Use of Nutraceuticals in Elderly to Fight Inflammation and Immuno-Senescence: A Randomized Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assay Methods
2.2. Statistical Analysis
3. Results
3.1. Within-Group Analysis
3.1.1. White Cell
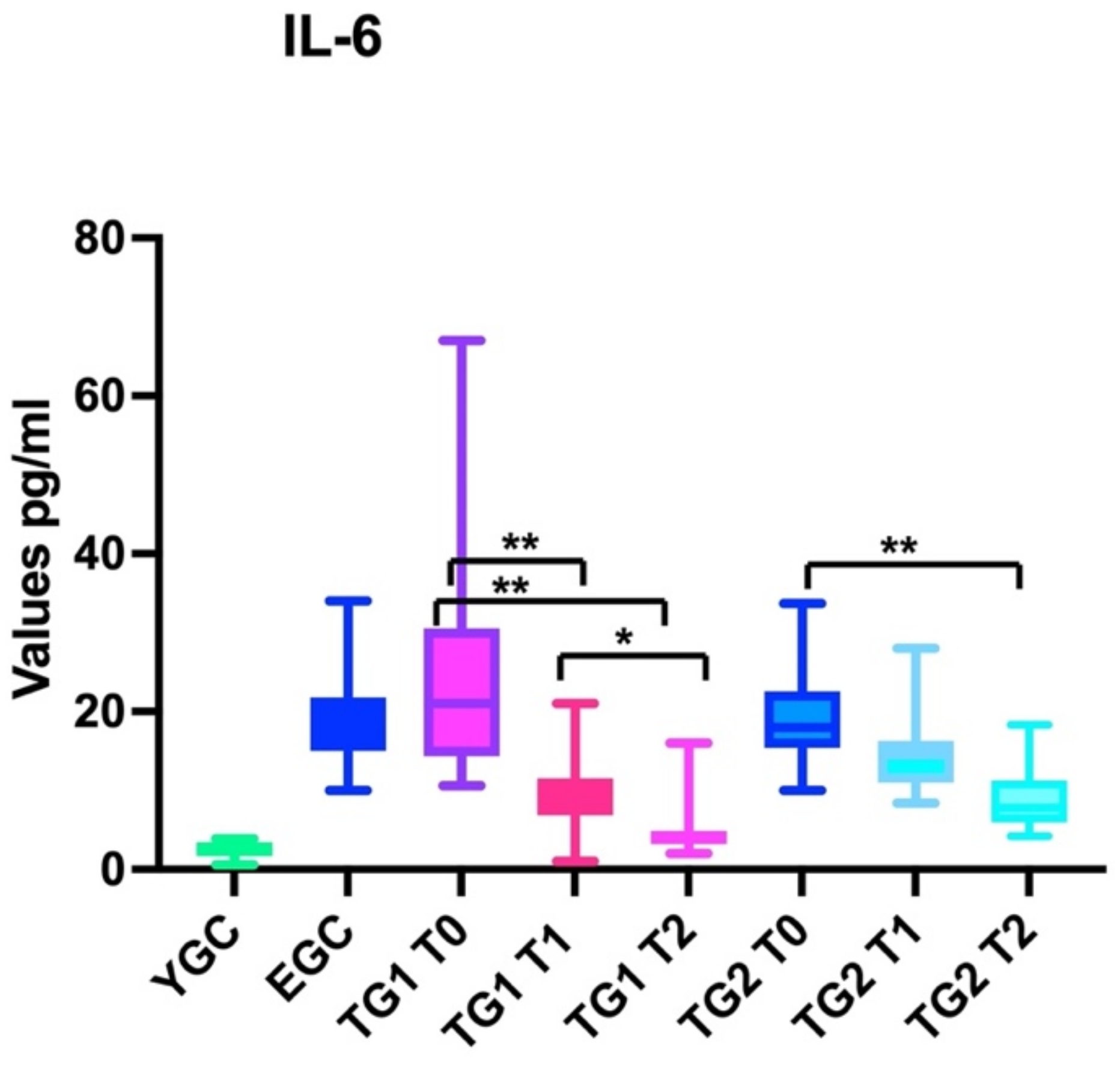
3.1.2. IL-6
3.1.3. CPR
3.2. Between Groups Analysis
3.2.1. White Cells
3.2.2. IL-6
3.2.3. CPR
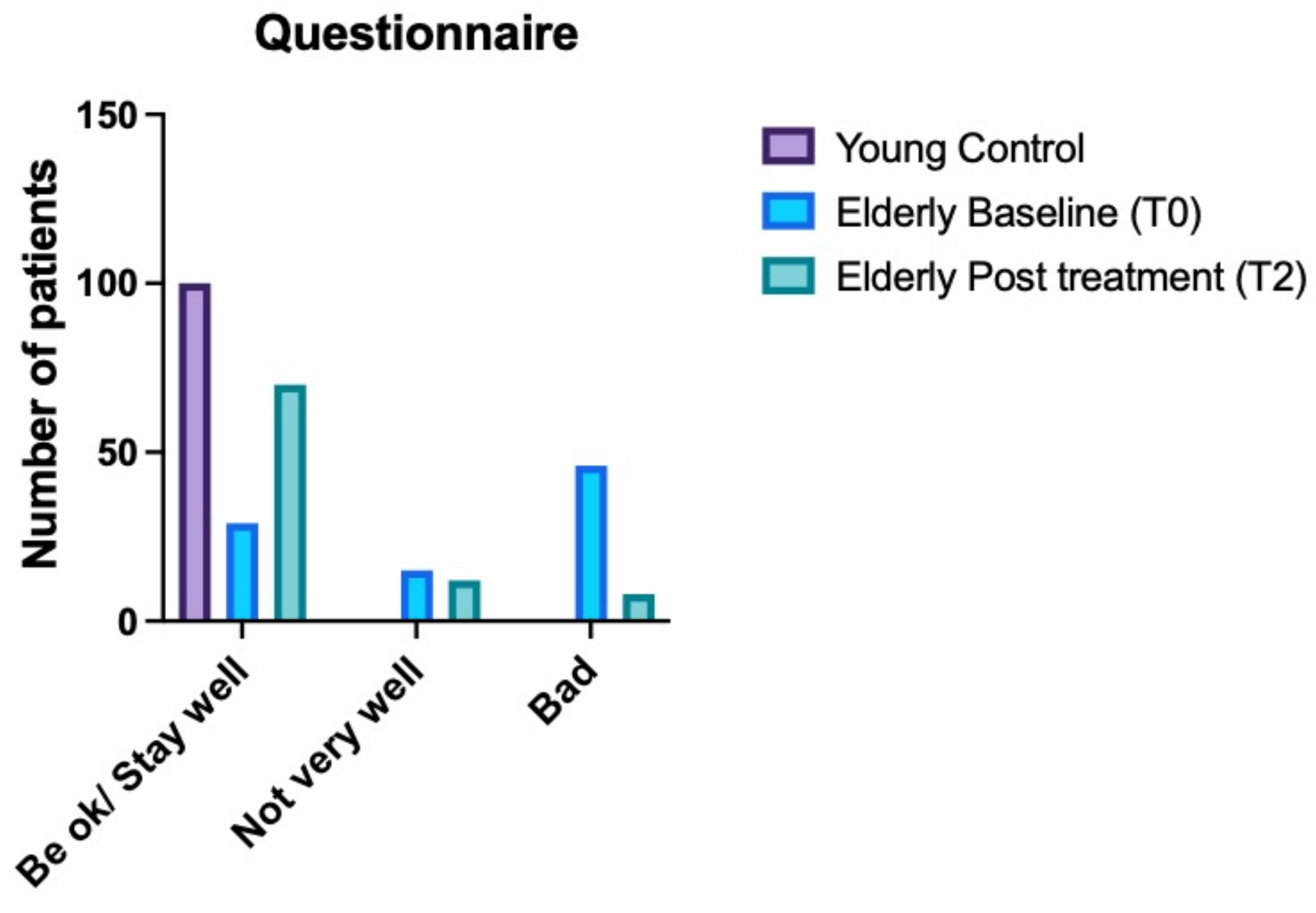
3.3. Health Assessment Questionnaire Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuentes, E.; Fuentes, M.; Alarcón, M.; Palomo, I. Immune System Dysfunction in the Elderly. An. Acad. Bras. Ciências 2017, 89, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.-A.; McGowan, S.J.; et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, R.L.; Peterson, P.K. Immunodeficiency of the Elderly. Clin. Infect. Dis. 1987, 9, 1127–1139. [Google Scholar] [CrossRef]
- Agarwal, S.; Busse, P.J. Innate and adaptive immunosenescence. Ann. Allergy Asthma Immunol. 2010, 104, 183. [Google Scholar] [CrossRef]
- Bagatini, M.D.; Cardoso, A.M.; Reschke, C.R.; Carvalho, F.B. Immune System and Chronic Diseases 2018. J. Immunol. Res. 2018, 2018, 8653572. [Google Scholar] [CrossRef] [Green Version]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef]
- Pae, M.; Wu, D. Nutritional modulation of age-related changes in the immune system and risk of infection. Nutr. Res. 2017, 41, 14–35. [Google Scholar] [CrossRef] [Green Version]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2017, 105, 4–9. [Google Scholar] [CrossRef]
- Ferrando-Martínez, S.; Romero-Sánchez, M.C.; Solana, R.; Delgado, J.; De La Rosa, R.; Muñoz-Fernández, M.; Ruiz-Mateos, E.; Leal, M. Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. AGE 2011, 35, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Sayed, N.; Gao, T.; Tibshirani, R.; Hastie, T.; Cui, L.; Kuznetsova, T.; Rosenberg-Hasson, Y.; Ostan, R.; Monti, D.; Lehallier, B.; et al. An Inflammatory Clock Predicts Multi-morbidity, Immunosenescence and Cardiovascular Aging in Humans. bioRxiv 2019. [Google Scholar]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [Green Version]
