Heart Rate Variability—An Index of the Efficacy of Complementary Therapies in Irritable Bowel Syndrome: A Systematic Review
Abstract
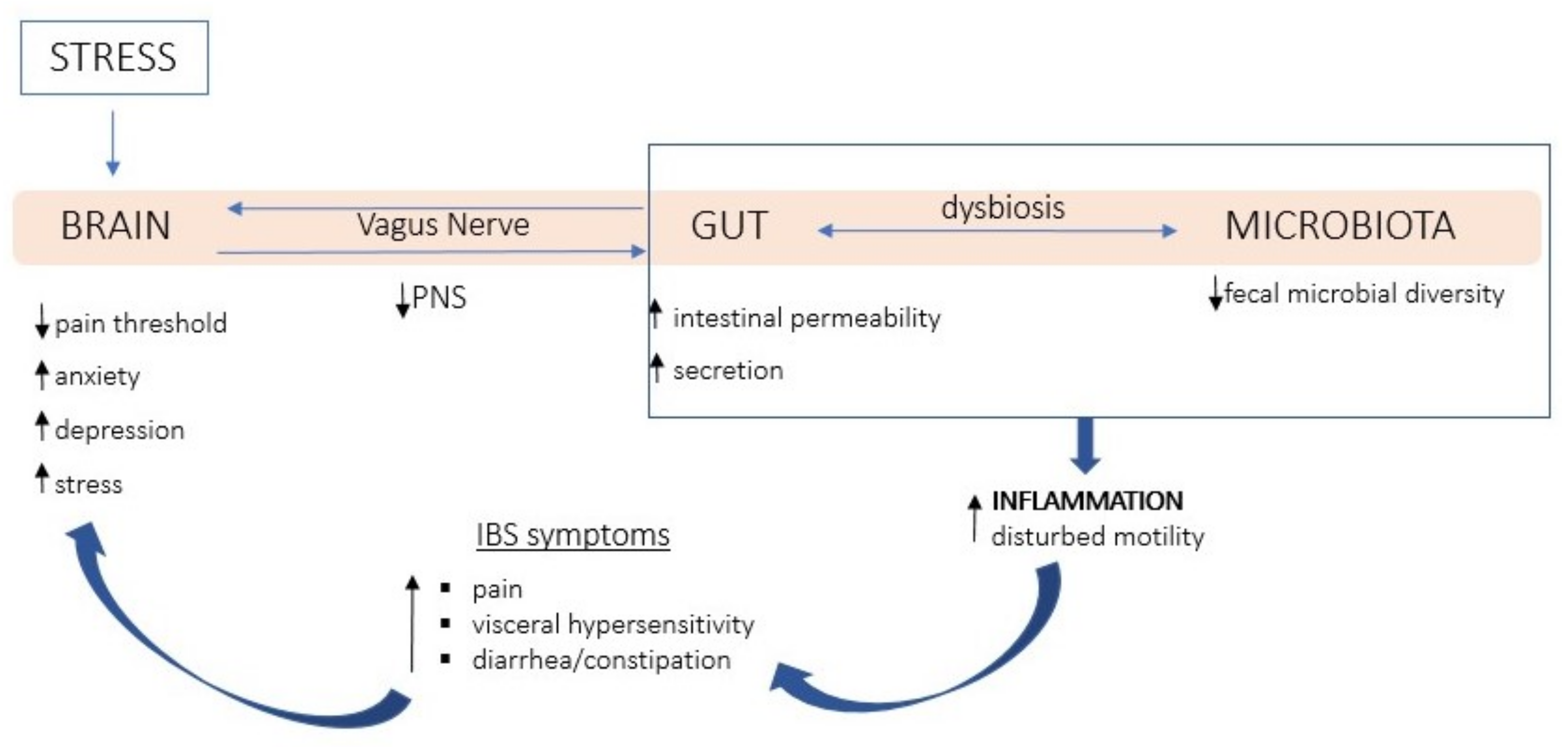
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Search Strategy
2.3. Primary Outcomes
2.4. Secondary Outcomes
2.5. Synthesis of Results
2.6. Evaluation of the Quality of Tests
3. Results
3.1. Characteristics of the Studies
| Study | Intervention (E/C) | Duration/Frequency/ Duration of One Session | Description |
|---|---|---|---|
| Jang et al. [46] | CBT/general information about IBS | E: 8 weeks/once a week/ 80 min (include 20 min of relaxation training); C: 1st week/only once/50 min | E: Group session of CBT (4–6 participants) with 60 min of thematic training and 20 min of relaxation training. C: One session with 50 min of general information about IBS (during the first week). Similarly to the experimental group—an interview on IBS symptoms in groups of 4–6 people (four times: at baseline; and at 8, 16, and 24 weeks). |
| Jurek et al. [47] | Slow deep breathing/normal activities | E: 4 weeks/5 times a week/20-min; C: normal activities | E: Self-directed Slow Deep Breathing with 20-min video. At least 4 times a week. C: maintenance regular activity. |
| Kavuri, Selvan, Malamud et al. [48] | Yoga/Combination/ Wait-List groups a | Y/CB—Yoga RYM: 12 weeks/ 3 times a week/60 min; WL-C -walking: 12 weeks/once a day/60 min | E: Each yoga session started with simple breathing practices, loosening practices, and simple postures with relaxation in between. The session ended with regulated breathing and meditation. C: maintenance of their regular activities; suggestion to walk for 60 min three times a week during their waiting period. |
| Park and Cha [49] | KHA/sham-KHA | 4 weeks/twice a week/25 min | E: 16 KHA reflection spots on both hands were stimulated. The needles were inserted at less than 1 mm depth. C: 16 spots that were unrelated to the crucial energy spots were inserted by the needles. Each reflection spot wasstimulated for 25 min in both groups. |
| Go and Park [50] | Auricular Acupressure/ no treatment | 4 weeks/5 days a week/5 times a day | E: Semen sinapis albae seeds were used to acupressure four auricular points: endocrine, large intestine, lung, and Shenmen. Stickers remained in place for 5 days, and sticker-attached areas were pressed 5 times a day. Acupressure stickers were applied weekly for 4 weeks with a 2-day break time between each treatment. C: No treatment. |
| Shi et al. [51] | taVNS/ sham-taVNS | 4 weeks/twice a day/30 min | E: “The taVNS treatment was performed at auricular cymba concha. One pair of electrodes was placed at bilateral auricular concha, via which trains of pulses were delivered from a watch-size digital stimulator” [51] p. 12. C: Sham-taVNS was performed with the same parameters as taVNS. Electrical stimulation was performed at sham point at the elbow area. |
3.2. Characteristics of HRV Measurements
| Study | Position and Length of Recordings | Time of HRV Recording | Frequency Ranges (Hz) | HRV Hardware | HRV Software | HRV Indicators |
|---|---|---|---|---|---|---|
| Jang et al. [46] | seated, 10 min | unclear | HF: 0.15–0.4 LF: 0.04–0.15 | QECG-3 monitoring system (Laxtha Inc., Daejeon, Korea) | TeleScan Ver.2.8; Laxtha Inc. | HF, LF/HF |
| Jurek et al. [47] | unclear | unclear | unclear | Polar heart rate monitor (Kempele, Finland) | Elite HRV app and Kubios software (Finland) | HF, LF/HF, PNS index, SNS index |
| Kavuri, Selvan, Malamud et al. [48] | lying, 5 min | unclear | unclear | ECG and respiration—Biopac MP 45 Data Acquisition System (BIOPAC, CA, USA) | Kubios (version 2.2, Finland) | HF, LF, LF/HF |
| Park & Cha [49] | seated, 5 min | unclear | unclear | SA-3000P (Medicore Co. Ltd., Seoul, Korea) | SDRR, PSI, TP, VLF, LF, HF, LF-Norm, HF-Norm, LF/HF | |
