Prevalence of Low Muscle Mass in the Computed Tomography at the Third Lumbar Vertebra Level Depends on Chosen Cut-Off in 200 Hospitalised Patients—A Prospective Observational Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Computed Tomography Selection Criteria
2.3. Selection of Cut-Offs for Low Muscle Mass
2.4. Statistical Analysis
3. Results
3.1. Description of Study Population and CT Scans
3.2. Diagnosis of Low Muscle Mass in Two Selected Patients
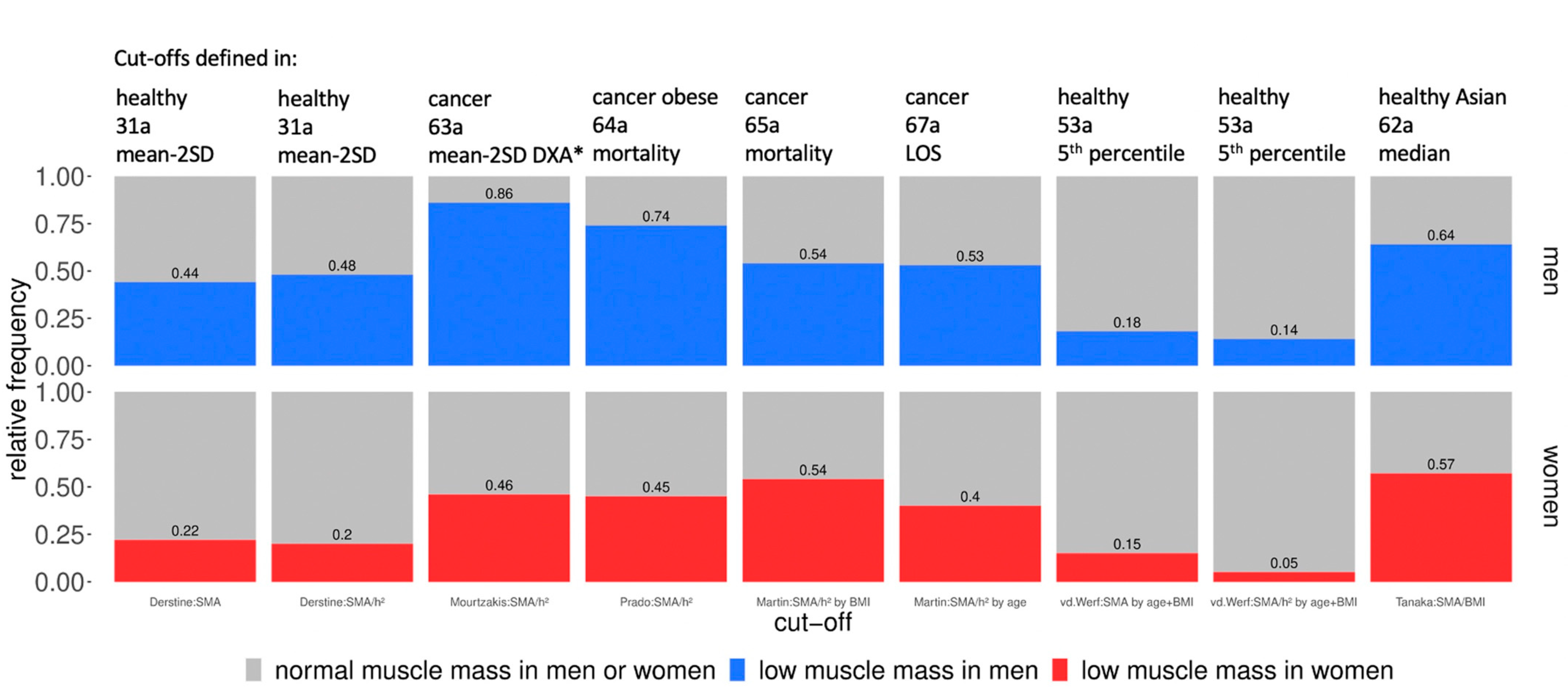
3.3. Statistical Calculation of the Cut-Off Influenced Prevalence Number
3.4. Adjustment of the Cut-Off-Influenced Prevalence Distribution Pattern across Age Classes
3.5. Adjustment of the Cut-Off-Influenced Prevalence Distribution Pattern across BMI Classes
4. Discussion
4.1. Prevalence of Low Muscle Mass in Men vs. Women
4.2. Statistical Calculation of the Cut-Off-Influenced Prevalence Number
4.3. Adjustment of the Cut-Off-Influenced Prevalence Distribution Pattern across Age Classes
4.4. Adjustment of the Cut-Off-Influenced Prevalence Distribution Pattern across BMI Classes
4.5. Prevalence Numbers of Low Muscle Mass in the Literature
4.6. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total Body Skeletal Muscle and Adipose Tissue Volumes: Estimation from a Single Abdominal Cross-Sectional Image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, L.; Geisler, C.; Pourhassan, M.; Braun, W.; Glüer, C.C.; Bosy-Westphal, A.; Müller, M.J. What Is the Best Reference Site for a Single MRI Slice to Assess Whole Body Skeletal Muscle and Adipose Tissue Volumes in Healthy Adults? Am. J. Clin. Nutr. 2015, 102, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Mok, M.; Allende, R.; Leipsic, J.; Altisent, O.A.J.; Del Trigo, M.; Campelo-Parada, F.; Delarochellière, R.; Dumont, E.; Doyle, D.; Côté, M.; et al. Prognostic Value of Fat Mass and Skeletal Muscle Mass Determined by Computed Tomography in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2016, 117, 828–833. [Google Scholar] [CrossRef]
- Tandon, P.; Low, G.; Mourtzakis, M.; Zenith, L.; Myers, R.P.; Abraldes, J.G.; Shaheen, A.A.M.; Qamar, H.; Mansoor, N.; Carbonneau, M.; et al. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1473–1480. [Google Scholar] [CrossRef]
- Weijs, P.J.M.; Looijaard, W.G.P.M.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Oudemans-van Straaten, H.M.; Beishuizen, A. Low Skeletal Muscle Area Is a Risk Factor for Mortality in Mechanically Ventilated Critically Ill Patients. Crit. Care 2014, 18, R12. [Google Scholar] [CrossRef]