- Caruso, C.; Ligotti, M.E.; Accardi, G.; Aiello, A.; Candore, G. An immunologist’s guide to immunosenescence and its treatment. Expert Rev. Clin. Immunol. 2022, 18, 961–981. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.; Hood, M.; Zhang, X.; Zhang, L.; Kan, J.; Du, J. A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. Nutrients 2020, 12, 1193. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Di Stadio, A.; Ishai, R.; Gambacorta, V.; Korsch, F.; Ricci, G.; Della Volpe, A.; Bernitsas, E. Nutraceuticals as immune-stimulating therapy to fight COVID-19. Combination of elements to improve the efficacy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9182–9187. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; Della Volpe, A.; Korsch, F.M.; De Lucia, A.; Ralli, M.; Martines, F.; Ricci, G. Difensil Immuno Reduces Recurrence and Severity of Tonsillitis in Children: A Randomized Controlled Trial. Nutrients 2020, 12, 1637. [Google Scholar] [CrossRef]
- Huang, H.Y.; Caballero, B.; Chang, S.; Alberg, A.; Semba, R.; Schneyer, C.; Wilson, R.F.; Cheng, T.Y.; Prokopowicz, G.; Barnes, G.J., 2nd; et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid. Rep. Technol. Assess. 2006, 139, 1–117. [Google Scholar]
- Ligthart, G.J.; Corberand, J.X.; Fournier, C.; Galanaud, P.; Hijmans, W.; Kennes, B.; Müller-Hermelink, H.K.; Steinmann, G.G. Admission criteria for immunogerontological studies in man: The senieur protocol. Mech. Ageing Dev. 1984, 28, 47–55. [Google Scholar] [CrossRef]
- Fenech, M.; Morley, A.A. Measurement of micronuclei in lymphocytes. Mutat. Res. Mutagen. Relat. Subj. 1985, 147, 29–36. [Google Scholar] [CrossRef]
- Ramey, D.R.; Fries, J.F.; Singh, G. Quality of Life and Pharmacoleconomics in Clinical Trials and The Health Assesment Questionnaire 1995—Status and Review; Spilker, B., Ed.; Lippincott-Raven Pub.: Philadelphia, PA, USA, 1996; pp. 227–237. [Google Scholar]
- Shibata, K.; Hirose, J.; Fukuwatari, T. Relationship between Urinary Concentrations of Nine Water-soluble Vitamins and their Vitamin Intakes in Japanese Adult Males. Nutr. Metab. Insights 2014, 7, NMI.S17245. [Google Scholar] [CrossRef]