| Go & Park [50] | seated, twice a for 5 min | unclear | unclear | SA-3000P (Medicore Co. Ltd., Seoul, Korea) | SDRR, PSI, TP, LF-Norm, HF-Norm, LF/HF | |
| Shi et al. [51] | lying, 30 min | unclear | HF: 0.15–0.50 LF: 0.04–0.15 | ECG-01A (Ningbo Maida Medical Device Inc., Ningbo, China) | unclear | LF-Norm, HF-Norm |
3.3. Evaluation of the Effectiveness of the Intervention
| Primary and Secondary Variables | Methods for Assessing Variables | Effect Sizes a | Significance Level | Conclusions |
|---|---|---|---|---|
| Shi et al. [51] | ||||
| IBS symptoms | IBS-SSS | d = 1.30 | p = 0.001 | taVNS improved HRV parameters—increased the vagal activity (HF-norm). taVNS reduced IBS symptoms, pain, anxiety, and depression, and improved quality of life. |
| HRV | HF-norm | d = −0.66 | p = 0.04 | |
| LF-norm | n/d | n/d | ||
| Pain | VAS | d = 1.17 | p = 0.001 | |
| Anxiety | SAS | d = 1.24 | p < 0.001 | |
| Depression | SDS | d = 0.84 | p = 0.011 | |
| Stress | not measured | |||
| Go and Park [50] | ||||
| IBS symptoms | BSSS-AD-F | d = 0.81 | n/d | Auricular acupressure effectively reduced IBS symptoms. The severity of loose stools, diarrhea, abdominal pain, and abdominal discomfort were lower. In the experimental group, HRV parameters significantly improved, indicating increased parasympathetic activity (increase in HFNorm), increased resistance to stress (increase in SDRR), and decreased LH/HF balance. The level of experienced stress in the experimental group decreased significantly. |
| BSSS-AD-DS. | d = 1.16 | n/d | ||
| BSSS-AD-DB | d = 1.52 | n/d | ||
| HRV | HF-norm | d = −1.1 | n/d | |
| LF-norm | d = 0.88 | n/d | ||
| LF/HF | d = 0.88 | n/d | ||
| PSI | d = 0.29 | n/d | ||
| SDRR | d = −0.59 | n/d | ||
| Pain | BSSS-AP-F | d = 0.42 | n/d | |
| BSSS-AP-DS. | d = −0.70 | n/d | ||
| BSSS-AP-DB | d = 0.97 | n/d | ||
| Anxiety | SCL-90R-K-A | d = 0.24 | n/d | |
| Depression | SCL-90R-K-D | d = 0.13 | n/d | |
| Stress | PSS | d = 1.07 | n/d | |
| Park and Cha [49] | ||||
| IBS symptoms | BSSS-AD-F | d = 0 | n/d | Some of the symptoms of IBS have improved—especially those related to abdominal pain: frequency of loose stools and abdominal pain, reduction of anxiety and perceived disability caused by abdominal pain, flatulence, and discomfort in the abdominal cavity. KHA was not effective in reducing stress and promoting mental health. There was no change in HRV. |
| BSSS-AD-DS. | d = −0.32 | n/d | ||
| BSSS-AD-DB | d = −0.24 | n/d | ||
| HRV | HF-norm | d = 0.18 | n/d | |
| HF | d = −0.39 | n/d | ||
| LF-norm | d = −0.18 | n/d | ||
| LF | d = −0.24 | n/d | ||
| LF/HF | d = −0.20 | n/d | ||
| PSI | d = 0.48 | n/d | ||
| SDRR | d = −0.42 | n/d | ||
| Pain | BSSS-AP-F | d = 0.19 | n/d | |
| BSSS-AP-DS. | d = 0.15 | n/d | ||
| BSSS-AP-DB | d = −0.32 | n/d | ||
| Anxiety | SCL-90R-K-A | d = −0.11 | n/d | |
| Depression | SCL-90R-K-D | d = −0.05 | n/d | |
| Stress | GARS | d = −0.51 | n/d | |
| Jurek et al. [47] | ||||
| IBS symptoms | IBS-SSS | d = −0.19 | n/d | There were no changes in the functioning of the autonomic system (no significant differences in HRV). The severity of IBS symptoms has not changed. |
| HRV | HF | d = 0 | n/d | |
| LF/HF | d = 0.16 eta2 = 0.47 | n/d | ||
| PNS index | d = 0.80 | n/d | ||
| SNS index | d = 0.18 | n/d | ||
| Pain | not measured | |||
| Anxiety | ||||
| Depression | ||||
| Stress | ||||
| Jang et al. [46] | ||||
| IBS symptoms | GSRS-IBS | n/d | p < 0.001 | Significant changes in the functioning of ANS were observed—CBT resulted in a significant increase in HF and a significant decrease in the LF/HF ratio. These changes coexisted with significant reductions in IBS symptoms, anxiety, depression, and stress. Differences in HF and the LF/HF ratio were significantly associated with changes in symptoms of IBS, anxiety, depression, and stress. |