- Du, Y.; Karvellas, C.J.; Baracos, V.; Williams, D.C.; Khadaroo, R.G. Sarcopenia Is a Predictor of Outcomes in Very Elderly Patients Undergoing Emergency Surgery. Surgery 2014, 156, 521–527. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal Muscle Cutoff Values for Sarcopenia Diagnosis Using T10 to L5 Measurements in a Healthy US Population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef]
- Tanaka, M.; Okada, H.; Hashimoto, Y.; Kumagai, M.; Nishimura, H.; Oda, Y.; Fukui, M. Relationship between Nonalcoholic Fatty Liver Disease and Muscle Quality as Well as Quantity Evaluated by Computed Tomography. Liver Int. 2020, 40, 120–130. [Google Scholar] [CrossRef]
- Van Der Werf, A.; Langius, J.A.E.; De Van Der Schueren, M.A.E.; Nurmohamed, S.A.; Van Der Pant, K.A.M.I.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for Skeletal Muscle Index, Area and Radiation Attenuation Based on Computed Tomography Imaging in a Healthy Caucasian Population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef]
- Gaillard, M.; Tranchart, H.; Maitre, S.; Perlemuter, G.; Lainas, P.; Dagher, I. Preoperative Detection of Sarcopenic Obesity Helps to Predict the Occurrence of Gastric Leak After Sleeve Gastrectomy. Obes. Surg. 2018, 28, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, J.L.A.; Buettner, S.; Alferink, L.J.M.; Bossche, N.; de Bruin, R.W.F.; Darwish Murad, S.; Polak, W.G.; Metselaar, H.J.; Ijzermans, J.N.M. Low Skeletal Muscle Mass Is Associated with Increased Hospital Costs in Patients with Cirrhosis Listed for Liver Transplantation–a Retrospective Study. Transpl. Int. 2018, 31, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A Practical and Precise Approach to Quantification of Body Composition in Cancer Patients Using Computed Tomography Images Acquired during Routine Care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Hirai, T.; Kobayashi, H.; Okuma, T.; Ishibashi, Y.; Ikegami, M.; Ohki, T.; Shinoda, Y.; Okajima, K.; Zhang, L.; Akiyama, T.; et al. Skeletal Muscle Measurements Predict Surgical Wound Complications but Not Overall Survival in Patients with Soft Tissue Sarcoma. Jpn. J. Clin. Oncol. 2020, 50, 1168–1174. [Google Scholar] [CrossRef]