- Puzianowska-Kuźnicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-reactive protein, successful aging, and mortality: The PolSenior study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Shen, H.; Schenten, D.; Shan, P.; Lee, P.J.; Goldstein, D.R. Aging Enhances the Basal Production of IL-6 and CCL2 in Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2012, 32, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegertjes, R.; Thielen, N.; van Caam, A.; van Laar, M.; van Beuningen, H.; Koenders, M.; van Lent, P.; van der Kraan, P.; van de Loo, F.; Davidson, E.B. Increased IL-6 receptor expression and signaling in ageing cartilage can be explained by loss of TGF-β-mediated IL-6 receptor suppression. Osteoarthr. Cartil. 2021, 29, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Ershler, W.B.; Keller, E.T. Age-Associated Increased Interleukin-6 Gene Expression, Late-Life Diseases, and Frailty. Annu. Rev. Med. 2000, 51, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Zaharuddin, L.; Mokhtar, N.M.; Nawawi, K.N.M.; Ali, R.A.R. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef] [Green Version]
- Labudzynskyi, D.; Shymanskyy, I.; Veliky, M. Role of vitamin D3 in regulation of interleukin-6 and osteopontin expression in liver of diabetic mice. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2916–2919. [Google Scholar]
- Bateman, R.M.; Sharpe, M.D.; Jagger, J.E.; Ellis, C.G.; Solé-Violán, J.; López-Rodríguez, M.; Herrera-Ramos, E.; Ruíz-Hernández, J.; Borderías, L.; Horcajada, J.; et al. 36th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 15–18 March 2016. Crit. Care 2016, 20, 94, Erratum in: Crit. Care. 2016, 20, 347. [Google Scholar] [CrossRef] [Green Version]
- Huey, K.A.; Fiscus, G.; Richwine, A.F.; Johnson, R.W.; Meador, B.M. In vivo vitamin E administration attenuates interleukin-6 and interleukin-1β responses to an acute inflammatory insult in mouse skeletal and cardiac muscle. Exp. Physiol. 2008, 93, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Cantorna, M.T.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [Green Version]