| HRV | HF | n/d | p = 0.017 | |
| LF/HF | n/d | p = 0.003 | ||
| Pain | not measured | |||
| Anxiety | HADS-A | n/d | p < 0.001 | |
| Depression | HADS-D | n/d | p < 0.001 | |
| Stress | GARS | n/d | p < 0.001 | |
| Kavuri, Selvan, Malamud et al. [48] | ||||
| IBS symptoms | IBS-SSS | Y vs. WL: d = 4.03 | p < 0.001 | The remedial yoga module (RYM) reduces symptoms of IBS, anxiety, and depression (in both groups: (Y), yoga with limited conventional treatment; and (CB), combination—yoga with conventional treatment). Only in the combination group (yoga with conventional treatment) were there significantly favourable changes in HRV parameters (indicating increased parasympathetic activity). |
| CB vs. WL: d = 3.12 | p < 0.001 | |||
| Y vs. CB d = 0.52 | p > 0.05 | |||
| HRV | HF | n/d | CB vs. WL: p < 0.01 | |
| LF | n/d | CB vs. WL: p < 0.05 | ||
| LF/HF | n/d | CB vs. WL: p < 0.01 | ||
| Pain | not measured | |||
| Anxiety | HADS-A | n/d | Y vs. WL *** | |
| n/d | CB vs. WL *** | |||
| n/d | Y vs. CB | |||
| Depression | HADS-D | n/d | Y vs. WL *** | |
| n/d | CB vs. WL *** | |||
| n/d | Y vs. CB | |||
| Stress | not measured | |||
3.4. Evaluation of the Quality of Tests
4. Discussion
4.1. Characteristics of IBS Patients under Non-Pharmacological Interventions
4.2. Heart Rate Variability Measurements
4.3. Limitations of the Review
4.4. Strengths of the Review
4.5. Similarities of Analyzed Interventions
- (a)
- stimulation of the vagus nerve, which is recommended in IBS therapy [10] (the CBT therapy with relaxation elements has the potential to affect the vagal tone as well);
- (b)
- the starting point of the observed changes was the stimulation of peripheral sensory nerve fibers in the skin, muscles, and viscera (bottom-up process). Taylor et al. [83] postulate that bottom-up processes in MBT interventions correct functional changes in central nervous processing, increase heart rate variability (HRV), and decrease the expression of proinflammatory cytokines;
- (c)
- they took place daily or enabled the daily implementation of acquired skills.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ma, H.; Zhu, J.Z.; Lu, C.; Yu, C.H.; Li, Y.M. The Role of Dietary Energy and Macronutrients Intake in Prevalence of Irritable Bowel Syndromes. Biomed Res. Int. 2019, 2019, 8967306. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.; Gray, J.; Lam, S.; Balshaw, R.; Khorasheh, S.; Barbeau, M.; Kelly, S.; McBurney, C.R. Health-Related Quality of Life, Work Productivity, and Health Care Resource Utilization of Subjects with Irritable Bowel Syndrome: Baseline Results from Logic (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a Naturalistic Study. Clin. Ther. 2006, 28, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Faresjö, Å.; Walter, S.; Norlin, A.K.; Faresjö, T.; Jones, M.P. Gastrointestinal Symptoms - An Illness Burden That Affects Daily Work in Patients with IBS. Health Qual. Life Outcomes 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Fadgyas-Stanculete, M.; Buga, A.-M.; Popa-Wagner, A.; Dumitrascu, D.L. The Relationship between Irritable Bowel Syndrome and Psychiatric Disorders: From Molecular Changes to Clinical Manifestations. J. Mol. Psychiatry 2014, 2, 4. [Google Scholar] [CrossRef]
- Faresjö, Å.; Grodzinsky, E.; Hallert, C.; Timpka, T. Patients with Irritable Bowel Syndrome Are More Burdened by Co-Morbidity and Worry about Serious Diseases than Healthy Controls- Eight Years Follow-up of IBS Patients in Primary Care. BMC Public Health 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota-Gut-Brain Axis in Visceral PaRelevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef]