- Sandini, M.; Patiňo, M.; Ferrone, C.R.; Alvarez-Pérez, C.A.; Honselmann, K.C.; Paiella, S.; Catania, M.; Riva, L.; Tedesco, G.; Casolino, R.; et al. Association between Changes in Body Composition and Neoadjuvant Treatment for Pancreatic Cancer. JAMA Surg. 2018, 153, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Hopkins, J.; Malietzis, G.; Jenkins, J.T.; Sawyer, M.B.; Brisebois, R.; MacLean, A.; Nelson, G.; Gramlich, L.; Baracos, V.E. Assessment of Computed Tomography (CT)-Defined Muscle and Adipose Tissue Features in Relation to Short-Term Outcomes After Elective Surgery for Colorectal Cancer: A Multicenter Approach. Ann. Surg. Oncol. 2018, 25, 2669–2680. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Choe, E.K.; Kang, H.Y.; Park, B.; Yang, J.I.; Kim, J.S. The Association between Nonalcoholic Fatty Liver Disease and CT-Measured Skeletal Muscle Mass. J. Clin. Med. 2018, 7, 310. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among Skeletal Muscle Mass Indices Derived from Height-, Weight-, and Body Mass Index-Adjusted Models in Assessing Sarcopenia. Korean J. Int. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A Biolog. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Peters, K.W.; Shardell, M.D.; McLean, R.R.; Dam, T.T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Cutpoints for Low Appendicular Lean Mass That Identify Older Adults with Clinically Significant Weakness. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 567–575. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Hertwig, A.; Hahn, R.; Anwar, M.; Siebenrock, T.; Pesta, M.; Liebau, K.; Timmermann, I.; Brugger, J.; Posch, M.; et al. Validation of Bedside Ultrasound to Predict Lumbar Muscle Area in the Computed Tomography in 200 Non-Critically Ill Patients: The USVALID Prospective Study. Clin. Nutr. 2022, 41, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Gifford, J.M.; Chandolu, S.; Colantuoni, E.; Pronovost, P.J.; Needham, D.M. The Functional Comorbidity Index Had High Inter-Rater Reliability in Patients with Acute Lung Injury. BMC Anesthesiol. 2012, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Hiesmayr, M.; Frantal, S.; Schindler, K.; Themessl-Huber, M.; Mouhieddine, M.; Schuh, C.; Pernicka, E.; Schneider, S.; Singer, P.; Ljunqvist, O.; et al. The Patient- And Nutrition-Derived Outcome Risk Assessment Score (PANDORA): Development of a Simple Predictive Risk Score for 30-Day In-Hospital Mortality Based on Demographics, Clinical Observation, and Nutrition. PLoS ONE 2015, 10, e0127316. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18–88 Yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- McGovern, J.; Dolan, R.D.; Horgan, P.G.; Laird, B.J.; McMillan, D.C. Computed Tomography-Defined Low Skeletal Muscle Index and Density in Cancer Patients: Observations from a Systematic Review. J. Cachexia Sarcopenia Muscle 2021, 12, 1408–1417. [Google Scholar] [CrossRef]
- Georgiou, A.; Papatheodoridis, G.V.; Alexopoulou, A.; Deutsch, M.; Vlachogiannakos, I.; Ioannidou, P.; Papageorgiou, M.V.; Papadopoulos, N.; Yannakoulia, M.; Kontogianni, M.D. Validation of Cutoffs for Skeletal Muscle Mass Index Based on Computed Tomography Analysis against Dual Energy X-Ray Absorptiometry in Patients with Cirrhosis: The KIRRHOS Study. Ann. Gastroenterol. 2020, 33, 80–86. [Google Scholar] [CrossRef]
- Naumann, P.; Eberlein, J.; Farnia, B.; Hackert, T.; Debus, J.; Combs, S.E. Continued Weight Loss and Sarcopenia Predict Poor Outcomes in Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers 2019, 11, 709. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, M.C.; Lyadov, V.K.; Liang, P.C.; Chen, C.M.; Shih, T.T.F.; Chang, Y.T. Comparing Western and Eastern Criteria for Sarcopenia and Their Association with Survival in Patients with Pancreatic Cancer. Clin. Nutr. 2019, 38, 862–869. [Google Scholar] [CrossRef]
- Pittelkow, E.M.; Debrock, W.C.; McLaughlin, B.E.; Hassanein, A.H.; Socas, J.; Lester, M.E.; Duquette, S.P. Preoperatively Identified Sarcopenia Leads to Increased Postoperative Complications, Hospital and ICU Length of Stay in Autologous Microsurgical Breast Reconstruction. J. Reconstr. Microsurg. 2020, 36, 059–063. [Google Scholar] [CrossRef]
- Ghaffarian, A.A.; Foss, W.T.; Donald, G.; Kraiss, L.W.; Sarfati, M.; Griffin, C.L.; Smith, B.K.; Brooke, B.S. Prognostic Implications of Diagnosing Frailty and Sarcopenia in Vascular Surgery Practice. J. Vasc. Surg. 2019, 70, 892–900. [Google Scholar] [CrossRef]
- Fischer, A.; Kiss, N.; Rudas, V.; Nieding, K.; Wildner, B.; Hiesmayr, M. Prevalence of Low Muscle Mass in Healthy Subjects and Patients According to Different Definitions of Cut-off Values for Low Muscle Mass on the Computed Tomography at the Third Lumbar Vertebra Level: A Systematic Review and Meta-Analysis. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020206919 (accessed on 28 July 2022).


