 | −0.02, 0.007 | −0.07 | 0.445 | 7 | 0.033 |
| Constant | 2.75 ± 0.04 | 2.68, 2.83 | <0.0001 | Overall fit statistic = 0.462 | ||
| Model and Variables | MPF Linear Regression Models | Dominance Analysis | ||||
|---|---|---|---|---|---|---|
| (A) | ||||||
| HCAZ 1 | Coeff ± SE | 95% CI | β | p-Value | Ranking | Standardized Dominance |
| t-Parity, n | 0.13 ± 0.05 | 0.02, 0.23 | 0.24 | 0.017 | 1 | 0.312 |
| t-Plateletcrit, % | −0.04 ± 0.02 | −0.08, −0.003 | −0.22 | 0.034 | 2 | 0.212 |
| t-Plasma volume, dL * | 0.10 ± 0.06 | −0.02, 0.22 | 0.16 | 0.103 | 3 | 0.176 |
| t-Ferritin, µg/L | −0.01 ± 0.005 | −0.02, 0.001 | −0.17 | 0.079 | 4 | 0.120 |
| t-Weeks post-partum | −0.04 ± 0.02 | −0.07, 0.001 | −0.20 | 0.059 | 5 | 0.107 |
| t-GA at birth, wks | 0.06 ± 0.07 | −0.08, 0.98 | 0.08 | 0.416 | 6 | 0.073 |
| Constant | −0.24 ± 0.12 | −0.48, −3.35 × 10−7 | 0.050 | Overall fit statistic = 0.201 | ||
| (B) | ||||||
| LAZ 2 | Coeff ± SE | 95% CI | β | p-Value | Ranking | Standardized Dominance |
| t-Plasma volume, mL | 0.32 ± 0.06 | 0.19, 0.45 | 0.44 | <0.0001 | 1 | 0.465 |
| t-Months on iron supplements | −0.12 ± 0.05 | −0.22, −0.02 | −0.22 | 0.014 | 2 | 0.134 |
| t-Platelets × 103/mm3 | −2.66 ± 1.33 | −5.31, −0.02 | −0.17 | 0.048 | 3 | 0.114 |
| t-Vitamin D, nmol/L | 0.02 ± 0.01 | 0.007, 0.04 | 0.23 | 0.008 | 4 | 0.108 |
| t-MNS, tbsp/d | 0.25 ± 0.09 | 0.07, 0.44 | 0.23 | 0.009 | 5 | 0.098 |
| t-Gestational age, wks | 0.14 ± 0.07 | −0.01, 0.28 | 0.16 | 0.064 | 6 | 0.081 |
| Constant | −0.67 ± 0.13 | −0.93, −0.41 | <0.0001 | Overall fit statistic 0.379 | ||
| (C) | ||||||
| WAZ 3 | Coeff ± SE | 95% CI | β | p-Value | Ranking | Standardized Dominance |
| t-Plasma volume, L | 0.19 ± 0.06 | 0.06, 0.32 | 0.27 | 0.004 | 1 | 0.273 |
| t- Animal-source foods/portions/wk | 0.12 ± 0.04 | 0.04, 0.21 | 0.26 | 0.005 | 2 | 0.239 |
| t-Platelets × 103/mm3 | −2.99 ± 1.36 | −5.69, −0.28 | −0.20 | 0.031 | 3 | 0.195 |
| t-Gestational age, wk | 0.17 ± 0.07 | 0.02, 0.31 | 0.21 | 0.026 | 4 | 0.185 |
| t-Wood smoke, h/d | −0.20 ± 0.11 | −0.43, 0.02 | −0.16 | 0.078 | 5 | 0.107 |
| Constant | 0.04 ± 0.13 | −0.22, 0.31 | 0.733 | Overall fit statistic = 0.277 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Fernández, D.; Nemeth, E.; Pons, E.d.C.; Sinisterra, O.T.; Rueda, D.; Starr, L.; Sangkhae, V.; Murillo, E.; Scott, M.E.; Koski, K.G. Multiple Indicators of Undernutrition, Infection, and Inflammation in Lactating Women Are Associated with Maternal Iron Status and Infant Anthropometry in Panama: The MINDI Cohort. Nutrients 2022, 14, 3497. https://doi.org/10.3390/nu14173497
González-Fernández D, Nemeth E, Pons EdC, Sinisterra OT, Rueda D, Starr L, Sangkhae V, Murillo E, Scott ME, Koski KG. Multiple Indicators of Undernutrition, Infection, and Inflammation in Lactating Women Are Associated with Maternal Iron Status and Infant Anthropometry in Panama: The MINDI Cohort. Nutrients. 2022; 14(17):3497. https://doi.org/10.3390/nu14173497
Chicago/Turabian StyleGonzález-Fernández, Doris, Elizabeta Nemeth, Emérita del Carmen Pons, Odalis Teresa Sinisterra, Delfina Rueda, Lisa Starr, Veena Sangkhae, Enrique Murillo, Marilyn E. Scott, and Kristine G. Koski. 2022. "Multiple Indicators of Undernutrition, Infection, and Inflammation in Lactating Women Are Associated with Maternal Iron Status and Infant Anthropometry in Panama: The MINDI Cohort" Nutrients 14, no. 17: 3497. https://doi.org/10.3390/nu14173497
APA StyleGonzález-Fernández, D., Nemeth, E., Pons, E. d. C., Sinisterra, O. T., Rueda, D., Starr, L., Sangkhae, V., Murillo, E., Scott, M. E., & Koski, K. G. (2022). Multiple Indicators of Undernutrition, Infection, and Inflammation in Lactating Women Are Associated with Maternal Iron Status and Infant Anthropometry in Panama: The MINDI Cohort. Nutrients, 14(17), 3497. https://doi.org/10.3390/nu14173497