- Pellissier, S.; Dantzer, C.; Mondillon, L.; Trocme, C.; Gauchez, A.S.; Ducros, V.; Mathieu, N.; Toussaint, B.; Fournier, A.; Canini, F.; et al. Relationship between Vagal Tone, Cortisol, TNF-Alpha, Epinephrine and Negative Affects in Crohn’s Disease and Irritable Bowel Syndrome. PLoS ONE 2014, 9, e105328. [Google Scholar] [CrossRef]
- Pellissier, S.; Dantzer, C.; Canini, F.; Mathieu, N.; Bonaz, B. Psychological Adjustment and Autonomic Disturbances in Inflammatory Bowel Diseases and Irritable Bowel Syndrome. Psychoneuroendocrinology 2010, 35, 653–662. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. The Vagus Nerve in the Neuro-Immune Axis: Implications in the Pathology of the Gastrointestinal Tract. Front. Immunol. 2017, 8, 1452. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- van Thiel, I.A.M.; de Jonge, W.J.; Chiu, I.M.; van den Wijngaard, R.M. Microbiota-Neuroimmune Cross Talk in Stress-Induced Visceral Hypersensitivity of the Bowel. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G1034–G1041. [Google Scholar] [CrossRef]
- Elsenbruch, S. Abdominal Pain in Irritable Bowel Syndrome: A Review of Putative Psychological, Neural and Neuro-Immune Mechanisms. Brain Behav. Immun. 2011, 25, 386–394. [Google Scholar] [CrossRef]
- Thakur, E.R.; Shapiro, J.; Chan, J.; Lumley, M.A.; Cully, J.A.; Bradford, A.; El-Serag, H.B. A Systematic Review of the Effectiveness of Psychological Treatments for IBS in Gastroenterology Settings: Promising but in Need of Further Study. Dig. Dis. Sci. 2018, 63, 2189–2201. [Google Scholar] [CrossRef]
- Peters, S.L.; Muir, J.G.; Gibson, P.R. Review Article: Gut-Directed Hypnotherapy in the Management of Irritable Bowel Syndrome and Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2015, 41, 1104–1115. [Google Scholar] [CrossRef]
- Shah, K.; Ramos-Garcia, M.; Bhavsar, J.; Lehrer, P. Mind-Body Treatments of Irritable Bowel Syndrome Symptoms: An Updated Meta-Analysis. Behav. Res. Ther. 2020, 128, 103462. [Google Scholar] [CrossRef]
- Gaylord, S.A.; Palsson, O.S.; Garland, E.L.; Faurot, K.R.; Coble, R.S.; Mann, J.D.; Frey, W.; Leniek, K.; Whitehead, W.E. Mindfulness Training Reduces the Severity of Irritable Bowel Syndrome in Women: Results of a Randomized Controlled Trial. Am. J. Gastroenterol. 2011, 106, 1678–1688. [Google Scholar] [CrossRef]
- Jun, H.; Ko, S.-J.; Kim, K.; Kim, J.; Park, J.-W. An Overview of Systematic Reviews of Herbal Medicine for Irritable Bowel Syndrome. Front. Pharmacol. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Zhang, J.; Sun, F.; Duan, L. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Black, C.J.; Ford, A.C. Best Management of Irritable Bowel Syndrome. Frontline Gastroenterol. 2020, 12, 303–315. [Google Scholar] [CrossRef]
- Nelkowska, D. Treating Irritable Bowel Syndrome through an Interdisciplinary Approach. Ann. Gastroenterol. 2020, 33, 1–8. [Google Scholar] [CrossRef]
- Clarke, G.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Irritable Bowel Syndrome: Towards Biomarker Identification. Trends Mol. Med. 2009, 15, 478–489. [Google Scholar] [CrossRef]
- Chen, M.; Ruan, G.; Chen, L.; Ying, S.; Li, G.; Xu, F.; Xiao, Z.; Tian, Y.; Lv, L.; Ping, Y.; et al. Neurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front. Endocrinol. 2022, 13, 23. [Google Scholar] [CrossRef]
- Malik, M.; John Camm, A.; Thomas Bigger, J.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Porges, S.W. The Polyvagal Perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Sadowski, A.; Dunlap, C.; Lacombe, A.; Hanes, D. Alterations in Heart Rate Variability Associated with Irritable Bowel Syndrome or Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2021, 12, e00275. [Google Scholar] [CrossRef]
- Mazurak, N.; Stein, J.; Kipphan, S.; Muth, E.R.; Teufel, M.; Zipfel, S.; Enck, P. Heart Rate Variability in Anorexia Nervosa and the Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2011, 23, e470–e478. [Google Scholar] [CrossRef]