| Characteristic | All (n = 200) | Male (n = 118) | Female (n = 82) |
|---|---|---|---|
| Age (years) | 61.3 (51.0–70.1) (19–86) | 63.6 (51.4–71.3) (19–86) | 58.9 (45.8–68.8) (21–85) |
| Weight (kg) | 73.9 ± 16.0 (41–118) | 79.1 ± 14.0 (47–110) | 66.3 ± 15.8 (41–118) |
| Height (cm) | 172.0 ± 9.4 (148–197) | 177.1 ± 7.3 (160–197) | 164.6 ± 6.8 (148–183) |
| BMI (kg/m2) | 24.9 ± 4.8 (16.2–42.0) | 25.2 ± 4.4 (16.5–38.3) | 24.5 ± 5.4 (16.2–42.0) |
| Functional comorbidity index (FCI) (points) [26] | 2 (1–3) (0–10) | 2 (1–3) (0–7) | 2 (1–4) (0–10) |
| Kidney injury | 21 (10.5) | 14 (11.9) | 7 (8.5) |
| Current presence of malignant tumour | 88 (44) | 48 (40.7) | 40 (48.8) |
| Surgical wards | 135 (67.5) | 77 (65) | 58 (70.7) |
| General surgery | 71 (35.5) | 44 (37.3) | 27 (32.9) |
| Urology | 35 (17.5) | 23 (19.5) | 12 (14.6) |
| Gynaecology | 13 (6.5) | - | 13 (15.9) |
| Cardiac surgery | 8 (4.0) | 4 (3.4) | 4 (4.9) |
| Vascular surgery | 5 (2.5) | 4 (3.4) | 1 (1.2) |
| Orthopaedic surgery | 2 (1.0) | 1 (0.8) | 1 (1.2) |
| Thoracic surgery | 1 (0.5) | 1 (0.8) | 0 (0) |
| Medical wards | 65 (32.5) | 41 (34.7) | 24 (29.3) |
| Gastroenterology | 41 (20.5) | 27 (22.9) | 14 (17.1) |
| Oncology | 11 (5.5) | 4 (3.4) | 7 (8.5) |
| Nephrology | 6 (3.0) | 4 (3.4) | 2 (2.4) |
| Cardiology | 5 (2.5) | 4 (3.4) | 1 (1.2) |
| Haematology | 2 (1.0) | 2 (1.7) | 0 (0) |
| Time between CT and ultrasound, hours | 22 (5–28) (1–48) | 21 (5–27) (1–48) | 22 (6–29) (1–48) |
| Clinical presence of peripheral oedema | 41 (20.5) | 24 (20.3) | 17 (20.7) |
| Patients with surgery prior to ultrasound examination | 73 (36.5) | 43 (36.4) | 30 (36.6) |
| Time between prior surgery and ultrasound, days | 5 (2–10) (0–59) | 5 (2–11) (0–40) | 4 (2–9) (0–59) |
| Hospital length of stay, days | 13 (6–23) (1–174) | 15 (6–26) (1–174) | 12 (6–23) (1–96) |
| Hospital mortality | 5 (2.5) | 3 (2.5) | 2 (2.4) |
| PANDORA score (points) [27] | 26.5 (19–34)(2–56) | 26 (20–33.8) (6–54) | 27.5 (19–35) (2–56) |
| All (n = 200) | Male (n = 118) | Female (n = 82) | |||||
|---|---|---|---|---|---|---|---|
| CT measurements | mean | SD | mean | SD | mean | SD | P |
| SMA (cm2) | 131.9 | 29.5 | 148.3 | 23.7 | 108.3 | 19.4 | <0.001 |
| SMA/height2 (cm2/m2) | 44.3 | 8.0 | 47.3 | 7.6 | 40.0 | 6.3 | <0.001 |
| SMA/BMI (cm2/(kg/m2)) | 5.4 | 1.2 | 6.0 | 1.0 | 4.6 | 1.0 | <0.001 |