- De Simone, C.; Ciardi, A.; Grassi, A.; Gardini, S.L.; Tzantzoglou, S.; Trinchieri, V.; Moretti, S.; Jirillo, E. Effect of Bifidobacterium bifidum and Lactobacillus acidophilus on gut mucosa and peripheral blood B lymphocytes. Immunopharmacol. Immunotoxicol. 1992, 14, 331–340. [Google Scholar] [CrossRef]
- Muhamed, P.K.; Vadstrup, S. Zinc is the most important trace element. Ugeskr. Laeger 2014, 176, V11120654. [Google Scholar]
- Bergman, P.; Lindh, A.U.; Björkhem-Bergman, L.; Lindh, J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef] [Green Version]
- Shaik-Dasthagirisaheb, Y.B.; Varvara, G.; Murmura, G.; Saggini, A.; Caraffa, A.; Antinolfi, P.; Tete’, S.; Tripodi, D.; Conti, F.; Cianchetti, E.; et al. Role of vitamins D, E and C in immunity and inflammation. J. Biol. Regul. Homeost. Agents 2013, 27, 291–295. [Google Scholar]
- Skowrońska, W.; Granica, S.; Czerwińska, M.E.; Osińska, E.; Bazylko, A. Sambucus nigra L. leaves inhibit TNF-α secretion by LPS-stimulated human neutrophils and strongly scavenge reactive oxygen species. J. Ethnopharmacol. 2022, 290, 115116. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [Green Version]
- Ellulu, M.S.; Rahmat, A.; Ismail, P.; Khaza’Ai, H.; Abed, Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Drug Des. Dev. Ther. 2015, 9, 3405–3412. [Google Scholar] [CrossRef] [Green Version]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’H, N.; Falgarone, G.; Boissier, M.-C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2018, 71, 487–494. [Google Scholar] [CrossRef]



| Age | Gender | Hospitalized | Non-Hospitalized | Surgery Perfomed within 6 Months | Infection | Hypertension | Cardiac Disease | Obesity | Ipercolesterol | Anemia | Diabetes | Thyroid Disorders | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YCG | 33.7 ± 8.7 | 17 women, 13 men | 14 | 16 | 5 | 4 | 18 | 6 | 7 | 6 | 4 | 6 | 8 |
| ECG | 74.3 ± 8.2 | 16 women, 14 men | 12 | 18 | 6 | 4 | 15 | 8 | 5 | 14 | 2 | 8 | 6 |
| TG1 | 73.8 ± 7.8 | 17 women, 13 men | 13 | 17 | 5 | 4 | 18 | 6 | 7 | 6 | 4 | 7 | 8 |
| TG2 | 71.4 ± 7.9 | 14 women, 16 men | 10 | 20 | 8 | 4 | 16 | 10 | 6 | 18 | 2 | 12 | 8 |