- Chalaye, P.; Goffaux, P.; Bourgault, P.; Lafrenaye, S.; Devroede, G.; Watier, A.; Marchand, S. Comparing Pain Modulation and Autonomic Responses in Fibromyalgia and Irritable Bowel Syndrome Patients. Clin. J. Pain 2012, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Mazurak, N.; Seredyuk, N.; Sauer, H.; Teufel, M.; Enck, P. Heart Rate Variability in the Irritable Bowel Syndrome: A Review of the Literature. Neurogastroenterol. Motil. 2012, 24, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kanazawa, M.; Palsson, O.S.; Van Tilburg, M.A.; Gangarosa, L.M.; Fukudo, S.; Drossman, D.A.; Whitehead, W.E. Increased Postprandial Colonic Motility and Autonomic Nervous System Activity in Patients With Irritable Bowel Syndrome: A Prospective Study. J. Neurogastroenterol. Motil. 2018, 24, 87. [Google Scholar] [CrossRef] [PubMed]
- Davydov, D.M.D.M.; Shahabi, L.; Naliboff, B. Cardiovascular Phenotyping for Personalized Lifestyle Treatments of Chronic Abdominal Pain in Irritable Bowel Syndrome: A Randomized Pilot Study. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2019, 31, e13710. [Google Scholar] [CrossRef]
- Park, H.-J. Heart Rate Variability as a Measure of Disease State in Irritable Bowel Syndrome. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2008, 2, 5–16. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Wang, E.M.; Yan, X.J.; Chen, S.L. Autonomic Functioning in Irritable Bowel Syndrome Measured by Heart Rate Variability: A Meta-Analysis. J. Dig. Dis. 2013, 14, 638–646. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The Irritable Bowel Severity Scoring System: A Simple Method of Monitoring Irritable Bowel Syndrome and Its Progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Cain, K.C.; Jarrett, M.E.; Burr, R.L.; Hertig, V.L.; Heitkemper, M.M. Heart Rate Variability Is Related to Pain Severity and Predominant Bowel Pattern in Women with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2007, 19, 110–118. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, 1–36. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic Reviews of Effectiveness. In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 9 July 2022).
- Wan, H.; Chen, Y. Effects of Antidepressive Treatment of Saint John’s Wort Extract Related to Autonomic Nervous Function in Women with Irritable Bowel Syndrome. Int. J. Psychiatry Med. 2010, 40, 45–56. [Google Scholar] [CrossRef]
- Lembo, T.; Naliboff, B.D.; Matin, K.; Munakata, J.; Parker, R.A.; Gracely, R.H.; Mayer, E.A. Irritable Bowel Syndrome Patients Show Altered Sensitivity to Exogenous Opioids. Pain 2000, 87, 137–147. [Google Scholar] [CrossRef]
- Kavuri, V.; Selvan, P.; Tabesh, A.; Raghuram, N.; Selvan, S.R. Remedial Yoga Module Improves Symptoms of Irritable Bowel Syndrome: Replication in the Wait-List Group and Sustained Improvements at 6 Months. Eur. J. Integr. Med. 2015, 7, 609–616. [Google Scholar] [CrossRef]
- Kim, T.; Chung, J.; Bae, S.; Lee, J.; Kim, J.; Lee, J.A.; Yoon, S.J.; Go, H.-Y.; Shin, S.-M. Efficacy and Safety of Different Doses of Moxibustion for Irritable Bowel Syndrome: A Randomised Controlled Pilot Trial. Eur. J. Integr. Med. 2018, 20, 79–83. [Google Scholar] [CrossRef]
- Edebol-Carlman, H.; Schrooten, M.; Ljótsson, B.; Boersma, K.; Linton, S.; Brummer, R.J.; Hanna, E.-C.; Martien, S.; Brjánn, L.; Katja, B.; et al. Cognitive Behavioral Therapy for Irritable Bowel Syndrome: The Effects on State and Trait Anxiety and the Autonomic Nervous System during Induced Rectal Distensions—An Uncontrolled Trial. Scand. J. Pain 2018, 18, 81–91. [Google Scholar] [CrossRef]