| Publication | Cut-Off Adjustment | Cut-Off Values Defined for Subgroups | Cut-Off Calculation | Study Population | Mean Age | Prevalence of Low Muscle Mass | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean BMI | ||||||||||||||
| Ethnicity | ||||||||||||||
| Derstine, 2018 [8] | SMA | Male: <144.3 cm2 Female: <92.2 cm2 | Mean-2 SD of a healthy, young population | n = 727 (410 female) healthy kidney donor candidates for CT at L3 level | 31 ± 6 years BMI: ~27 ± 16 NR (study conducted in the USA) | Male: NR Female: NR | ||||||||
| Derstine, 2018 [8] | SMA/height2 | Male: <45.4 cm2/m2 Female: <34.4 cm2/m2 | Mean-2 SD of a healthy, young population | n = 727 (410 female) healthy kidney donor candidates for CT at L3 level | 31 ± 6 years BMI: ~27 ± 16 NR (study conducted in the USA) | Male: NR Female: NR | ||||||||
| Mourtzakis, 2008 [13] | SMA/height2 | Male: < 55.4 cm2/m2 Female: < 38.9 cm2/m2 | Equation to predict DXA cut-offs [28] for low muscle mass | n = 31 (12 female) non-small cell lung or colorectal cancer patients | 63 ± 10 years BMI: 26.9 ± 6.2 96% Caucasian | Male: NR Female: NR | ||||||||
| Prado, 2008 [17] | SMA/height2 | Male: <52.4 cm2/m2 Female: <38.5 cm2/m2 | Optimal stratification related to mortality | n = 250 (114 female) respiratory or gastrointestinal cancer patients with BMI ≥ 30 | 64 ± 10 years BMI: 34.4 ± 4.4 NR (study conducted in Canada) | Male: 21% Female: 9% | ||||||||
| Martin, 2013 [3] | SMA/height2 | Male with BMI < 25: 43 cm2/m2 Male with BMI ≥ 25: 53 cm2/m2 Female (all BMI): <41 cm2/m2 | Optimal stratification related to mortality | n = 1473 (645 female) respiratory or gastrointestinal cancer patients (same initial patient cohort as Prado’s study [17]) | 65 ± 11 years BMI: ~25.5 NR (study conducted in Canada) | Male: 31% Female: 53% | ||||||||
| Martin, 2018 [16] | SMA/height2 | Age (years) | Male (cm2/m2) | Female (cm2/m2) | Generalized linear model with a negative binomial distribution related to hospital length of stay | n = 2100 (830 female) Colorectal cancer patients | 67 ± 12 yearsBMI: 27.7 ± 5.6NR (study conducted in Canada and UK) | Male: NRFemale: NR | ||||||
| <50 | <50.6 | <39.6 | ||||||||||||
| 50–59 | <49.3 | <37.6 | ||||||||||||
| 60–69 | <46.8 | <37.1 | ||||||||||||
| 70–79 | <43.4 | <35.2 | ||||||||||||
| ≥80 | <38.7 | <33.5 | ||||||||||||
| van der Werf, 2018 [10] | SMA | Male | Female | Predicted 5th percentile of SMA from BMI and age in a regression equation | n = 420 (246 female) healthy kidney donors | 53 ± 12 yearsBMI: 25.7 ± 3.5Caucasian | Male: 5%Female: 5% | |||||||