| YCG | ECG | TG1 | TG2 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Lymphocytes | T0 | 6.6 ± 1.7 | 13.9 ± 1.7 | 12.9 ± 1.7 | 15 ± 1.8 | >0.05 (ECG. TG1 TG2) | |
| T1 | 13.6 ±1.8 | 11.7 ± 1.5 | 13 ± 1.7 | <0.001 | |||
| T2 | 13.5 ± 1.5 | 10 ± 1.5 | 10.5 ± 2 | <0.001 | |||
| IL-6 | T0 | 2.4 ± 0.9 | 21.5 ± 8.6 | 23.4 ± 11.7 | 19.7 ± 6.7 | >0.05 (ECG. TG1 TG2) | |
| T1 | 21 ± 10.3 | 9.8 ± 4.2 | 14.8 ± 5.1 | <0.001 | |||
| T2 | 20.5 ± 11 | 4.8 ± 3.2 | 8.9 ± 3.5 | <0.001 | |||
| CRP | T0 | 0.4 ± 0.1 | 6.1 ± 1.8 | 4.5 ± 1.4 | 7.4 ± 2.3 | >0.05 (ECG. TG1 TG2) | |
| T1 | 5.8 ±1.5 | 1 ± 2.8 | 5.5 ± 1.7 | <0.01 | |||
| T2 | 5.5 ± 1.4 | 2.1 ± 0.8 | 4.5 ± 1.7 | <0.01 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maselli del Giudice, A.; La Mantia, I.; Barbara, F.; Ciccarone, S.; Ragno, M.S.; de Robertis, V.; Cariti, F.; Barbara, M.; D’Ascanio, L.; Di Stadio, A. Use of Nutraceuticals in Elderly to Fight Inflammation and Immuno-Senescence: A Randomized Case-Control Study. Nutrients 2022, 14, 3476. https://doi.org/10.3390/nu14173476
Maselli del Giudice A, La Mantia I, Barbara F, Ciccarone S, Ragno MS, de Robertis V, Cariti F, Barbara M, D’Ascanio L, Di Stadio A. Use of Nutraceuticals in Elderly to Fight Inflammation and Immuno-Senescence: A Randomized Case-Control Study. Nutrients. 2022; 14(17):3476. https://doi.org/10.3390/nu14173476
Chicago/Turabian StyleMaselli del Giudice, Alessandro, Ignazio La Mantia, Francesco Barbara, Silvana Ciccarone, Maria Sterpeta Ragno, Valentina de Robertis, Francesco Cariti, Michele Barbara, Luca D’Ascanio, and Arianna Di Stadio. 2022. "Use of Nutraceuticals in Elderly to Fight Inflammation and Immuno-Senescence: A Randomized Case-Control Study" Nutrients 14, no. 17: 3476. https://doi.org/10.3390/nu14173476
APA StyleMaselli del Giudice, A., La Mantia, I., Barbara, F., Ciccarone, S., Ragno, M. S., de Robertis, V., Cariti, F., Barbara, M., D’Ascanio, L., & Di Stadio, A. (2022). Use of Nutraceuticals in Elderly to Fight Inflammation and Immuno-Senescence: A Randomized Case-Control Study. Nutrients, 14(17), 3476. https://doi.org/10.3390/nu14173476