- Jang, A.; Hwang, S.-K.; Padhye, N.S.; Meininger, J.C. Effects of Cognitive Behavior Therapy on Heart Rate Variability in Young Females with Constipation-Predominant Irritable Bowel Syndrome: A Parallel-Group Trial. J. Neurogastroenterol. Motil. 2017, 23, 435–445. [Google Scholar] [CrossRef]
- Jurek, M.; Seavey, H.; Guidry, M.; Slomka, E.; Hunter, S.D.; Punyabati, O.; Deepak, K.K.; Sharma, M.P.; Dwivedi, S.N.; Frøkjaer, J.B.; et al. The Effects of Slow Deep Breathing on Microvascular and Autonomic Function and Symptoms in Adults with Irritable Bowel Syndrome: A Pilot Study. Neurogastroenterol. Motil. 2021, 19, e14275. [Google Scholar] [CrossRef]
- Kavuri, V.; Selvan, P.; Malamud, A.; Raghuram, N.; Selvan, S.R. Remedial Yoga Module Remarkably Improves Symptoms in Irritable Bowel Syndrome Patients: A 12-Week Randomized Controlled Trial. Eur. J. Integr. Med. 2015, 7, 595–608. [Google Scholar] [CrossRef]
- Park, H.J.; Cha, C. The Effect of Korean Hand Acupuncture on Young, Single Korean Students with Irritable Bowel Syndrome. Gastroenterol. Nurs. 2012, 35, 403–414. [Google Scholar] [CrossRef]
- Go, G.Y.; Park, H. Effects of Auricular Acupressure on Women with Irritable Bowel Syndrome. Gastroenterol. Nurs. 2020, 43, E24–E34. [Google Scholar] [CrossRef]
- Shi, X.D.; Hu, Y.D.; Zhang, B.; Li, W.N.; Chen, J.D.Z.; Liu, F. Ameliorating Effects and Mechanisms of Transcutaneous Auricular Vagal Nerve Stimulation on Abdominal Pain and Constipation. JCI Insight 2021, 6, 1–18. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Dobbin, A.; Dobbin, J.; Ross, S.C.; Graham, C.; Ford, M.J. Randomised Controlled Trial of Brief Intervention with Biofeedback and Hypnotherapy in Patients with Refractory Irritable Bowel Syndrome. J. R. Coll. Physicians Edinb. 2013, 43, 15–23. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Brignall, M.; Hamilton, M.; Beardsley, J.; Batson, R.D.; Hawrelak, J.; Lichtenstein, B.; Johnston, B.C. Biofeedback for treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2019, 11, 1–71. [Google Scholar] [CrossRef]
- Schumann, D.; Anheyer, D.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Effect of Yoga in the Therapy of Irritable Bowel Syndrome: A Systematic Review. Clin. Gastroenterol. Hepatol. 2016, 14, 1720–1731. [Google Scholar] [CrossRef]
- Vaschillo, E.; Lehrer, P.; Rishe, N.; Konstantinov, M. Heart Rate Variability Biofeedback as a Method for Assessing Baroreflex Function: A Preliminary Study of Resonance in the Cardiovascular System. Appl. Psychophysiol. Biofeedback 2002, 27, 1–27. [Google Scholar] [CrossRef]
- Gevirtz, R. The Promise of Heart Rate Variability Biofeedback: Evidence-Based Applications. Biofeedback 2013, 41, 110–120. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Gevirtz, R. Heart Rate Variability Biofeedback: How and Why Does It Work? Front. Psychol. 2014, 5, 756. [Google Scholar] [CrossRef]
- Szulczewski, M.T. Transcutaneous Auricular Vagus Nerve Stimulation Combined With Slow Breathing: Speculations on Potential Applications and Technical Considerations. Neuromodulation 2022, 25, 380–394. [Google Scholar] [CrossRef]
- Wu, C.; Liu, P.; Fu, H.; Chen, W.; Cui, S.; Lu, L.; Tang, C. Transcutaneous Auricular Vagus Nerve Stimulation in Treating Major Depressive Disorder: A Systematic Review and Meta-Analysis. Medicine 2018, 97, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Lu, D.; Rong, P.; Cheng, J.; Li, M.; Gong, Y.; Sun, C.; Wei, W.; Lin, L.; et al. Transcutaneous Auricular Vagal Nerve Stimulation Improves Functional Dyspepsia by Enhancing Vagal Efferent Activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G700–G711. [Google Scholar] [CrossRef] [PubMed]
- Mion, F.; Pellissier, S.; Garros, A.; Damon, H.; Roman, S.; Bonaz, B. Transcutaneous Auricular Vagus Nerve Stimulation for the Treatment of Irritable Bowel Syndrome: A Pilot, Open-Label Study. Bioelectron. Med. 2020, 3, 5–12. [Google Scholar] [CrossRef]