| BMI: 17–20 | BMI: 20–25 | BMI: 25–30 | BMI: 30–35 | BMI: 17–20 | BMI: 20–25 | BMI: 25–30 | BMI: 20–35 | |||||||
| 20–29 years | 131.4 | 145.4 | 162.6 | 179.3 | 88.2 | 102.7 | 119.4 | 134.7 | ||||||
| 30–39 years | 124.3 | 138.3 | 155.5 | 172.2 | 86.8 | 97.9 | 111.2 | 123.7 | ||||||
| 40–49 years | 117.1 | 131.2 | 148.3 | 165.0 | 85.1 | 93.1 | 102.9 | 112.3 | ||||||
| 50–59 years | 109.8 | 123.8 | 141.0 | 157.7 | 83.0 | 88.2 | 94.4 | 100.6 | ||||||
| 60–69 years | 102.3 | 116.4 | 133.6 | 150.3 | 80.7 | 83.1 | 85.9 | 88.4 | ||||||
| 70–79 years | 94.8 | 108.8 | 126.0 | 142.7 | 78.0 | 78.0 | 77.3 | 75.9 | ||||||
| van der Werf, 2018 [10] | SMA/height2 | Male | Female | Predicted 5th percentile of SMA/height2 from BMI and age in a regression equation | n = 420 (246 female) healthy kidney donors | 53 ± 12 yearsBMI: 25.7 ± 3.5Caucasian | Male: 5%Female: 5% | |||||||
| BMI: 17–20 | BMI: 20–25 | BMI: 25–30 | BMI: 30–35 | BMI: 17–20 | BMI: 20–25 | BMI: 25–30 | BMI: 20–35 | |||||||
| 20–29 years | 37.4 | 42.5 | 48.7 | 54.8 | 28.5 | 33.7 | 39.6 | 45.1 | ||||||
| 30–39 years | 35.9 | 41.0 | 47.2 | 53.3 | 28.7 | 32.8 | 37.6 | 42.2 | ||||||
| 40–49 years | 34.3 | 39.4 | 45.6 | 51.7 | 28.8 | 31.8 | 35.6 | 39.2 | ||||||
| 50–59 years | 32.7 | 37.7 | 43.9 | 50.0 | 28.7 | 30.9 | 33.5 | 36.1 | ||||||
| 60–69 years | 31.0 | 36.1 | 42.3 | 48.4 | 28.5 | 29.9 | 31.4 | 32.9 | ||||||
| 70–79 years | 29.3 | 34.4 | 40.6 | 46.7 | 28.2 | 28.8 | 29.3 | 29.5 | ||||||
| Tanaka, 2020 [9] | SMA/BMI | Male: <6.309 cm2/kg/m2 Female: <4.66 cm2/kg/m2 | Median of study population | n = 632 (279 female) employees undergoing CT health examinations | ~62 years BMI: ~24 Asian | Male: 50% Female: 50% | ||||||||
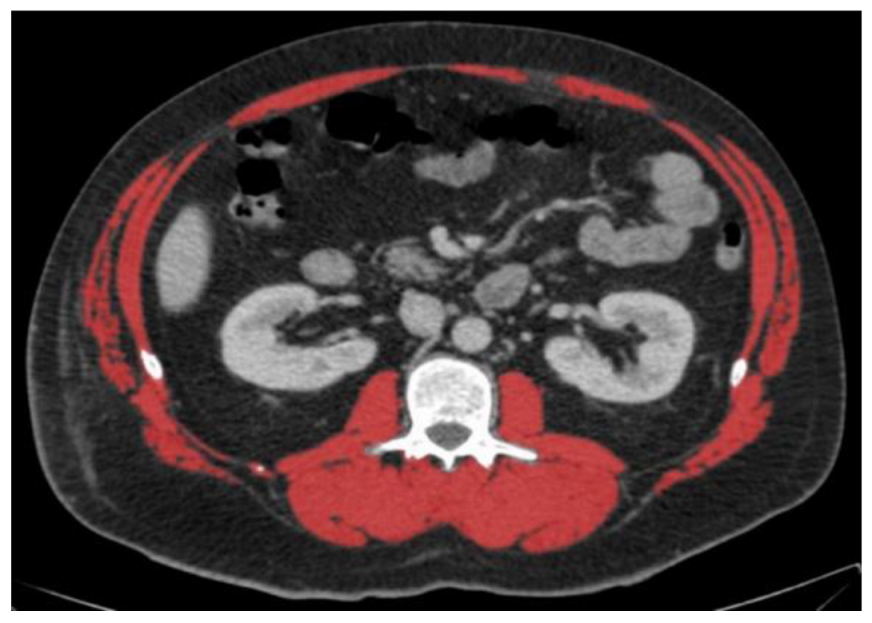 | 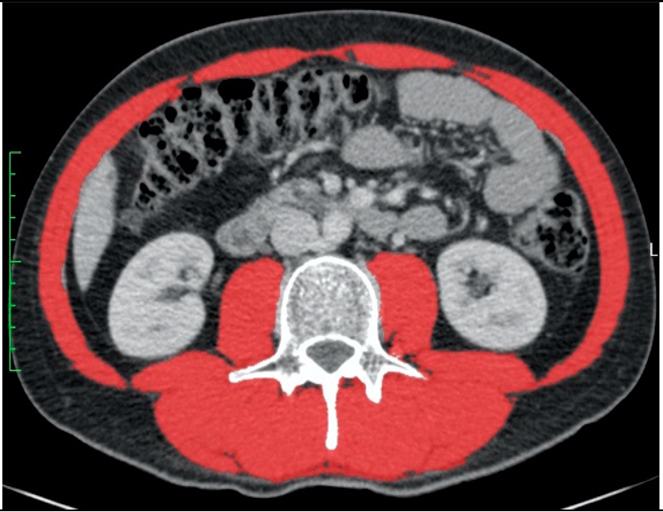 | All male patients (n = 118) * | |
| Sex | Male | Male | Male |
| Age (years) | 51 | 31 | 63.6 (51.4–71.3) |
| Height (cm) | 160 | 197 | 177.1 ± 7.3 |
| Weight (kg) | 93 | 85 | 79.1 ± 14.0 |
| BMI (kg/m2) | 36.3 | 21.9 | 25.2 ± 4.4 |
| CT area (cm2) | 939.8 | 592.4 | 749.8 ± 187.6 |
| A: Diagnosis of low or normal muscle mass according to sex-specific cut-offs set at the mean of our study population | |||
| SMA (cm2) | 150.6 (normal) | 162.9 (normal) | 148.3 ± 23.7 |
| SMA/height2 (cm2/m2) | 58.8 (normal) | 42.0 (low) | 47.3 ± 7.6 |