- Usichenko, T.; Hacker, H.; Lotze, M. Transcutaneous Auricular Vagal Nerve Stimulation (TaVNS) Might Be a Mechanism behind the Analgesic Effects of Auricular Acupuncture. Brain Stimul. 2017, 10, 1042–1044. [Google Scholar] [CrossRef]
- Wolf, V.; Kühnel, A.; Teckentrup, V.; Koenig, J.; Kroemer, N.B. Does Transcutaneous Auricular Vagus Nerve Stimulation Affect Vagally Mediated Heart Rate Variability? A Living and Interactive Bayesian Meta-Analysis. Psychophysiology 2021, 58, e13933. [Google Scholar] [CrossRef]
- Christodoulou, G.; Salami, N.; Black, D.S. The Utility of Heart Rate Variability in Mindfulness Research. Mindfulness 2020, 11, 554–570. [Google Scholar] [CrossRef]
- Hippel, C.V.; Hole, G.; Kaschka, W.P. Autonomic Profile under Hypnosis as Assessed by Heart Rate Variability and Spectral Analysis. Pharmacopsychiatry 2001, 34, 111–113. [Google Scholar] [CrossRef]
- Sakakibara, M.; Takeuchi, S.; Hayano, J. Effect of Relaxation Training on Cardiac Parasympathetic Tone. Psychophysiology 1994, 31, 223–228. [Google Scholar] [CrossRef]
- Lucini, D.; Covacci, G.; Milani, R.; Mela, G.S.; Malliani, A.; Pagani, M. A Controlled Study of the Effects of Mental Relaxation on Autonomic Excitatory Responses in Healthy Subjects. Psychosom. Med. 1997, 59, 541–552. [Google Scholar] [CrossRef]
- Laird, K.T.; Tanner-Smith, E.E.; Russell, A.C.; Hollon, S.D.; Walker, L.S. Short-Term and Long-Term Efficacy of Psychological Therapies for Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 937–947.e4. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- McCorry, L.K. Physiology of the Autonomic Nervous System. Am. J. Pharm. Educ. 2007, 71, 1–11. [Google Scholar] [CrossRef]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A Meta-Analysis of Heart Rate Variability and Neuroimaging Studies: Implications for Heart Rate Variability as a Marker of Stress and Health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Muscatello, M.R.A.; Bruno, A.; Mento, C.; Pandolfo, G.; Zoccali, R.A. Personality Traits and Emotional Patterns in Irritable Bowel Syndrome. World J. Gastroenterol. 2016, 22, 6402. [Google Scholar] [CrossRef]
- Scully, P.; McKernan, D.P.; Keohane, J.; Groeger, D.; Shanahan, F.; Dinan, T.G.; Quigley, E.M.M. Plasma Cytokine Profiles in Females with Irritable Bowel Syndrome and Extra-Intestinal Co-Morbidity. Am. J. Gastroenterol. 2010, 105, 2235–2243. [Google Scholar] [CrossRef]
- Gao, J. Correlation between Anxiety-Depression Status and Cytokines in Diarrhea-Predominant Irritable Bowel Syndrome. Exp. Ther. Med. 2013, 6, 93–96. [Google Scholar] [CrossRef]
- Hill, L.K.; Siebenbrock, A. Are All Measures Created Equal? Heart Rate Variability and Respiration-Biomed 2009. Biomed. Sci. Instrum. 2009, 45, 71–76. [Google Scholar]
- Alvares, G.A.; Quintana, D.S.; Hickie, I.B.; Guastella, A.J. Autonomic Nervous System Dysfunction in Psychiatric Disorders and the Impact of Psychotropic Medications: A Systematic Review and Meta-Analysis. J. Psychiatry Neurosci. 2016, 41, 89–104. [Google Scholar] [CrossRef]
- BouSaba, J.; Sannaa, W.; Camilleri, M. Pain in Irritable Bowel Syndrome: Does Anything Really Help? Neurogastroenterol. Motil. 2022, 34, e14305. [Google Scholar] [CrossRef]
- Kułak-Bejda, A.; Bejda, G.; Waszkiewicz, N. Antidepressants for Irritable Bowel Syndrome—A Systematic Review. Pharmacol. Rep. 2017, 69, 1366–1379. [Google Scholar] [CrossRef]
- Sims, S.T.; Ware, L.; Capodilupo, E.R. Patterns of Endogenous and Exogenous Ovarian Hormone Modulation on Recovery Metrics across the Menstrual Cycle. BMJ Ppen Sport Exerc. Med. 2021, 7, e001047. [Google Scholar] [CrossRef]
- Wilczak, A.; Marciniak, K.; Kłapciński, M.; Rydlewska, A.; Danel, D.; Jankowska, E.A. Relations between Combined Oral Contraceptive Therapy and Indices of Autonomic Balance (Baroreflex Sensitivity and Heart Rate Variability) in Young Healthy Women. Ginekol. Pol. 2013, 84, 915–921. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Mertgen, A. Vagal Tank Theory: The Three Rs of Cardiac Vagal Control Functioning - Resting, Reactivity, and Recovery. Front. Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef]
- Taylor, A.G.; Goehler, L.E.; Galper, D.I.; Innes, K.E.; Bourguignon, C. Top-Down and Bottom-Up Mechanisms in Mind-Body Medicine: Development of an Integrative Framework for Psychophysiological Research. EXPLORE 2010, 6, 29–41. [Google Scholar] [CrossRef]
- Jarrett, M.E.; Cain, K.C.; Barney, P.G.; Burr, R.L.; Naliboff, B.D.; Shulman, R.; Zia, J.; Heitkemper, M.M. Balance of Autonomic Nervous System Predicts Who Benefits from a Self-Management Intervention Program for Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2016, 22, 102–111. [Google Scholar] [CrossRef]
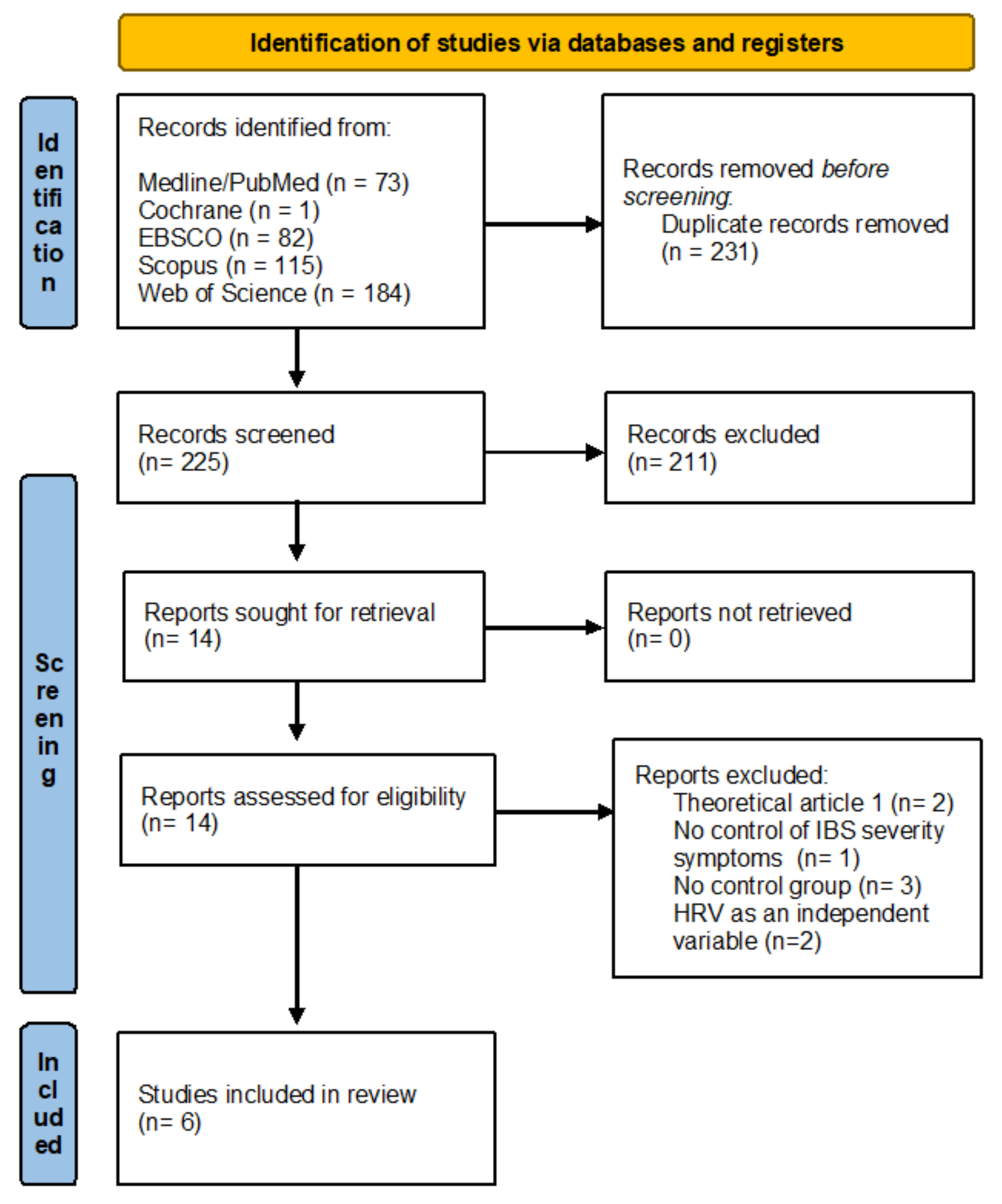
| Author | Year | Country | N (E/C) | % Women | Rome Criteria | IBS Type | Methods for Assessing IBS Symptoms | Methods for Assessing Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|
| Jang et al. [46] | 2017 | Korea | 21/17 | 100 | III | IBS-C | GI Symptom Rating Scale (GSRS-IBS) | Anxiety, Depression: HADS; Stress: GARS |
| Jurek et al. [47] | 2021 | USA | 7/6 | 69.2 | n/d | n/d | IBS Severity Scoring System (IBS-SSS) | none |
| Kavuri, Selvan, Malamud et al. [48] | 2015 | USA | 25/26/27 | 83.3 | III | IBS-C/D/M | IBS Severity Scoring System (IBS-SSS) | Anxiety, Depression: HADS |
| Park and Cha [49] | 2012 | Korea | 21/21 | 100 | III | n/d | IBS module from Rome III Questionnaire (10 items); Bowel Symptom Severity Scale (BSSS) | Stress: GARS; Mental Health: SCL-90R-K |
| Go and Park [50] | 2019 | South Korea | 29/27 | 100 | III | IBS-C/D/M | Bowel Symptom Severity Scale (BSSS) | Stress: PSS; Mental Health: SCL-90R-K |
| Shi et al. [51] | 2021 | China | 21/19 | 75 | IV | IBS-C | IBS-SSS, Bristol stool form scale (BSFS), the bowel diary with Visual Analogue Scale (VAS) for abdominal pain | Anxiety/Depression: SAS/SDS |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Overall a |
|---|---|---|---|---|---|---|---|---|---|---|
| Park & Cha [49] | + | + | ? | + | + | − | + | ? | + | 6.00 |
| Go & Park [50] | + | + | + | + | + | ? | + | ? | + | 7.00 |
| Jurek et al. [47] | + | − | ? | + | + | ? | + | ? | + | 5.00 |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Overall a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jang et al. [46] | + | ? | + | ? | - | + | − | ? | − | + | ? | + | + | 6.00 |
| Kavuri, Selvan, Malamud et al. [48] | + | + | + | ? | + | + | − | ? | + | + | ? | + | + | 9.00 |
| Shi et al. [51] | + | − | + | + | − | − | + | + | + | + | ? | + | + | 9.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mróz, M.; Czub, M.; Brytek-Matera, A. Heart Rate Variability—An Index of the Efficacy of Complementary Therapies in Irritable Bowel Syndrome: A Systematic Review. Nutrients 2022, 14, 3447. https://doi.org/10.3390/nu14163447
Mróz M, Czub M, Brytek-Matera A. Heart Rate Variability—An Index of the Efficacy of Complementary Therapies in Irritable Bowel Syndrome: A Systematic Review. Nutrients. 2022; 14(16):3447. https://doi.org/10.3390/nu14163447
Chicago/Turabian StyleMróz, Magdalena, Marcin Czub, and Anna Brytek-Matera. 2022. "Heart Rate Variability—An Index of the Efficacy of Complementary Therapies in Irritable Bowel Syndrome: A Systematic Review" Nutrients 14, no. 16: 3447. https://doi.org/10.3390/nu14163447
APA StyleMróz, M., Czub, M., & Brytek-Matera, A. (2022). Heart Rate Variability—An Index of the Efficacy of Complementary Therapies in Irritable Bowel Syndrome: A Systematic Review. Nutrients, 14(16), 3447. https://doi.org/10.3390/nu14163447