| SMA/BMI (cm2/(kg/m2)) | 4.1 (low) | 7.4 (normal) | 6.0 ± 1.0 |
| B: Diagnosis of low or normal muscle mass according to published cut-offs for low muscle mass | |||
| Derstine, 2018: SMA by sex [8] | Normal | Normal | |
| Derstine, 2018: SMA/height2 by sex [8] | Normal | Low | |
| Mourtzakis, 2008: SMA/height2 by sex [13] | Normal | Low | |
| Prado, 2008: SMA/height2 by sex [17] | Normal | Low | |
| Martin, 2013: SMA/height2 by sex and BMI [3] | Normal | Low | |
| Martin, 2018: SMA/height2 by sex and age [16] | Normal | Low | |
| van der Werf, 2018: SMA by sex, age and BMI [10] | Low | Normal | |
| van der Werf, 2018: SMA/height2 by sex, age and BMI [10] | Normal | Normal | |
| Tanaka, 2020: SMA/BMI by sex [9] | Low | Normal | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.; Kiss, N.; Rudas, V.-A.; Nieding, K.; Veraar, C.; Timmermann, I.; Liebau, K.; Pesta, M.; Siebenrock, T.; Anwar, M.; et al. Prevalence of Low Muscle Mass in the Computed Tomography at the Third Lumbar Vertebra Level Depends on Chosen Cut-Off in 200 Hospitalised Patients—A Prospective Observational Trial. Nutrients 2022, 14, 3446. https://doi.org/10.3390/nu14163446
Fischer A, Kiss N, Rudas V-A, Nieding K, Veraar C, Timmermann I, Liebau K, Pesta M, Siebenrock T, Anwar M, et al. Prevalence of Low Muscle Mass in the Computed Tomography at the Third Lumbar Vertebra Level Depends on Chosen Cut-Off in 200 Hospitalised Patients—A Prospective Observational Trial. Nutrients. 2022; 14(16):3446. https://doi.org/10.3390/nu14163446
Chicago/Turabian StyleFischer, Arabella, Noemi Kiss, Valerie-Anna Rudas, Kristina Nieding, Cecilia Veraar, Isabel Timmermann, Konstantin Liebau, Maximilian Pesta, Timo Siebenrock, Martin Anwar, and et al. 2022. "Prevalence of Low Muscle Mass in the Computed Tomography at the Third Lumbar Vertebra Level Depends on Chosen Cut-Off in 200 Hospitalised Patients—A Prospective Observational Trial" Nutrients 14, no. 16: 3446. https://doi.org/10.3390/nu14163446
APA StyleFischer, A., Kiss, N., Rudas, V.-A., Nieding, K., Veraar, C., Timmermann, I., Liebau, K., Pesta, M., Siebenrock, T., Anwar, M., Hahn, R., Hertwig, A., Brugger, J., Ringl, H., Tamandl, D., & Hiesmayr, M. (2022). Prevalence of Low Muscle Mass in the Computed Tomography at the Third Lumbar Vertebra Level Depends on Chosen Cut-Off in 200 Hospitalised Patients—A Prospective Observational Trial. Nutrients, 14(16), 3446. https://doi.org/10.3390/nu14163446






